José Luis Álvarez-Vásquez1,2,* , Nathaly Fernanda Parra-Solano1,2
, Nathaly Fernanda Parra-Solano1,2 , Gabriela Elizabeth Saavedra-Cornejo1,2
, Gabriela Elizabeth Saavedra-Cornejo1,2 and Ximena Elizabeth Espinosa-Vásquez1,2
and Ximena Elizabeth Espinosa-Vásquez1,2
1Especialización en Endodoncia, Facultad de Odontología, Universidad de Cuenca, Ecuador.
2Facultad de Odontología, Universidad de Cuenca, Cuenca, Ecuador.
Corresponding Author E-mail: jose.alvarezv@ucuenca.edu.ec
DOI : https://dx.doi.org/10.13005/bpj/2421
Abstract
Toothache is one of the most common global health problems, and medicinal plants are widely used to relieve the associated pain and inflammation. Several studies have been conducted on the use of plants to treat toothache, but no study has comprehensively assessed the types of plants and the mechanisms of action of the phytochemical compounds involved in their analgesic effect. This review aims to bridge this gap. This is the first review to collect a large volume of data on the global use of medicinal plants used in the treatment of toothache. It presents the relevant information for dentists, researchers, and academics on using medicinal plants to treat toothache. We found that preclinical studies and state-of-the-art technology hold promise for furthering our knowledge of this important topic. In total, 21 species of medicinal plants used to treat toothache were found in America, 29 in Europe, 192 in Africa, 112 in Asia, and 10 in Oceania. The most common species were Allium sativum, Allium cepa, Acmella oleracea, Jatropha curcas, Jatropha gossypiifolia, and Syzygium aromaticum. The most commonly found family of medicinal plants was Asteraceae, followed by Solanaceae, Fabaceae, Lamiaceae, Euphorbiaceae, Rutaceae, and Myrtaceae. The most common phytochemicals found were flavonoids, terpenes, polyphenols, and alkaloids. The reported mechanisms of action involved in toothache analgesia were antioxidant effects, effects mediated by transient receptor potential channels, the γ-aminobutyric acid mechanism, and the cyclooxygenase/lipoxygenase anti-inflammatory mechanism.
Keywords
Dental Pain; Flavonoids; Medicinal Plants; Phytochemicals; Toothache
Download this article as:| Copy the following to cite this article: Álvarez-Vásquez J. L, Parra-Solano N. F, Saavedra-Cornejo G. E, Espinosa-Vásquez X. E. Global use of Ethnomedicinal Plants to Treat Toothache. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Álvarez-Vásquez J. L, Parra-Solano N. F, Saavedra-Cornejo G. E, Espinosa-Vásquez X. E. Global use of Ethnomedicinal Plants to Treat Toothache. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3I2SVtf |
Introduction
Toothache is an unpleasant sensory and emotional experience1 originating in the tooth or adjacent structures and is caused by factors such as caries, periodontal disease, trauma, or dentoalveolar abscess2. It is one of the most common health problems worldwide3. Toothache has a higher prevalence in lower socioeconomic groups, in whom this disease is not always adequately treated4, and in developing countries, where access to healthcare is limited. This has led many local communities to resort to using alternatives for toothache relief, such as medicinal plants3.
Medicinal plants are widely used in dental practices. The World Health Organization has reported that between 65% and 80% of the population in developing countries use them to reduce inflammation, inhibit oral pathogen growth, and trigger anti-inflammatory, antiseptic, antioxidant, and analgesic effects3,4. Several phytochemical studies conducted on these plants have identified compounds such as flavonoids, alkaloids, and terpenes, which reduce toothache through their mechanism of action3,5-7.
Phytotherapy is the use of plants to treat diseases or as health-promoting agents. When used for this purpose, their original composition and integrity are generally preserved, so that an entire plant or a desired percentage of its components may be used for medicinal purposes, fulfilling a specific mechanism of action, generally, a specific pathway to relieve pain8. However, to our knowledge, no study has comprehensively tackled the mechanisms of action of the phytochemical compounds contained in medicinal plants used to treat toothache. This integrative review aimed to bridge this gap by compiling and analyzing the different studies available in the literature.
Materials and Methods
The available literature in PubMed, PMC, and Scopus databases was searched to identify relevant articles on medicinal plants used to relieve toothache, published in English until July 31, 2021, using the search terms toothache, dental pain, medicinal plants, medicinal herbs, and phytochemicals. Articles unrelated to the use of plants that relieved toothache or lacking data for at least one of the following characteristics were excluded: family, scientific name, plant parts used, and method of preparation.
Of a total of 300 articles, 80 met the inclusion criteria and were comprehensively analyzed for this review. In addition, we performed a manual search of the reference lists of the initially selected articles to complement the available information and found 294 additional articles. Ten books with relevant information were also included. Regional medicinal plant types retrieved from the articles and books were summarized by continents. Finally, owing to length restrictions, this review did not include information related to the possible adverse reactions and drug interactions resulting from the use of the plants included in this review.
Medicinal Plants for Toothache Treatment
For several millennia, plants have been used in traditional dentistry to treat toothache, periodontal disease, herpetic ulcers, stomatitis, maxillary sinusitis, and other ailments6. In recent years, advances in science and technology have identified the phytochemical compounds in some of these plants and their mechanisms of action3. Phytochemicals are a large group of plant-derived chemical substances that have various biochemical and physiological effects that are beneficial for human health and nutrition6,9.
Phytochemicals found in plants vary greatly in number, structural heterogeneity, and distribution, and they are classified into polyphenols, carotenoids, alkaloids, terpenes, and terpenoids10,11. All the tables in this review outline the phytochemicals described in previous reports on medicinal plants used to treat toothache, focusing on their analgesic mechanisms of action.
Plant Parts and Preparation Method
As mentioned above, plants are used to treat diseases through phytotherapy, using either the entire plant or a desired percentage of its components8. The most commonly used parts of medicinal plants are the leaves, seeds, flowers, and roots. The roots, in particular, are highly important because they are higher in bioactive compound content than other plant parts3, 12-14.
Leaves contain high concentrations of secondary metabolites, phytochemicals, and essential oils that have various health benefits14. Hence, most of the research studies support the use of leaves instead of roots because root extraction threatens the conservation of several plant species, especially those that are widely used3,14.
There is considerable variation in the preparation methods of plants used to treat toothache, and the most common methods of administration are: using the plant extract, chewing, crushing, and drinking a decoction3.
Mechanisms of action of phytochemical compounds
Phytochemicals such as flavonoids, alkaloids, and terpenes3,5 are biologically active compounds found in plants that work through various mechanisms of action15,16. Based on the information gathered in this review, the most salient mechanisms of action of phytochemicals used to treat toothache were antioxidant activity9,17, action on transient receptor potential channels (TRP)18, γ-aminobutyric acid (GABA) mechanism19,20, and anti-inflammatory mechanisms (cyclooxygenase (COX) and lipoxygenase (LOX) pathways)21.
Antioxidant Activity
In living organisms, reactive oxygen species (ROS) are generated during metabolism and do not generally cause oxidative damage to cellular components due to the action of antioxidants present in these organisms22.
Natural antioxidants are found in various plants and play a key role in stopping the generation of free radicals by preventing the oxidation of biomolecules in the body. Therefore, they are valuable therapeutic agents for preventing diseases caused by oxidative stress. The latter causes an imbalance that favors the production of prooxidants, represented by ROS, such as superoxide anions (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH–) [23], which damage key cellular components, such as DNA, proteins, and membrane lipids, and can even trigger cell death17,24-27.
Conversely, during inflammatory processes, free radicals balance themselves by attacking the nearest stable molecule and “stealing” an electron. The attacked molecule then becomes a free radical by losing its electron and initiating a cascade of cell-damaging reactions24. Additionally, leukocytes present in damaged regions cause a “respiratory burst” from enhanced oxygen uptake, and inflammatory cells generate inflammatory mediators that act on the infection site to release more reactive species24,28,29.
Therefore, the role of antioxidants is to delay, prevent, or eliminate oxidative damage of target molecules by controlling the levels of free radicals and other reactive species30. Plants are responsible for our oxygenated environment, and because they are exposed to high intracellular levels of oxygen and ROS, they have developed specialized defense systems (antioxidants) to protect their structures and tissues. Antioxidant activity is inherent to all plants as they act to prevent, destroy, or neutralize free radicals17.
These antioxidant defense systems can be enzymatic complexes and non-enzymatic systems. Some enzymatic complexes are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR). Non-enzymatic systems consist of low-molecular-weight antioxidants such as ascorbic acid, glutathione, proline, carotenoids, phenolic acids, and flavonoids; and high-molecular-weight secondary metabolites, such as tannins, which efficiently prevent the toxic effects of free radicals31,32.
The phytochemicals in plants can act as antioxidants by directly eliminating ROS, chelating metals (Fe, Zn, Mg, and Mn), quenching the mitochondrial respiratory chain, and increasing the levels of endogenous antioxidant enzymes, such as SOD, CAT, and GPx9,31.
ROS and reactive nitrogen species (RNS) are key players in various types of pain33. Evidence suggests that tissue injury induces the production of both ROS and RNS, which cause pain by promoting neuronal excitability in pain pathways through neural interactions and by triggering mitochondrial dysfunction and neuro-inflammation26,34.
Peroxynitrite (ONOO–) (PN) and its precursor superoxide (SO) are critical in the development of chronic pain and in the transition from acute to chronic pain35. An increase in SO/PN production triggers thermal hyperalgesia associated with acute and chronic inflammation in response to the activation of the N-methyl-d-aspartate receptor (NMDAR), leading to the development of orofacial pain36.
PN improves protein kinase C (PKC) activity. This kinase is activated by peripheral and central sensitization and optimizes the translocation of regulatory subunits of NADPH oxidase to the cell membrane to increase the production of SO derived from NADPH oxidase. These two mechanisms together amplify the formation of SO-derived PN, leading to the development of central sensitization35.
Thus, antioxidants can be administered for pain management to prevent the negative impact of ROS and RNS on nociception, both of which play key roles in neuro-inflammatory processes both at the central and peripheral levels, leading to increased nociceptive and inflammatory responses26,33,37,38.
In addition to their antioxidant activity, flavonoids and phenolic compounds exhibit effective anti-inflammatory biological properties by blocking two main signaling pathways, NF-𝜅B and mitogen-activated protein kinase (MAPK)24. These pathways initiate a cascade of phosphorylation events and result in the production of several pro-inflammatory mediators that mediate the transmission of extracellular signals from the membrane to the nucleus24,27,39.
Action on TRP Channels
TRP channels are involved in various homeostatic and sensory functions, such as nociception and temperature sensation, and are expressed in both neuronal and non-neuronal cells. They are grouped into six subfamilies: TRP ankyrin (TRPA), TRP canonical (TRPC), TRP melastatin (TRPM), TRP mucolipin (TRPML), TRP polycystin (TRPP), and TRP vanilloid (TRPV). They are mostly non-selective cation channels expressed on the cell membrane, including the TRPA1 channel, a Ca2+-permeable channel expressed in sensory neurons and is activated by phytochemicals and multiple products of oxidative stress18.
The Ca2+-permeable TRP channels of presynaptic terminals can modulate synaptic transmission independent of action potentials. Thus, the TRP channels, TRPV1, and TRPA1 can cause the release of neurotransmitters at sensory nerve terminals where these channels are highly co-expressed and participate in inflammatory hyperalgesia18,40. Capsaicin (hot pepper), allicin (garlic), camphor (Cinnamomum camphora), rosemary, and menthol (peppermint) are all analgesics that excite and desensitize nociceptive sensory neurons by acting on the TRPA1 and TRPV1 channels41–43.
Other phytochemicals also activate the TRP channels. For example, curcumin (Curcuma longa) activates TRPA1 channels; eugenol activates the TRPV1 and TRPV3 channels; menthol activates TRPM8 channels; ginger components activate the TRPV1 and TRPA1 channels; and the thymol and linalool compounds of thyme (Thymus vulgaris) activate the TRPV3 and TRPA1 channels18.
GABA Mechanism
GABA is a major inhibitory neurotransmitter44 involved in most inhibitory actions in the central and peripheral nervous systems (CNS and PNS). GABA exerts its action through two types of receptors: ionotropic (GABAA and GABAC) and metabotropic (GABAB) receptors. GABAA and GABAC are ion channels found in CNS neurons that are permeable to chloride ions when activated by GABA. GABAB receptors belong to the superfamily of G protein-coupled receptors and are present at different levels of the pain neuraxis where they regulate nociceptive transmission and pain19,45,46.
Some phytochemicals, including flavonoids and terpenes, modulate the function of ionotropic GABA receptors and can act as positive, negative, and neutralizing allosteric modulators. Thus, herbal preparations such as Heliopsis longipes, Acmella caulirhiza, Ginkgo biloba, Panax ginseng, and Scutellaria lateriflora may help modulate toothache by crossing the blood–brain barrier and influencing brain function. Past research has suggested that an increase in GABAergic activity in the rostral agranular insular cortex may induce analgesia by enhancing the descending inhibition of spinal cord nociceptive neurons19,47.
Spilanthes acmella is a flowering herb species, also known as the “toothache plant”48–50. It has been used for centuries to treat oral pain owing to its analgesic, anti-inflammatory, and anesthetic properties attributed to its bioactive compounds, especially phytosterols, phenolic compounds, and N-alkylamides48,50,51. Spilanthol, which is mainly present in the flowers and shoots of S. acmella, is the most representative compound found in this genus. This plant species and other species such as H. longipes are used worldwide as traditional remedies for their analgesic, antinociceptive, antioxidant, and anti-inflammatory effects. The analgesic effect of this compound is attributed to GABA release in the temporal cerebral cortex, whereas the antinociceptive effect is caused by the activation of the opioid-adrenergic, serotonergic, and GABAergic systems52.
The flavonoid baicalein, which can be extracted from S. lateriflora, exerts sedative and anxiolytic effects by binding to GABAA receptors and, hence, could be used to manage orofacial pain. This flavonoid is also believed to modulate both intra- and extracellular calcium levels, which play key roles in pain signaling and transmission44.
GABA receptor systems are found in peripheral pathways and the spinal cord, which are both important sites for pain impulse formation and transmission; they are located in the marginal zone and substantia gelatinosa of the dorsal horn, which are essential for interpreting and responding to pain signals. These findings indicate that GABA plays a key role in nociceptive processing. Consequently, agents that modify the function of this inhibitory neurotransmitter are used as analgesics46.
Anti-inflammatory Mechanism (COX and LOX Pathways)
Inflammation is mediated by several families of mediators such as eicosanoids, which are lipid mediators produced through arachidonic acid metabolism, primarily in the COX and LOX pathways53. The COX pathway leads to the formation of prostanoids (prostaglandins (PG), prostacyclin, and thromboxane), whereas the LOX pathway leads to the production of leukotrienes (LTs)54.
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit the COX pathway, whereas other drugs such as licofelone are dual inhibitors that block both COX and LOX54,55. However, the selective inhibition of the two COX isoforms by NSAIDs has several reported side effects. This has encouraged the search for a dual inhibitor of both COX-2 and 5-LOX that possesses improved anti-inflammatory potency and fewer side effects53,56.
This anti-inflammatory effect leads to the elimination of harmful stimuli and the restoration of normal physiology through the complex molecular cascade mentioned above3,21. This is thought to be the mechanism by which herbal extracts act in the treatment of toothache21. Accordingly, medicinal plants, particularly herbs whose main component is curcumin, such as C. longa, seem to provide several advantages through their mediating action on the COX and LOX pathways. As a dual inhibitor, curcumin exhibits synergistic effects and optimal anti-inflammatory activity57. Allium cepa (onion), which also contains polyphenols and flavonoids, inhibits the COX and LOX pathways and prevents the formation of LTs, thromboxane B2 (TXB2), and prostaglandin E2 (PGE2)58,59.
Additionally, various ginger compounds, such as gingerols, shogaols, zingerones, gingerdiols, and paradols, exhibit antioxidant, analgesic, and anti-inflammatory activities. More specifically, they act through the inhibition of COX and LOX in addition to their antioxidant activity resulting in an analgesic effect60. Allium sativum also has antioxidant and anti-inflammatory properties, and its efficacy in reducing pro-inflammatory responses is based on its nature as a COX and LOX inhibitor59.
Bioavailability of Medicinal Plants
In humans, most phytochemicals exhibit low bioavailability after ingestion9. Hence, polyphenols have a rather low bioavailability because they exert most of their antioxidant activity in the gastrointestinal tract61. Additionally, a challenge with flavonoids is their low water solubility, which leads to decreased absorption and consequently decreased bioavailability following oral administration62.
Interindividual variability, which depends on several factors such as diet, genetic background, composition, and activity of the intestinal microbiota, must also be considered. For example, polyphenols are relatively poorly absorbed (0.3–43%), resulting in low circulating plasma concentrations of their metabolites63. Additionally, the quantity and composition of phytochemicals in plants are influenced by species, age, plant part, cultivation method, harvesting season, conservation method, and geographic distribution9,64.
To improve bioavailability, proper decoction practices and various plants combinations have been suggested65 due to their different phytochemical components and because they may provide different health benefits without requiring an increase in the dose9. For example, Piper sarmentosum combined with ginger is used to soothe toothache66. Medicinal plants containing hundreds of phytochemicals can produce many metabolites in the body, exerting more efficient beneficial effects than individual phytochemicals9. However, their combination can also directly affect their bioavailability in the body via mechanisms such as the first-pass effect18,67.
Medicinal Plants vs Pharmaceutical Drugs
Comparisons between the analgesic effects of medicinal plants and pharmaceutical drugs have shown that the rhizome of Zingiber officinale (ginger) has long been used in traditional Chinese and Indian medicine to treat a wide range of ailments, including toothache68. Fresh ginger extracts have been subjected to chromatographic purification, and the resulting fractions were analyzed to assess their effect on PG synthesis. Through this method, plant extracts belonging to the Zingiberaceae family were found to inhibit PG synthesis in vitro69.
The rhizome of Z. officinale has pharmacological properties similar to those of dual-action NSAIDs55. It inhibits both COX and LOX and has significantly fewer side effects than conventional NSAIDs69,70. Licofelone is an example of a dual-action NSAID (5-LOX/COX) that is currently in phase III clinical development55. Studies have shown that orally administered dry ginger or ginger extract can reduce acute inflammation68,71, and in vitro and in vivo comparisons have confirmed the anti-inflammatory and analgesic actions of ginger extract69,70.
However, most in vitro studies analyzed phytochemical profiles using indices such as the half-maximal inhibitory concentration (IC50), and the medicinal plant extracts have been tested for only a single biological target, COX or LOX. This is insufficient to validate their anti-inflammatory and analgesic properties and hinders direct comparisons between plants and dual-action NSAIDs72.
The anti-inflammatory properties of ginger extracts come from a mixture of biologically active components such as gingerols, shogaols, and paradols, which are phenolic compounds73. The inhibitory effects of ginger on PG synthesis can be attributed to the presence of hydroxymethoxyphenyl compounds in gingerols and shogaols, which in turn inhibit arachidonic acid metabolism via the COX pathway69,74. Moreover, ginger components inhibit several genes encoding cytokines and chemokines involved in inflammatory responses69,75.
Essential oil from the fruit of the plant Dennettia tripetala (DT), commonly known as pepper fruit, has analgesic effects like those induced by opioids morphine, aspirin, and indomethacin. The analgesic mechanism of DT has been inferred from studies showing that naloxone, which inhibits the analgesic effect of morphine, could also inhibit DT. These findings suggest that DT can also be used for toothache relief22.
Plant Combinations
Mixtures of medicinal plants are a key field of research which accounts for a large volume of information because their polyvalent effects can be used to cure multicausal diseases76. In different regions and cultures, plants are used as the entire plant, a combination of plants, or a combination of a plant and a drug. When medicinal plants are mixed, side effects are more likely to happen because interactions can occur between individual components. The most desirable interactions provide additional therapeutic benefits. However, natural extracts also contain multiple components. Therefore, the effects of interactions between two plants are often unpredictable and complex77.
Additionally, a combination of two or more phytochemicals does not always enhance a specific effect. Combining two or more active chemical substances can produce additive, synergistic, or antagonistic effects9,78. An example of synergism in the use of medicinal plants is Iberogast®, a phyto-preparation used in European countries consisting of nine plant extracts. It is considered to have a multi-target effect (at the gastrointestinal level). Such a multi-target effect has advantages over that of synthetic single-target drugs79,80.
Another example is the phytotherapeutic drug Lenidase®, which, when compared to ibuprofen, more efficiently and safely controls postoperative pain and discomfort following third molar extraction. Lenidase® contains a blend of herbal extracts, such as baicalin (190 mg), bromelain (50 mg), and escin (30 mg)81, which exhibit anti-inflammatory activities. Bromelain inhibits pain mediators such as PGE2 and substance P and exhibits anti-edematous activity. Baicalin regulates several genes associated with inflammation, such as COX, LOX, and the inducible nitric oxide synthase gene. Escin exerts anti-inflammatory and anti-edematous effects through antihistaminic and antiserotonergic activities81.
Results and Discussion
As discussed above, medicinal plants, their phytochemicals, and their mechanisms of action are key subjects of scientific research because they are used to treat and prevent various diseases. Further, plants are the basis of many drugs. Although they are highly complex compounds and are not always suitable substitutes for synthetic agents82, phytochemicals have been used to provide relief from toothache in various regions of the world, as outlined in the five tables included in this review.
The components of the medicinal plants used and their preparations vary by location and between species. For example, a study conducted in America revealed that leaves were the most commonly used plant parts and that the most common preparations were pastes, extracts, and rinses83. Another study conducted in Africa found that Datura stramonium L. roots, leaves, stems, and seeds were often used to provide relief from toothache3. These findings demonstrate the need for further phytochemical and pharmacological studies to identify the plant part that is most effective for toothache treatment and the optimal application method to increase its action.
The parts used may vary with the medicinal plant; however, we found that the most commonly used plant parts were leaves and roots, followed by the bark, stem, and seeds (Fig. 1). Preferentially using the leaves instead of roots can also prevent detrimental effects on the plants.
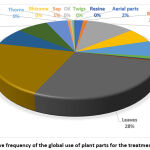 |
Figure 1: Relative Frequency of the Global use of Plant Parts for the Treatment of Toothache. |
The most common phytochemicals involved in the mechanism of action of medicinal plants for toothache treatment are polyphenols, more specifically, flavonoids and terpenes, which are the most abundant secondary metabolites and antioxidants in the human diet3,5-7. Flavonoids are the most ubiquitous group of all plant phenolics, which could explain the implicit antioxidant capacity of all medicinal plants84. Furthermore, flavonoids can modulate the function of ionotropic GABA receptors, suggesting that these phytochemicals can exert different mechanisms of action to relieve pain20.
Polyphenols are strong antioxidants that neutralize free radicals by donating an electron or a hydrogen atom61, thus exerting antioxidant effects in plants and organisms that consume them. However, polyphenols decrease the concentrations of ROS and RNS far from the site of the primary response because the local concentrations of these radicals around the inflammatory site are substantially high (> 1 mM). Therefore, polyphenols are highly unlikely to be effective where these free radicals are produced but could be quantitatively more effective as antioxidants in the surrounding unaffected tissues85.
Substances such as capsaicin, allicin, camphor, and menthol cause a state of activation and desensitization in the TRP receptor pathway through which pain may be reduced18,43. Of these phytochemicals, only allicin was analyzed in the studies included in this review. Moreover, allicin and spilanthol, compounds present in A. caulirhiza, have various biological and pharmacological effects, which may cause analgesia3,52. Further studies should be performed to better understand the roles of these phytochemicals.
The mechanisms of action of phytochemicals in toothache relief are only partly understood. This review shows that these mechanisms involve antioxidant activity, action on TRP receptors, GABA mechanism, and COX/LOX inhibitory activity. The tables in this review outline 163 medicinal plants with antioxidant mechanisms of action, 20 with an anti-inflammatory mechanism (COX/LOX), four with GABA mechanism, and two with TRP mechanism. Some plants have two reported mechanisms of action. However, in general, there is insufficient literature addressing each mechanism responsible for toothache relief.
Several reports cite the use of various plants for toothache treatment; however, in many of these reports, the mechanism of action underlying pain relief is not specified, as indicated in the tables in this review. Moreover, in several studies, the phytochemicals potentially responsible for the analgesic effect were not reported. Accordingly, future studies should focus on identifying the exact mechanisms that contribute to dental analgesia and the phytochemicals involved. Additionally, the “common names” of medicinal plants were not included in this research, considering the extensive information involved in the preparation of this manuscript.
As mentioned above, we found only one study comparing the pharmacological properties of medicinal plants with those of conventional pharmaceutical drugs81. However, 30 plants with dual anti-inflammatory mechanisms (COX/LOX) were identified, as outlined in the tables for each continent. This information could be useful in future comparative studies of conventional or dual NSAIDs.
Although dual inhibition of microsomal PGE2 synthetase (mPGES-1) and 5-LOX has not been described as a mechanism of action in the reports included in this review, several plants (some of which are indicated in our tables) contain acylphloroglucinols, phenolic compounds, and non-phenolic acidic structures that exhibit such dual action [86]. mPGES-1 is an inducible enzyme at inflammatory sites that preferentially receives its substrate from co-induced COX-2 and is responsible for the excessive formation of PGE2 during acute and chronic inflammation87,88; thus, its inhibition could be a promising strategy for toothache treatment with medicinal plants. Furthermore, natural mPGES-1 inhibitors have advantages over NSAIDs since they are non-synthetic and safer because they do not inhibit COX-derived homeostatic eicosanoids86.
However, in several in vitro and in vivo studies (utilizing indices such as IC50), the vast majority of medicinal plant extracts have been tested only against one biological target (COX or LOX), which is insufficient to validate their anti-inflammatory and analgesic properties and hinders direct comparisons between plants and conventional or dual-action NSAIDs72. Therefore, further studies should be conducted to address this gap in research and gather more relevant information.
Multiple experimental studies of COX-1/COX-2 inhibition have used IC50 (the concentration at which an NSAID produces 50% inhibition of both COX enzymes) to rank the relative inhibitory activity of NSAIDs on these enzymes, and consequently, establish their selectivity over COX, correlating this in vitro inhibition with clinical efficacy and toxicity levels89. However, IC50 values do not indicate the mechanism of enzyme inhibition and vary with substrate concentration. Furthermore, these values are not directly comparable unless identical experimental conditions are used, and they must be analyzed carefully when inhibition is time-dependent89. These drawbacks also (91) hinder direct comparison between medicinal plants and NSAIDs.
Only two studies on plant combinations were found for this review. The study on phytotherapeutic Lenidase®81 was already described above. The other reported on the use of seven popular medicinal plant mixtures for toothache in Europe (Catalonia), including several species76 whose use was not found in other continents.
Although medicinal plants are distributed throughout the world90, biodiversity could affect how intensely such plants are used for toothache, and thus the discovery of new drugs91. The 17 most megadiverse countries in the world are Brazil, Colombia, Mexico, Peru, Ecuador, Venezuela, the United States of America, Indonesia, Australia, Madagascar, China, the Philippines, India, New Guinea, Malaysia, South Africa, and the Democratic Republic of Congo; most of these are in the American continent92. However, in this present review, most of the information on medicinal plants was gathered from Asia (Table 4) and Africa (Table 5), possibly because herbal medicines remain a key component of healthcare systems in the developing cultures of these continents90. Oceania has only two of the 17 megadiverse countries, which may explain the scarcity of plants in this continent (Table 5). Nevertheless, in some regions, much of the traditional knowledge about medicinal plants is only spread verbally and, thus, remains unexplored and unreported93.
In terms of plants used in different continents, A. sativum was found in America (Table 1), Europe (Table 2), and Africa (Table 3); A. cepa and Syzygium aromaticum were found in Europe, Africa, and Asia; and Acmella oleracea, Jatropha curcas, and Jatropha gossypiifolia were all found in America (Table 1), Africa (Table 3), and Asia (Table 4). Conversely, some species are found only in one continent, such as Thymus schimperi Ronniger in Africa, perhaps because this species is a rare plant highly localized in and endemic to Ethiopia94.
 |
Table 1: America. |
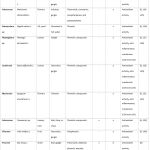 |
Table 2: Europe. |
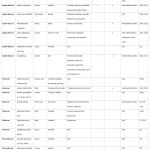 |
Table 3: Africa. |
 |
Table 4. Asia. |
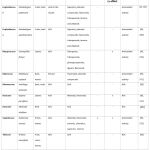 |
Table 5: Oceania. |
Among the families of medicinal plants used worldwide, Asteraceae was the most common, followed by Solanaceae, Fabaceae, Lamiaceae, Euphorbiaceae, Rutaceae, and Myrtaceae (Fig. 2). The first three of these families have been widely reported as those most commonly used to treat inflammation and various types of pain44,44,76.
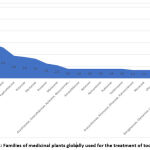 |
Figure 2: Families of medicinal plants globally used for the treatment of toothache. |
Notably, the present review discusses several medicinal plants for toothache treatment, which have been globally classified by continents, unlike all the referenced studies, which were approached separately. Moreover, the five tables provide details about the parts used, preparation, phytochemicals, analgesic/anti-inflammatory effect, and mechanisms of action, contrary to most studies that do not include such details. Additionally, this review analyzes all the mechanisms of action of the medicinal plants that have been ascribed until now for toothache treatment, unlike many studies that only cite these mechanisms. Furthermore, we have included a section comparing medicinal plants and pharmaceutical drugs, unlike all the referenced studies that do not provide such a comparison. Finally, this review discusses the use of medicinal plants for the treatment of dental pain, while most articles deal with this topic on a general basis.
Future Perspectives
Although phytotherapy has a long history, natural medicines are considered a hidden source of drugs because many medicinal plants have not been studied in depth79. Accordingly, further studies should be conducted to better understand the role and benefits of phytotherapeutic drugs81 for toothache treatment, particularly when combined based on the multi-objective therapeutic principle of phytotherapy79,95. This principle would be analogous to the multimodal analgesic approach used for NSAIDs96-98.
Aromatherapy also has non-pharmacological therapeutic potential for reducing toothache by combining highly complex mixtures of essential oils to produce a therapeutic effect95. Therefore, further research should be conducted in this field of alternative medicine.
Although polyphenols in organic food extracts (extractable polyphenols) have already been analyzed, significant amounts of potentially bioactive polyphenols that remain in the residues (non-extractable polyphenols) have been overlooked. Additionally, significant amounts of non-extractable polyphenols are found in foods and vegetables61,99. Therefore, these compounds should be considered for future studies.
A promising therapeutic option for the administration of flavonoids that may increase their bioavailability is to develop protective systems, such as microcapsules, nanoparticles, and nano-formulations, which improve water solubility, dissolution, absorption, and thermal stability. Accordingly, in the near future, such systems should be developed and administered for pain management62.
Finally, human clinical trials are essential to confirm the effectiveness of traditional phytotherapy for toothache and to investigate the pharmacodynamic and pharmacokinetic interactions between medicinal plants and other synthetic drugs. Similarly, predictive (in silico) models, phytochemical analyses, and ethnopharmacological studies could be milestones for drug discovery in traditional medicinal plants for toothache treatment, because many of them lack information or have not been studied.
Conclusion
This is the first review to compile a large volume of data on the global use of medicinal plants for the treatment of toothache. A total of 21 species of medicinal plants were found in America (Table 1), 29 in Europe (Table 2), 192 in Africa (Table 3), 112 in Asia (Table 4), and 10 in Oceania (Table 5). Asia and Africa are the continents where the most research has been done on this topic. Asteraceae was the most commonly found plant family in this review, followed by Solanaceae, Fabaceae, Lamiaceae, Euphorbiaceae, Rutaceae, and Myrtaceae.
In total, 364 medicinal plants used for toothache treatment were identified, of which 139 have not yet been scientifically studied, highlighting opportunities for ethnopharmacological research on toothache treatments. The most common species were A. sativum, A. cepa, A. oleracea, J. curcas, J. gossypiifolia, and S. aromaticum. These families and species were more commonly found in Africa and Asia, corroborating our previously reported findings. As determined in this review, the most commonly used plant parts were the leaves and roots, followed by the bark, stems, and seeds.
We identified four mechanisms of action of medicinal plants implied in toothache treatment, namely, the antioxidant effect, effects mediated through TRP receptors, GABA mechanism, and the anti-inflammatory mechanism (COX/LOX). Flavonoids, terpenes, polyphenols, and alkaloids are the phytochemicals most commonly associated with toothache treatment. Many of the plants analyzed in this review have the potential to be used as agents for toothache treatment. Therefore, future studies must prioritize the analysis of their pharmacodynamic and pharmacokinetic interactions.
Finally, to more precisely clarify the usefulness of medicinal plants as a valid option for toothache treatment, comparative studies between medicinal plants and commonly used pharmaceutical drugs should be conducted. In addition, studies published in Spanish should be included in future reviews since we only analyzed studies published in English, and this may have limited our ability to gather additional information.
Acknowledgments
None.
Ethical Statement
Ethical approval was not required for this review article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heir GM. The Emerging Specialty of Orofacial Pain. J Indian Prosthodont Soc 2013; 13: 140–141.
CrossRef - Cohen LA, Bonito AJ, Akin DR, et al. Toothache pain: behavioral impact and self-care strategies. Spec Care Dent 2009; 29: 85–95.
CrossRef - Megersa M, Jima TT, Goro KK. The Use of Medicinal Plants for the Treatment of Toothache in Ethiopia. Evid Based Complement Alternat Med 2019; 2019: 2645174.
CrossRef - Palombo EA. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid Based Complement Alternat Med 2011; 2011: 680354.
CrossRef - Sharifi‐Rad M, Yılmaz YB, Antika G, et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother Res 2021; 35: 95–121.
CrossRef - Ilic DV, Radicevic BA, Nedelcheva A, et al. Traditional dentistry knowledge among Serbs in several Balkan countries. J Intercult Ethnopharmacol 2017; 6: 223–233.
CrossRef - Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011; 3: 10–14.
- Falzon CC, Balabanova A. Phytotherapy: An Introduction to Herbal Medicine. Prim Care 2017; 44: 217–227.
CrossRef - Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J Nutr Biochem 2019; 69: 19–30.
CrossRef - K. Tiwari, Nigel P. Bruton, Charles S. Brennan. Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction. 2013.
CrossRef - Prakash Bhanu. Functional and Preservative Properties of Phytochemicals. 1st ed. Elsevier, 2020.
CrossRef - Mahmood A, Mahmood A, Malik RN, et al. Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J Ethnopharmacol 2013; 148: 714–723.
CrossRef - Kunwar RM, Nepal BK, Kshhetri HB, et al. Ethnomedicine in Himalaya: a case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J Ethnobiol Ethnomed 2006; 2: 27.
CrossRef - Farooq A, Amjad MS, Ahmad K, et al. Ethnomedicinal knowledge of the rural communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. J Ethnobiol Ethnomed 2019; 15: 45.
CrossRef - Luna-Vázquez FJ, Ibarra-Alvarado C, Rojas-Molina A, et al. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013; 18: 5814–5857.
CrossRef - Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004; 134: 3479S-3485S.
CrossRef - Benzie IF, Choi SW. Antioxidants in food: content, measurement, significance, action, cautions, caveats, and research needs. Adv Food Nutr Res 2014; 71: 1–53.
CrossRef - Premkumar LS. Transient receptor potential channels as targets for phytochemicals. ACS Chem Neurosci 2014; 5: 1117–1130.
CrossRef - Johnston GAR, Hanrahan JR, Chebib M, et al. Modulation of ionotropic GABA receptors by natural products of plant origin. Adv Pharmacol 2006; 54: 285–316.
CrossRef - Hanrahan JR, Chebib M, Johnston GAR. Interactions of flavonoids with ionotropic GABA receptors. Adv Pharmacol 2015; 72: 189–200.
CrossRef - Tasneem S, Liu B, Li B, et al. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol Res 2019; 139: 126–140.
CrossRef - Iseghohi SO. A Review of the Uses and Medicinal Properties of Dennettia tripetala (Pepperfruit). Med Sci Basel 2015; 3: 104–111.
CrossRef - Nilius B, Flockerzi V. Mammalian Transient Receptor Potential (TRP) Cation Channels. Handb Exp Pharmacol, 2014. Epub ahead of print 2014. DOI: 10.1007/978-3-319-05161-1.
CrossRef - Arulselvan P, Fard MT, Tan WS, et al. Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longev 2016; 2016: 5276130.
CrossRef - Becker EM, Nissen LR, Skibsted LH. Antioxidant evaluation protocols: Food quality or health effects. Eur Food Res Technol 2004; 219: 561–571.
CrossRef - Tobore TO. Towards a Comprehensive Theory of Non-Cancer Acute and Chronic Pain Management: The Critical Role of Reactive Oxygen and Nitrogen Species in Pain, and Opioid Dependence, Addiction, Hyperalgesia, and Tolerance. Adv Redox Res 2021; 2: 100003.
CrossRef - Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000; 279: L1005–L1028.
CrossRef - Federico A, Morgillo F, Tuccillo C, et al. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 2007; 121: 2381–2386.
CrossRef - Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003; 3: 276–285.
CrossRef - Tang SY, Halliwell B. Medicinal plants and antioxidants: what do we learn from cell culture and Caenorhabditis elegans studies? Biochem Biophys Res Commun 2010; 394: 1–5.
CrossRef - Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr J Microbiol Res 2009; 3: 981–996.
- Kasote DM, Katyare SS, Hegde MV, et al. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int J Biol Sci 2015; 11: 982–991.
CrossRef - Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids 2012; 42: 75–94.
CrossRef - Grace PM, Gaudet AD, Staikopoulos V, et al. Nitroxidative Signaling Mechanisms in Pathological Pain. Trends Neurosci 2016; 39: 862–879.
CrossRef - Salvemini D, Little JW, Doyle T, et al. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med 2011; 51: 951–966.
CrossRef - Yeo JF, Ling SF, Tang N, et al. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Exp Brain Res 2008; 184: 435–438.
CrossRef - Bruno R, Ghiadoni L. Polyphenols, Antioxidants and the Sympathetic Nervous System. Curr Pharm Des 2018; 24: 130–139.
CrossRef - Rokyta R, Holecek V, Pekárkova I, et al. Free radicals after painful stimulation are influenced by antioxidants and analgesics. Neuro Endocrinol Lett 2003; 24: 304–309.
- Boutros T, Chevet E, Metrakos P. Mitogen-Activated Protein (MAP) Kinase/MAP Kinase Phosphatase Regulation: Roles in Cell Growth, Death, and Cancer. Pharmacol Rev 2008; 60: 261–310.
CrossRef - Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and Proc Natl Acad Sci U S A 2001; 98: 6951–6956.
CrossRef - Ruparel NB, Patwardhan AM, Akopian AN, et al. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 2008; 135: 271–279.
CrossRef - Bautista DM, Movahed P, Hinman A, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 2005; 102: 12248–12252.
CrossRef - Hossain MZ, Bakri MM, Yahya F, et al. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int J Mol Sci 2019; 20: 526.
CrossRef - Uritu CM, Mihai CT, Stanciu GD, et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res Manag 2018; 2018: 7801543.
CrossRef - Goudet C, Magnaghi V, Landry M, et al. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev 2009; 60: 43–56.
CrossRef - Enna SJ, McCarson KE. The Role of GABA in the Mediation and Perception of Pain. Adv Pharmacol 2006; 54: 1–27.
CrossRef - Jasmin L, Rabkin SD, Granato A, et al. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature 2003; 424: 316–320.
CrossRef - Dubey S, Maity S, Singh M, et al. Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A Review. Adv Pharmacol Sci 2013; 2013: 423750.
CrossRef - Prachayasittikul V, Prachayasittikul S, Ruchirawat S, et al. High therapeutic potential of Spilanthes acmella: A review. EXCLI J 2013; 12: 291–312.
- Abdul Rahim R, Jayusman PA, Muhammad N, et al. Potential Antioxidant and Anti-Inflammatory Effects of Spilanthes acmella and Its Health Beneficial Effects: A Review. Int J Environ Res Public Health 2021; 18: 3532.
CrossRef - Rondanelli M, Fossari F, Vecchio V, et al. Acmella oleracea for pain management. Fitoterapia 2020; 140: 104419.
CrossRef - Barbosa AF, Carvalho MG de, Smith RE, et al. Spilanthol: occurrence, extraction, chemistry and biological activities. Rev Bras Farmacogn 2016; 26: 128–133.
CrossRef - Julémont F, Dogné JM, Pirotte B, et al. Recent development in the field of dual COX / 5-LOX inhibitors. Mini Rev Med Chem 2004; 4: 633–638.
CrossRef - Yahfoufi N, Alsadi N, Jambi M, et al. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018; 10: 1618.
CrossRef - Martel-Pelletier J, Lajeunesse D, Reboul P, et al. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 2003; 62: 501–509.
CrossRef - Leval X d, Julemont F, Delarge J, et al. New trends in dual 5-LOX/COX inhibition. Curr Med Chem 2002; 9: 941–962.
CrossRef - Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol 2007; 595: 213–226.
CrossRef - Marefati N, Ghorani V, Shakeri F, et al. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm Biol 2021; 59: 287–302.
CrossRef - Wilson EA, Demmig‐Adams B. Antioxidant, anti‐inflammatory, and antimicrobial properties of garlic and onions. Nutr Food Sci 2007; 37: 178–183.
CrossRef - Rondanelli M, Fossari F, Vecchio V, et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phytother Res 2020; 34: 2843–2856.
CrossRef - Belščak-Cvitanović A, Durgo K, Huđek A, et al. Overview of polyphenols and their properties. In: Galanakis CM (ed) Polyphenols: Properties, Recovery, and Applications. Woodhead Publishing, 2018, pp. 3–44.
CrossRef - Ferraz CR, Carvalho TT, Manchope MF, et al. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020; 25: 762.
CrossRef - Rein MJ, Renouf M, Cruz‐Hernandez C, et al. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 2013; 75: 588–602.
CrossRef - Lim W, Mudge KW, Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium). J Agric Food Chem 2005; 53: 8498–8505.
CrossRef - Platel K, Srinivasan K. Bioavailability of Micronutrients from Plant Foods: An Update. Crit Rev Food Sci Nutr 2016; 56: 1608–1619.
- Sun X, Chen W, Dai W, et al. Piper sarmentosum Roxb.: A review on its botany, traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol 2020; 263: 112897.
CrossRef - Hewlings SJ, Kalman DS. Curcumin: A Review of Its Effects on Human Health. Foods 2017; 6: 92.
CrossRef - Rayati F, Hajmanouchehri F, Najafi E. Comparison of anti-inflammatory and analgesic effects of Ginger powder and Ibuprofen in postsurgical pain model: A randomized, double-blind, case-control clinical trial. Dent Res J Isfahan 2017; 14: 1–7.
CrossRef - Grzanna R, Lindmark L, Frondoza CG. Ginger–an herbal medicinal product with broad anti-inflammatory actions. J Med Food 2005; 8: 125–132.
CrossRef - Ali BH, Blunden G, Tanira MO, et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 2008; 46: 409–420.
CrossRef - Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005; 12: 684–701.
CrossRef - Khumalo GP, Van Wyk BE, Feng Y, et al. A review of the traditional use of southern African medicinal plants for the treatment of inflammation and inflammatory pain. J Ethnopharmacol 2022; 283: 114436.
CrossRef - Mao QQ, Xu XY, Cao SY, et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019; 8: 185.
CrossRef - Tjendraputra E, Tran VH, Liu-Brennan D, et al. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem 2001; 29: 156–163.
CrossRef - Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med 1986; 24: 195–198.
CrossRef - Gras A, Parada M, Rigat M, et al. Folk medicinal plant mixtures: Establishing a protocol for further studies. J Ethnopharmacol 2018; 214: 244–273.
CrossRef - Che CT, Wang ZJ, Chow MSS, et al. Herb-Herb Combination for Therapeutic Enhancement and Advancement: Theory, Practice and Future Perspectives. Molecules 2013; 18: 5125–5141.
CrossRef - Vijayalakshmi G, Adinarayana M, Jayaprakash Rao P. A synergistic approach to kinetic and mechanistic studies of regeneration of β-carotene from tert-butoxyl radical induced β-carotene radical cation by chlorogenic acid. Int J Pharm Life Sci 2014; 5: 942–950.
- Rather MA, Bhat BA, Qurishi MA. Multicomponent phytotherapeutic approach gaining momentum: Is the ‘one drug to fit all’ model breaking down?. Phytomedicine 2013; 21: 1–14.
CrossRef - Allescher HD. Functional dyspepsia–a multicausal disease and its therapy. Phytomedicine 2006; 13 Suppl 5: 2–11.
CrossRef - Isola G, Matarese M, Ramaglia L, et al. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: a randomized, triple-blind, controlled clinical trial. Clin Oral Investig 2019; 23: 2443–2453.
CrossRef - Szyszkowska A, Koper J, Szczerba J, et al. The use of medicinal plants in dental treatment. Herba Pol 2010; 56: 97–107.
- Cruz Martínez C, Diaz Gómez M, Oh MS. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: a review. Pharm Biol 2017; 55: 1992–1998.
CrossRef - Campos-Vega R, Oomah BD. Chemistry and classification of phytochemicals. In: Tiwari BK, Brunton NP, Brennan CS (eds) Handbook of Plant Food Phytochemicals. John Wiley & Sons Ltd, 2013, pp. 5–48.
CrossRef - Mark S. Meskin, Wayne R. Bidlack, Audra J. Davies, et al. Phytochemicals: Mechanisms of Action. 1st ed. 2003.
CrossRef - Koeberle A, Werz O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol Adv 2018; 36: 1709–1723.
CrossRef - Koeberle A, Northoff H, Werz O. Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase-1. Mol Cancer Ther 2009; 8: 2348–2355.
CrossRef - Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 2007; 59: 207–224.
CrossRef - Knights KM, Mangoni AA, Miners JO. Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert Rev Clin Pharmacol 2010; 3: 769–776.
CrossRef - Cock I. Medicinal and aromatic plants – Australia. 2011.
- Cordell GA. Biodiversity and drug discovery–a symbiotic relationship. Phytochemistry 2000; 55: 463–480.
CrossRef - Harding K, Benson EE, Nunes E da C, et al. Can Biospecimen Science Expedite the Ex Situ Conservation of Plants in Megadiverse Countries? A Focus on the Flora of Brazil. Crit Rev Plant Sci 2013; 32: 411–444.
CrossRef - Jamir K, Seshagirirao K, Meitei MD. Indigenous oral knowledge of wild medicinal plants from the Peren district of Nagaland, India in the Indo Burma hot-spot. Acta Ecol Sin 2021; 18.
CrossRef - Desta KT, Kim GS, Abd El-Aty AM, et al. Flavone polyphenols dominate in Thymus schimperi Ronniger: LC-ESI-MS/MS characterization and study of anti-proliferative effects of plant extract on AGS and HepG2 cancer cells. J Chromatogr B Analyt Technol Biomed Life Sci 2017; 1053: 1–8.
CrossRef - Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets 2011; 12: 122–132.
CrossRef - Manworren RC. Multimodal pain management and the future of a personalized medicine approach to pain. AORN J 2015; 101: 308–318.
CrossRef - Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin 2012; 30: 91–100.
CrossRef - Helander EM, Menard BL, Harmon CM, et al. Multimodal Analgesia, Current Concepts, and Acute Pain Considerations. Curr Pain Headache Rep 2017; 21: 3.
CrossRef - Gavarić N, Kladar N, Mišan A, et al. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind Crops Prod 2015; 74: 457–464.
CrossRef - Al-Snafi A. Chemical constituents and pharmacological effects of Asclepias curassavica – A review. Asian J Pharm Res 2015; 5: 83–87.
- Rezende CM, Fraga SRG. Chemical and aroma determination of the pulp and seeds of murici (Byrsonima crassifolia L.). J Braz Chem Soc 2003; 14: 425–428.
CrossRef - Maldini M, Sosa S, Montoro P, et al. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J Ethnopharmacol 2009; 122: 430–433.
CrossRef - Otunola GA, Oloyede OB, Oladiji AT, et al. Comparative analysis of the chemical composition of three spices – Allium sativum L. Zingiber officinale Rosc. and Capsicum frutescens L. commonly consumed in Nigeria. Afr J Biotechnol 2010; 9: 6927–6931.
CrossRef - Peraza-Sánchez SR, Cen-Pacheco F, Noh-Chimal A, et al. Leishmanicidal evaluation of extracts from native plants of the Yucatan peninsula. Fitoterapia 2007; 78: 315–318.
CrossRef - Hernández I, Márquez L, Martínez I, et al. Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. J Ethnopharmacol 2009; 124: 649–652.
CrossRef - Srianthie D, Udayangani DN, Chamari H. Antioxidant, antibacterial and anti-inflammatory potential of the aqueous extract of the raw leaves of sri lankan variety of persea americana miller (avocado). Int J Ayurveda Pharma Res 2020; 8: 1–11.
- Chaves OS, Gomes RA, Tomaz AC de A, et al. Secondary Metabolites from Sida rhombifolia L. (Malvaceae) and the Vasorelaxant Activity of Cryptolepinone. Molecules 2013; 18: 2769–2777.
CrossRef - Sabandar CW, Ahmat N, Jaafar FM, et al. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry 2013; 85: 7–29.
CrossRef - Pudji A. The ability of anti-inflammatory jatropha curcas leaf extract at cox-2 expression on monocytes were exposed LPS. UNEJ E-Proceeding 2017; 154–157.
- Shankland WE 2nd. Four common herbs seen in dental practice: properties and potential adverse effects. Cranio 2009; 27: 118–124.
CrossRef - Kim S, Kim DB, Jin W, et al. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat Prod Res 2018; 32: 1193–1197.
CrossRef - Salehi B, Valussi M, Morais-Braga MFB, et al. Tagetes spp. Essential Oils and Other Extracts: Chemical Characterization and Biological Activity. Molecules 2018; 23: 2847.
CrossRef - Céspedes CL, Avila JG, Martínez A, et al. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J Agric Food Chem 2006; 54: 3521–3527.
CrossRef - Vergara Barragán E, Bach H, Meza-Reyes S, et al. Bioactivities of Flavonoids from Lopezia racemosa. BioMed Res Int 2019; 2019: 3286489.
CrossRef - Cruz Paredes C, Bolívar Balbás P, Gómez-Velasco A, et al. Antimicrobial, antiparasitic, anti-inflammatory, and cytotoxic activities of Lopezia racemosa. ScientificWorldJournal 2013; 2013: 237438.
CrossRef - Srinath J. Therapeutic Potential of Spilanthes acmella – A Dental Note. Int J Pharm Sci Rev Res 2014; 151–153.
- Pareek S, Sagar NA, Sharma S, et al. Onion (Allium cepa L.). In: Yahia EM (ed) Fruit and Vegetable Phytochemicals. Chichester, UK: John Wiley & Sons, Ltd, 2017, pp. 1145–1162.
CrossRef - Babotă M, Mocan A, Vlase L, et al. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018; 23: 409.
CrossRef - Gafrikova M, Galova E, Sevcovicova A, et al. Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide. Molecules 2014; 19: 3160–3172.
CrossRef - Singh J, Upadhyay AK, Bahadur A, et al. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). Sci Hortic 2006; 108: 233–237.
CrossRef - Singh N, Rao AS, Nandal A, et al. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem 2021; 338: 127773.
CrossRef - Dezsi Ș., Bădărău AS, Bischin C, et al. Antimicrobial and Antioxidant Activities and Phenolic Profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johnson Leaves. Molecules 2015; 20: 4720–4734.
CrossRef - Al-Snafi A. Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger and Hyoscyamus reticulates)-A review. IOSR J Pharm 2018; 8: 18–32.
- Singh O, Khanam Z, Misra N, et al. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev 2011; 5: 82–95.
CrossRef - Roby MHH, Sarhan MA, Selim KA-H, et al. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind Crops Prod 2013; 44: 437–445.
CrossRef - Cheikh-Rouhou S, Besbes S, Hentati B, et al. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem 2007; 101: 673–681.
CrossRef - Beara IN, Lesjak MM, Orčić DZ, et al. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT – Food Sci Technol 2012; 47: 64–70.
CrossRef - Beara IN, Orcić DZ, Lesjak MM, et al. Liquid chromatography/tandem mass spectrometry study of anti-inflammatory activity of Plantain (Plantago L.) species. J Pharm Biomed Anal 2010; 52: 701–706.
CrossRef - Afonso AF, Pereira OR, Fernandes Â, et al. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019; 24: 4327.
CrossRef - Ghasemian M, Owlia S, Owlia MB. Review of Anti-Inflammatory Herbal Medicines. Adv Pharmacol Sci 2016; 2016: 9130979.
CrossRef - Kaur K, Kaushal S. Phytochemistry and pharmacological aspects of Syzygium aromaticum: A review. J Pharmacogn Phytochem 2019; 8: 398–406.
- Déciga-Campos M, Beltrán-Villalobos KL, Aguilar-Mariscal H, et al. Synergistic Herb-Herb Interaction of the Antinociceptive and Anti-Inflammatory Effects of Syzygium aromaticum and Rosmarinus officinalis Combination. Evid Based Complement Alternat Med 2021; 2021: 8916618.
CrossRef - Kisiel W, Barszcz B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia 2000; 71: 269–273.
CrossRef - Moldovan ML, Carpa R, Fizeșan I, et al. Phytochemical Profile and Biological Activities of Tendrils and Leaves Extracts from a Variety of Vitis vinifera L. Antioxidants 2020; 9: 373.
CrossRef - Nicolì F, Negro C, Vergine M, et al. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019; 24: 1998.
CrossRef - Günaydin K, Savci S. Phytochemical studies on Ruta chalepensİs (Lam.) Lamarck. Nat Prod Res 2005; 19: 203–210.
CrossRef - Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int 2014; 2014: 842674.
CrossRef - Choi EM, Hwang JK. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004; 75: 557–565.
CrossRef - Maroyi A. Euclea undulata Thunb.: Review of its botany, ethnomedicinal uses, phytochemistry and biological activities. Asian Pac J Trop Med 2017; 10: 1030–1036.
CrossRef - Tefera BN, Kim YD. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed 2019; 15: 25.
CrossRef - Buyinza D, Chalo DM, Derese S, et al. Flavonoids and Isoflavonoids of Millettia dura and Millettia ferruginea: Phytochemical review and chemotaxonomic values. Biochem Syst Ecol 2020; 91: 104053.
CrossRef - Wondimieneh S, Asres K. In Vivo Anti-inflammatory and Antinociceptive Activities of Salvia nilotica and Rosa abyssinica. Ethiop Pharm J 2008; 26: 75–82.
CrossRef - Itou RDGE, Sanogo R, Ossibi AWE, et al. Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Stem Bark of Ceiba pentandra Gaertn. Pharmacol Pharm 2014; 05: 1113–1118.
CrossRef - Issa TO, Mohamed YS, Yagi S, et al. Ethnobotanical investigation on medicinal plants in Algoz area (South Kordofan), Sudan. J Ethnobiol Ethnomed 2018; 14: 31.
CrossRef - Dirar AI, Adhikari-Devkota A, Kunwar RM, et al. Genus Blepharis (Acanthaceae): A review of ethnomedicinally used species, and their phytochemistry and pharmacological activities. J Ethnopharmacol 2021; 265: 113255.
CrossRef - Tesfaye S, Belete A, Engidawork E, et al. Ethnobotanical Study of Medicinal Plants Used by Traditional Healers to Treat Cancer-Like Symptoms in Eleven Districts, Ethiopia. Evid Based Complement Alternat Med 2020; 2020: 7683450.
CrossRef - Jimoh MO, Afolayan AJ, Lewu FB. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci Rep 2019; 9: 12965.
CrossRef - Ashu Agbor M, Naidoo S. Ethnomedicinal Plants Used by Traditional Healers to Treat Oral Health Problems in Cameroon. Evid Based Complement Alternat Med 2015; 2015: 649832.
CrossRef - Pareek S, Sagar N, Sharma S, et al. Onion (Allium cepa L.): Chemistry and Human Health. In: Fruit and Vegetable Phytochemicals. 2017, pp. 1145–1162.
CrossRef - Elgorashi EE, McGaw LJ. African plants with in vitro anti-inflammatory activities: A review. South Afr J Bot 2019; 126: 142–169.
CrossRef - Matata DZ, Moshi MJ, Machumi F, et al. Isolation of a new cytotoxic compound, 3-((Z)-heptadec-14-enyl) benzene – 1-ol from Rhus natalensis root extract. Phytochem Lett 2020; 36: 120–126.
CrossRef - Kidane B, van Andel T, van der Maesen LJG, et al. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J Ethnobiol Ethnomed 2014; 10: 46.
CrossRef - Alqasoumi SI, Basudan OA, Alam P, et al. Antioxidant study of flavonoid derivatives from the aerial parts of Rhus natalensis growing in Saudi Arabia. Pak J Pharm Sci 2016; 29: 97–103.
- Martins MR, Arantes S, Candeias F, et al. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol 2014; 151: 485–492.
CrossRef - Bringmann G, Rüdenauer S, Irmer A, et al. Antitumoral and antileishmanial dioncoquinones and ancistroquinones from cell cultures of Triphyophyllum peltatum (Dioncophyllaceae) and Ancistrocladus abbreviatus (Ancistrocladaceae). Phytochemistry 2008; 69: 2501–2509.
CrossRef - Zougagh S, Belghiti A, Rochd T, et al. Medicinal and Aromatic Plants Used in Traditional Treatment of the Oral Pathology: The Ethnobotanical Survey in the Economic Capital Casablanca, Morocco (North Africa). Nat Prod Bioprospect 2019; 9: 35–48.
CrossRef - Khalil N, Bishr M, Desouky S, et al. Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules 2020; 25: 301.
CrossRef - Laribi B, Kouki K, M’Hamdi M, et al. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015; 103: 9–26.
CrossRef - Tugume P, Kakudidi EK, Buyinza M, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J Ethnobiol Ethnomed 2016; 12: 5.
CrossRef - Woode E, Ansah C, Ainooson GK, et al. Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (forsk.) Vahl (apocynaceae). J Sci Technol Ghana 2007; 27: 5–15.
CrossRef - Farkhondeh T, Kianmehr M, Kazemi T, et al. Toxicity effects of Nerium oleander, basic and clinical evidence: A comprehensive review. Hum Exp Toxicol 2020; 39: 773–784.
CrossRef - Shafiq Y, Naqvi SBS, Rizwani GH, et al. A mechanistic study on the inhibition of bacterial growth and inflammation by Nerium oleander extract with comprehensive in vivo safety profile. BMC Complement Med Ther 2021; 21: 135.
CrossRef - Megersa M, Asfaw Z, Kelbessa E, et al. An ethnobotanical study of medicinal plants in Wayu Tuka District, East Welega Zone of Oromia Regional State, West Ethiopia. J Ethnobiol Ethnomed 2013; 9: 68.
CrossRef - Wangteeraprasert R, Lipipun V, Gunaratnam M, et al. Bioactive Compounds from Carissa spinarum. Phytother Res 2012; 26: 1496–1499.
CrossRef - Punia DP. A review on varieties of Arka Calotropis procera (AITON) Dryand and Calotropis gigantea (L.) Dryand. Global J Res Med Plants & Indigen Med 2013; 2: 392–400.
- Ghosh PK, Bhattacharjee P, Mitra S, et al. Physicochemical and Phytochemical Analyses of Copra and Oil of Cocos nucifera L. (West Coast Tall Variety). Int J Food Sci 2014; 2014: 310852.
CrossRef - Lima EB, Sousa CN, Meneses LN, et al. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz J Med Biol Res 2015; 48: 953–964.
CrossRef - Chithra MA, Ijinu TP, Kharkwal H, et al. Phenolic rich Cocos nucifera inflorescence extract ameliorates inflammatory responses in LPS-stimulated RAW264.7 macrophages and toxin-induced murine models. Inflammopharmacology 2020; 28: 1073–1089.
CrossRef - Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed 2013; 9: 65.
CrossRef - Misganaw D, Sahile S, Negash W. Invitro antimicrobial effects of gomphocarpus purpurascens a. rich against standard and clinically isolated microorganisms. Glob J Sci Res 2019; 7: 121–136.
- Hassan H, Ahmadu AA, Hassan AS. Analgesic and anti-inflammatory activities of Asparagus africanus root extract. Afr J Tradit Complement Altern Med 2007; 5: 27–31.
CrossRef - Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed 2014; 10: 40.
CrossRef - Yimer T, Birru EM, Adugna M, et al. Evaluation of Analgesic and Anti-Inflammatory Activities of 80% Methanol Root Extract of Echinops kebericho M. (Asteraceae). J Inflamm Res 2020; 13: 647–658.
CrossRef - Liu X, Wang X, Chen Z, et al. De novo assembly and comparative transcriptome analysis: novel insights into terpenoid biosynthesis in Chamaemelum nobile L. Plant Cell Rep 2019; 38: 101–116.
CrossRef - Msaada K, Salem N, Bachrouch O, et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood ( Artemisia absinthium) Essential Oils and Phenolics. J Chem 2015; 2015: 1–12.
CrossRef - Bouabid K, Lamchouri F, Toufik H, et al. Phytochemical investigation, in vitro and in vivo antioxidant properties of aqueous and organic extracts of toxic plant: Atractylis gummifera L. J Ethnopharmacol 2020; 253: 112640.
CrossRef - Ngueguim TF, Djouwoug Noussi C, Donfack JH, et al. Acute and sub-acute toxicity of a lyophilized aqueous extract of the aerial part of Spilanthes africana Delile in rats. J Ethnopharmacol 2015; 172: 145–154.
CrossRef - Okunade AL. Ageratum conyzoides L. (Asteraceae). Fitoterapia 2002; 73: 1–16.
CrossRef - Mohammed T, Teshale C. Preliminary phytochemical screening and evaluation of antibacterial activity of Dichrocepala integrifolia (L.f) O. kuntze. J Intercult Ethnopharmacol 2012; 1: 30–34.
CrossRef - Uthpala TGG, Navaratne SB. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source – Review. Food Rev Int 2021; 37: 399–414.
CrossRef - Letha N, Ganesan K, Kumar S, et al. Studies on phytochemical screening and in vitro antioxidant activity of Ethiopian indigenous medicinal plants, Artemisia abyssinica Sch.Bip. Ex A.Rich. World J Pharm Res 2016; 5: 1048–1058.
- Tariku Y, Hymete A, Hailu A, et al. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem Biodivers 2011; 8: 614–623.
CrossRef - Studzińska-Sroka E, Dudek-Makuch M, Chanaj-Kaczmarek J, et al. Anti-inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018; 23: 2133.
CrossRef - Ali S, Zameer S, Yaqoob M. Ethnobotanical, phytochemical and pharmacological properties of Galinsoga parviflora (Asteraceae): A review. Trop J Pharm Res 2017; 16: 3023–3033.
- Albejo B, Endale M, Kibret B, et al. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. J Pharm Pharmacogn Res 2015; 3: 141–147.
- Speroni E, Cervellati R, Innocenti G, et al. Anti-inflammatory, anti-nociceptive and antioxidant activities of Balanites aegyptiaca (L.) J Ethnopharmacol 2005; 98: 117–125.
CrossRef - Traore KT, Ouédraogo N, Belemnaba L, et al. Anti-inflammatory and analgesic activities of extracts from Balanites aegyptiaca L. Delile (Balanitaceae) root bark: Plant used against liver diseases in Bukina Faso. Afr J Pharm Pharmacol 2019; 13: 322–329.
- Compaoré M, Lamien-Meda A, Mogoşan C, et al. Antioxidant, diuretic activities and polyphenol content of Stereospermum kunthianum Cham. (Bignoniaceae). Nat Prod Res 2011; 25: 1777–1788.
CrossRef - Ogundajo A, Ashafa AT. Phytochemical Compositions and In vitro Assessments of Antioxidant and Antidiabetic Potentials of Fractions from Ehretia cymosa Thonn. Pharmacogn Mag 2017; 13: S470–S480.
CrossRef - Yismaw YE, Abdelwuhab M, Ambikar DB, et al. Phytochemical and Antiulcer Activity Screening of Seed Extract of Cordia africana Lam (Boraginaceae) in Pyloric Ligated Rats. Clin Pharmacol Adv Appl 2020; 12: 67–73.
CrossRef - Riazullah, Hussain I, Badrullah. Phytochemical and anti-microbial activity of Lepidium sativum L. J Med Plants Res 2012; 6: 4358–4361.
CrossRef - Maroyi A. Boscia salicifolia: review of its botany, medicinal uses, phytochemistry and biological activities. J Pharm Sci 2019; 11: 3055–3060.
- Tekulu GH, Hiluf T, Brhanu H, et al. Anti-inflammatory and anti-nociceptive property of Capparis tomentosa Lam. root extracts. J Ethnopharmacol 2020; 253: 112654.
CrossRef - Abdulaziz Al-Hamoud G, Saud Orfali R, Sugimoto S, et al. Four New Flavonoids Isolated from the Aerial Parts of Cadaba rotundifolia Forssk. (Qadab). Molecules 2019; 24: 2167.
CrossRef - Martial N, Dah-Nouvlessounon D, Christine N tcha, et al. Phytochemistry and biological activities of crateva adansonii extracts. Int J Pharm Pharm Sci 2018; 10: 62–67.
CrossRef - Zunjar V, Mammen D, Trivedi B, et al. Pharmacognostic, Physicochemical and Phytochemical Studies on Carica papaya Linn. Leaves. Pharmacogn J 2011; 3: 5–8.
CrossRef - Kashyap K, Sarkar P, Kalita MC, et al. A review on the widespread therapeutic application of the traditional herb Drymaria cordata. Int J Pharma Bio Sci 2014; 5: 696–705.
- Kokanova-Nedialkova Z, Nedialkov PT, Nikolov SD. The Genus Chenopodium: Phytochemistry, Ethnopharmacology and Pharmacology. Pharmacogn Rev 2009; 3: 280–306.
- Kumar R, Mishra AK, Dubey NK, et al. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol 2007; 115: 159–164.
CrossRef - Muriithi E, Bojase-Moleta G, Majinda RRT. Benzophenone derivatives from Garcinia livingstonei and their antioxidant activities. Phytochem Lett 2016; 18: 29–34.
CrossRef - Yang H, Figueroa M, To S, et al. Benzophenones and biflavonoids from Garcinia livingstonei fruits. J Agric Food Chem 2010; 58: 4749–4755.
CrossRef - Ashokkumar K. Gloriosa superba (L.): A Brief Review of its Phytochemical Properties and Pharmacology. Int J Pharmacogn Phytochem Res 2015; 7: 1190–1193.
- Arbab A. Review on anogeissus leiocarpus a potent african traditional drug. Int J Res Pharm Chem 2014; 4: 496–500.
- Okoli CO, Akah PA, Nwafor SV, et al. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol 2007; 109: 219–225.
CrossRef - Alara OR, Abdurahman NH, Mudalip SKA, et al. Phytochemical and pharmacological properties of Vernonia amygdalina: A review. J Chem Eng Ind Biotechnol 2017; 2: 80–96.
CrossRef - Kriplani P, Guarve K, Baghael US. Arnica montana L. – a plant of healing: review. J Pharm Pharmacol 2017; 69: 925–945.
CrossRef - Fernandes JM, Cunha LM, Azevedo EP, et al. Kalanchoe laciniata and Bryophyllum pinnatum: an updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev Bras Farmacogn 2019; 29: 529–558.
CrossRef - Soh D, Bakang BT, Tchouboun EN, et al. New cucurbitane type triterpenes from Momordica foetida Schumach. (Cucurbitaceae). Phytochem Lett 2020; 38: 90–95.
CrossRef - Guimarães R, Barros L, Carvalho AM, et al. Aromatic plants as a source of important phytochemicals: Vitamins, sugars and fatty acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii leaves. Ind Crops Prod 2009; 30: 427–430.
CrossRef - Salih AM, Al-Qurainy F, Khan S, et al. Mass propagation of Juniperus procera Hoechst. Ex Endl. From seedling and screening of bioactive compounds in shoot and callus extract. BMC Plant Biol 2021; 21: 192.
CrossRef - Ilodibia* CV, Ugwu RU, Okeke CU, et al. Phytochemical evaluation of various parts of Dracaena arborea Link. and Dracaena mannii Bak. Afr J Plant Sci 2015; 9: 287–292.
CrossRef - Kilonzo M, Rubanza C, Richard U, et al. Antimicrobial activities and phytochemical analysis of extracts from Ormocarpum trichocarpum (Taub.) and Euclea divinorum (Hiern) used as traditional medicine in Tanzania. Tanzan J Health Res 2019; 21: 1–12.
CrossRef - Jena J, Gupta A. Ricinus communis linn: A phytopharmacological review. Int J Pharm Pharm Sci 2012; 4: 25–29.
- Martínez CA, Mosquera OM, Niño J. Medicinal plants from the genus Alchornea (Euphorbiaceae): A review of their ethnopharmacology uses and phytochemistry. Bol Latinoam Caribe Plant Med Aromat 2017; 16: 162–205.
- Yakubu OF, Adebayo AH, Iweala EEJ, et al. Anti-inflammatory and antioxidant activities of fractions and compound from Ricinodendron heudelotii (Baill.). Heliyon 2019; 5: e02779.
CrossRef - Koech S, Maoga J, Sindani A, et al. Anti-Inflammatory Activity of Dichloromethanolic Root Extract of Clutia abyssinica in Swiss Albino Mice. J Pharmacogn Nat Prod 2017; 3: 1000132.
- Seebaluck R, Gurib-Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae)–A review of their ethnopharmacology and phytochemistry. J Ethnopharmacol 2015; 159: 137–157.
CrossRef - Bruno T, Soh D, Ernestine N, et al. Phytochemical Composition and Biological Activity of Faidherbia albida (Mimosaceae) Roots and Leaves. Int J Pharm Sci Rev Res 2020; 65(1): 124–130.
CrossRef - Hebbar SS, Harsha VH, Shripathi V, et al. Ethnomedicine of Dharwad district in Karnataka, India—plants used in oral health care. J Ethnopharmacol 2004; 94: 261–266.
CrossRef - Kalaivani T, Mathew L. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree. Food Chem Toxicol 2010; 48: 298–305.
CrossRef - Ali A, Naveed A, Khan B, et al. Acacia nilotica: A plant of multipurpose medicinal uses. J Med Plants 2012; 6: 1492–1496.
CrossRef - Mariita RM, Orodho JA, Okemo PO, et al. Antifungal, antibacterial and antimycobacterial activity of Entada abysinnica Steudel ex A. Rich (Fabaceae) methanol extract. Pharmacogn Res 2010; 2: 163–168.
CrossRef - Gurmessa GT, Kusari S, Laatsch H, et al. Chemical constituents of root and stem bark of Erythrina brucei. Phytochem Lett 2018; 25: 37–42.
CrossRef - Narnoliya LK, Jadaun JS, Singh SP. The Phytochemical Composition, Biological Effects and Biotechnological Approaches to the Production of High-Value Essential Oil from Geranium. In: Malik S (ed) Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production. 2019, pp. 327–352.
CrossRef - Adesuyi A, Elumm I, Adaramola F, et al. Nutritional and Phytochemical Screening of Garcinia kola. Adv J Food Sci Technol 2012; 4: 9–14.
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry 2003; 62: 121–125.
CrossRef - Njeru SN, Obonyo M, Nyambati S, et al. Antimicrobial and cytotoxicity properties of the organic solvent fractions of Clerodendrum myricoides (Hochst.) R. Br. ex Vatke: Kenyan traditional medicinal plant. J Intercult Ethnopharmacol 2016; 5: 226–232.
CrossRef - Alemayehu K, Anza M, Engdaw D, et al. Chemical constituents, physicochemical properties and antibacterial activity of leaves essential oil of Ocimum urticifolium. J Coast Life Med 2016; 4: 955–960.
CrossRef - Idris S, Ndukwe G, Gimba C. Preliminary phytochemical screening and antimicrobial activity of seed extracts of Persea americana (avocado pear). Bayero J Pure Appl Sci 2009; 2: 173–176.
CrossRef - Tirfe M, Gebrehiwot M, Gebrelibanos M, et al. Radical Scavenging Activity and Preliminary Phytochemical Screening of Pods of Cassia arereh Del. (Fabaceae). Momona Ethiop J Sci 2015; 7: 125–133.
CrossRef - Jeremiah C, Aliyu N, Dijie H, et al. Pharmacognostic and Elemental Analysis of the Leaves of Tapinanthus globifer (A. Rich). Tiegh. Res J Pharmacol 2018; 6: 11–18.
- Abiche E, Habila J. Phytochemistry, pharmacology and medicinal uses of Cola (Malvaceae) family: a review. Med Chem Res 2020; 29: 2089–2105.
CrossRef - Imam H, et al. Neem (Azadirachta indica A. Juss)-A Nature’s Drugstore: An overview. Int Res J Biol Sci 2012; 1: 76–79.
- Sahrawat A, Sharma J, Rahul S, et al. Phytochemical analysis and Antibacterial properties of Azadirachta indica (Neem) leaves extract against E.coli. J Pharmacogn Phytochem 2018; 7: 1368–1371.
- Lakshmi T, Krishnan V, Rajendran R, et al. Azadirachta indica: A herbal panacea in dentistry – An update. Pharmacogn Rev 2015; 9: 41–44.
CrossRef - Qureshi H, Arshad M, Akram A, et al. Ethnopharmacological and phytochemical account of paradise tree (Melia azedarach L.: Meliaceae). Pure Appl Biol 2015; 5: 5–14.
CrossRef - Aćimović M, Jeremić K, Salaj N, et al. Marrubium vulgare L.: A Phytochemical and Pharmacological Overview. Molecules 2020; 25: 2898.
CrossRef - Pascal DrMK, my el abbes F, Meddah B, et al. Assessment of methanolic extract of Marrubium vulgare for antiinflammatory, analgesic and anti-microbiologic activities. J Chem Pharm Res 2011; 3: 199–204.
- Bouyahya A, Chamkhi I, Benali T, et al. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J Ethnopharmacol 2021; 265: 113318.
CrossRef - Negi A, Dobhal K, Ghildiyal P. Antioxidant Potential and Effect of Extraction Solvent on Total Phenol Content, Flavonoids Content and Tannin Content of Ficus palmata Forssk. Int J Pharm Sci Rev Res 2018; 49: 19–24.
- Anibasa G. Antimicrobial And Phytochemical Screening Activities Of Ficus Sur (Forssk). N Y Sci J 2011; 4(1): 15–18.
- Taviano MF, Rashed K, Filocamo A, et al. Phenolic profile and biological properties of the leaves of Ficus vasta Forssk. (Moraceae) growing in Egypt. BMC Complement Altern Med 2018; 18: 161.
CrossRef - Paikra BK, Dhongade H kumar J, Gidwani B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J Pharmacopuncture 2017; 20: 194–200.
CrossRef - Messaoud C, Laabidi A, Boussaid M. Myrtus communis L. infusions: the effect of infusion time on phytochemical composition, antioxidant, and antimicrobial activities. J Food Sci 2012; 77: C941–C947.
CrossRef - Kaushal S. Phytochemistry and pharmacological aspects of Syzygium aromaticum: A review. J Pharmacogn Phytochem 2019; 8: 398–406.
- Kamath JV, Rahul N, Kumar CKA, et al. Psidium guajava L: A review. Int J Green Pharm; 2. Epub ahead of print 2008. DOI: 10.22377/ijgp.v2i1.386.
CrossRef - Dhakad AK, Pandey VV, Beg S, et al. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J Sci Food Agric 2018; 98: 833–848.
CrossRef - Al-Snafi A. The pharmacological and therapeutic importance of Eucalyptus species grown in Iraq. IOSR J Pharm 2017; 7: 72–91.
CrossRef - Fatma B, Fatiha M, Elattafia B, et al. Phytochemical and antimicrobial study of the seeds and leaves of Peganum harmala L. against urinary tract infection pathogens. Asian Pac J Trop Dis 2016; 6: 822–826.
CrossRef - Ghasemi Pirbalouti A, Momeni M, Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan Districts, Ilam Province, Iran. Afr J Tradit Complement Altern Med 2013; 10: 368–385.
CrossRef - Mina CN, Farzaei MH, Gholamreza A. Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med 2015; 35: 104–109.
CrossRef - Almeida ML, Freitas WE, de Morais PL, et al. Bioactive compounds and antioxidant potential fruit of Ximenia americana L. Food Chem 2016; 192: 1078–1082.
CrossRef - Balkrishna A, Rohela A, Kumar A, et al. Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management. Plants 2021; 10: 1089.
CrossRef - Sekharan TR, Mohan MS, Venkatnarayanan R, et al. Pharmacognostical and Preliminary Phytochemical Screening the Leaves of Jasminum grandiflorum Linn. Res J Pharmacogn Phytochem 2010; 2: 438–440.
- Rathore S, Bhatt S, Dhyani D, et al. Preliminary phytochemical screening of medicinal plant Ziziphus mauritiana Lam fruits. Int J Curr Pharm Res 2012; 4: 160–162.
- Aruna K, Devi P, Rajeswari R, et al. Quantitative phytochemical analysis of oxalis corniculata l. (oxalidaceae). World J Pharm Pharm Sci 2014; 3: 711–716.
- Desta KT, Abd El-Aty AM. Triterpenoid and Saponin Rich Phytolacca dodecandra L’Herit (Endod): A Review on Its Phytochemistry and Pharmacological Properties. Mini Rev Med Chem 2021; 21: 23–34.
CrossRef - Nakalembe L, Kasolo JN, Nyatia E, et al. Analgesic and Anti-Inflammatory Activity of Total Crude Leaf Extract of Phytolacca dodecandra in Wistar Albino Rats. Neurosci Med 2019; 10: 259–271.
CrossRef - Manu P, Lal A, Rana S, et al. Plumbago zeylanica L.: A mini review. Int J Pharm Appl 2012; 3: 399-405.
- Rajakrishnan R, Lekshmi R, Benil PB, et al. Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephroprotective agent. Saudi J Biol Sci 2017; 24: 760–766.
CrossRef - Ojewole JA. Analgesic, anti-inflammatory and hypoglycaemic effects of Securidaca longepedunculata (Fresen.) [Polygalaceae] root-bark aqueous extract. Inflammopharmacology 2008; 16: 174–181.
CrossRef - Mekonnen T, Urga K, Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J Ethnopharmacol 2010; 127: 433–439.
CrossRef - Kumar S, Singh PK. Phytochemical investigation and antioxidant characterization of essential oil from roots of Rumex nepalensis Spreng high altitude of North India. Mater Today Proc 2020; 26: 3442–3448.
CrossRef - Hawaze S, Deti H, Suleman S. In vitro Antimicrobial Activity and Phytochemical Screening of Clematis Species Indigenous to Ethiopia. Indian J Pharm Sci 2012; 74: 29–35.
CrossRef - Tadele A, Asres K, Melaku D, et al. In vivo anti-inflammatory and antinociceptive activities of the leaf extracts of Clematis simensis Fresen. Ethiop Pharm J 2010; 27: 33–41.
CrossRef - Madivoli ES, Maina EG, Kairigo PK, et al. In vitro antioxidant and antimicrobial activity of Prunus africana (Hook. f.) Kalkman (bark extracts) and Harrisonia abyssinica Oliv. extracts (bark extracts): A comparative study. J Med Plants Econ Dev 2018; 2: 1–9.
CrossRef - Bento C, Gonçalves AC, Silva B, et al. Peach (Prunus Persica): Phytochemicals and Health Benefits. Food Rev Int 2020; 1–32.
CrossRef - Venditti A, Guarcini L, Ballero M, et al. Iridoid glucosides from Pentas lanceolata (Forssk.) Deflers growing on the Island of Sardinia. Plant Syst Evol 2015; 301: 685–690.
CrossRef - Lawal IO, Grierson DS, Afolayan AJ. Phytochemical and antioxidant investigations of a Clausena anisata hook, a South African medicinal plant. Afr J Tradit Complement Altern Med 2015; 12: 28–37.
CrossRef - Nantongo JS, Odoi JB, Abigaba G, et al. Variability of phenolic and alkaloid content in different plant parts of Carissa edulis Vahl and Zanthoxylum chalybeum Engl. BMC Res Notes 2018; 11: 125.
CrossRef - Dutta S, Shaikh A. The Active chemical constituent and biological activity of salvadora persica (Miswak). Int J Curr Pharm Rev Res 2012; 3: 1–14.
- Rani MS, Pippalla RS, Mohan K. Dodonaea Viscosa Linn. – An Overview. Asian J Pharm Res Health Care 2009; 1: 97–112.
- Jima T, Megersa M. Ethnobotanical Study of Medicinal Plants Used to Treat Human Diseases in Berbere District, Bale Zone of Oromia Regional State, South East Ethiopia. Evid Based Complement Alternat Med 2018; 2018: 8602945.
CrossRef - Mergia E, Shibeshi W, Terefe G, et al. Antitrypanosomal activity of Verbascum sinaiticum Benth. (Scrophulariaceae) against Trypanosoma congolense isolates. BMC Complement Altern Med 2016; 16: 362.
CrossRef - Guluma T, G NB, Teju E, et al. Phytochemical investigation and evaluation of antimicrobial activities of Brucea antidysenterica leaves. Chem Data Collect 2020; 28: 100433.
CrossRef - Soni P, Siddiqui AA, Dwivedi J, et al. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pac J Trop Biomed 2012; 2: 1002–1008.
CrossRef - Sayyed A. Phytochemistry, pharmacological and traditional uses of Datura stramonium L. J Pharmacogn Phytochem 2013; 2: 123–125.
- Oyekunle I, Nwogu U, Orababa O, et al. Phytochemical, Antimicrobial and Proximate Composition of Nicotiana tabacum Leaves Extract. Int J Innov Res Sci Eng Technol; 4.
- Sambo H, Olatunde A, Kiyawa S. Phytochemical, Proximate and Mineral Analyses of Solanum incanum Fruit. Int J Chem Mater Environ Res 2016; 3: 8–13.
- Sbhatu D, Abraha H. Preliminary Antimicrobial Profile of Solanum incanum L.: A Common Medicinal Plant. Evid Based Complement Alternat Med 2020; 2020: 3647065.
CrossRef - Jaspers MW, Bashir AK, Zwaving JH, et al. Investigation of Grewia bicolor juss. J Ethnopharmacol 1986; 17: 205–211.
CrossRef - Dianita R, Jantan I. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Premna: a review. Pharm Biol 2017; 55: 1715–1739.
CrossRef - Awah FM, Uzoegwu PN, Oyugi JO, et al. Free radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extract. Food Chem 2010; 119: 1409–1416.
CrossRef - Seyfe S, Toma A, Etisso A, et al. Journal of Medicinal Plants Research Phytochemical screening and in vivo antimalarial activities of crude extracts of Lantana trifolia root and Premna oligotricha leaves in Plasmodium berghei infected mice. J Med Plants Res 2017; 11: 763–769.
- Njeru SN, Obonyo MA, Nyambati SO, et al. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complement Altern Med 2015; 15: 295.
CrossRef - Chidambara Murthy KN, Vanitha A, Mahadeva Swamy M, et al. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J Med Food 2003; 6: 99–105.
CrossRef - Kumar S, Yadav M, Yadav A, et al. Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. South Afr J Bot 2017; 111: 50–59.
CrossRef - Ahmed H. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J Ethnobiol Ethnomed 2016; 12: 8.
CrossRef - Salehi B, Albayrak S, Antolak H, et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int J Mol Sci 2018; 19: 2843.
CrossRef - Sánchez-Machado DI, López-Cervantes J, Sendón R, et al. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci Technol 2017; 61: 94–102.
CrossRef - Eyob S, Martinsen BK, Tsegaye A, et al. Antioxidant and antimicrobial activities of extract and essential oil of korarima (Aframomum corrorima (Braun) P.C.M. Jansen). Afr J Biotechnol 2008; 7: 2585–2592.
- Kumar G, Loganathan K, Rao B. A Review on Pharmacological and Phytochemical Properties of Zingiber officinale Roscoe (Zingiberaceae). J Pharm Res 2011; 4: 2963–2966.
- Kumar H, Agrawal R, Kumar V. Barleria cristata: perspective towards phytopharmacological aspects. J Pharm Pharmacol 2018; 70: 475–487.
CrossRef - Muñoz-Acevedo A, Martinez JL, Rai M. Ethnobotany: Local Knowledge and Traditions. 1st ed. CRC Press, 2019.
CrossRef - Pasaribu G, Budianto E, Cahyana H, et al. A Review on Genus Saurauia: Chemical Compounds and their Biological Activity. Pharmacogn J 2020; 12: 657–666.
CrossRef - Hong L, Guo Z, Huang K, et al. Ethnobotanical study on medicinal plants used by Maonan people in China. J Ethnobiol Ethnomed 2015; 11: 32.
CrossRef - Ye CL, Dai DH, Hu WL. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013; 30: 48–53.
CrossRef - Ghamari S, Mohammadrezaei Khorramabadi R, Mardani M, et al. An overview of the most important medicinal plants with anti-toothache property based on ethnobotanical sources in Iran. J Pharm Sci Res 2017; 9: 796–799.
- Bor M, Özdemir F, Türkan I. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 2003; 164: 77–84.
CrossRef - Srivastava R. A review on phytochemical, pharmacological, and pharmacognostical profile of Wrightia tinctoria: Adulterant of kurchi. Pharmacogn Rev 2014; 8: 36–44.
CrossRef - Zhang SX, Tani T, Yamaji S, et al. Glycosyl flavonoids from the roots and rhizomes of Asarum longerhizomatosum. J Asian Nat Prod Res 2003; 5: 25–30.
CrossRef - Hu R, Lin C, Xu W, et al. Ethnobotanical study on medicinal plants used by Mulam people in Guangxi, China. J Ethnobiol Ethnomed 2020; 16: 40.
CrossRef - Doss A, Anand SP. Preliminary phytochemical screening of Asteracantha longifolia and Pergularia daemia. World Appl Sci J 2012; 18: 233–235.
- Abd-Alla HI, Shalaby NMM, Hamed MA, et al. Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch Pharm Res 2016; 39: 10–20.
CrossRef - Sukumaran P, Nair AG, Chinmayee DM, et al. Phytochemical Investigation of Bidens biternata (Lour.) Merr. and Sheriff. – -a nutrient-rich leafy vegetable from Western Ghats of India. Appl Biochem Biotechnol 2012; 167: 1795–1801.
CrossRef - Patel S. Harmful and beneficial aspects of Parthenium hysterophorus: an update. 3 Biotech 2011; 1: 1–9.
CrossRef - Lunlun G, Wei N, Yang G, et al. Ethnomedicine study on traditional medicinal plants in the Wuliang Mountains of Jingdong, Yunnan, China. J Ethnobiol Ethnomed 2019; 15: 20.
CrossRef - Farsam H, Amanlou M, Reza Dehpour A, et al. Anti-inflammatory and analgesic activity of Biebersteinia multifida DC. root extract. J Ethnopharmacol 2000; 71: 443–447.
CrossRef - Chi YM, Nakamura M, Zhao XY, et al. A monoterpene alkaloid from incarvillea sinensis. Chem Pharm Bull (Tokyo) 2005; 53: 1178–1179.
CrossRef - Wurchaih, Huar, Menggenqiqig, et al. Medicinal wild plants used by the Mongol herdsmen in Bairin Area of Inner Mongolia and its comparative study between TMM and TCM. J Ethnobiol Ethnomed 2019; 15: 32.
CrossRef - Yu CH, Tang WZ, Peng C, et al. Diuretic, anti-inflammatory, and analgesic activities of the ethanol extract from Cynoglossum lanceolatum. J Ethnopharmacol 2012; 139: 149–154.
CrossRef - Joshi K. Cynoglossum L.: A review on phytochemistry and chemotherapeutic potential. J Pharmacogn Phytochem 2016; 5: 32–39.
- Jeeva K, Thiyagarajan M, Elangovan V, et al. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crops Prod 2014; 52: 714–720.
CrossRef - Jiménez-López J, Ruiz-Medina A, Ortega-Barrales P, et al. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem 2018; 250: 54–59.
CrossRef - Zhang H, Ma ZF. Phytochemical and Pharmacological Properties of Capparis spinosa as a Medicinal Plant. Nutrients 2018; 10: 116.
CrossRef - Mishra S, Moharana S, Dash M. Review on Cleome gynandra. Int J Res Pharm Chem 2011; 1: 681–689.
- Uddin G, Rauf A, Siddiqui BS, et al. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 2014; 21: 954–959.
CrossRef - Rauf A. Phytochemical screening and biological activity of the aerial parts of Elaeagnus umbellata. Sci Res Essays 2012; 7: 3690–3694.
- Ho JC, Chen CM. Flavonoids from the aquatic plant Eriocaulon buergerianum. Phytochemistry 2002; 61: 405–408.
CrossRef - Tantray MA, Khan R, Shawl AS, et al. Phenolic glycosides from Lespedeza juncea. Chem Nat Compd 2008; 44: 591–593.
CrossRef - Sroka Z, Bodalska HR, Mażol I. Antioxidative Effect of Extracts from Erodium cicutarium L. Z Naturforsch C J Biosci 1994; 49: 881–884.
CrossRef - Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol 2015; 79: 578–592.
CrossRef - Raak C, Büssing A, Gassmann G, et al. A systematic review and meta-analysis on the use of Hypericum perforatum (St. John’s Wort) for pain conditions in dental practice. Homeopathy 2012; 101: 204–210.
CrossRef - Wei Y, Shu P, Hong J, et al. Qualitative and quantitative evaluation of phenolic compounds in Iris dichotoma Pall. Phytochem Anal 2012; 23: 197–207.
CrossRef - Prajapati MS, Patel JB, Modi K, et al. Leucas aspera: A review. Pharmacogn Rev 2010; 4: 85–87.
CrossRef - Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae). Phytother Res 2012; 26: 1581–1593.
CrossRef - Sadeghi H, Zarezade V, Sadeghi H, et al. Anti-inflammatory Activity of Stachys Pilifera Benth. Iran Red Crescent Med J 2014; 16: e19259.
CrossRef - Lukhoba CW, Simmonds MSJ, Paton AJ. Plectranthus: A review of ethnobotanical uses. J Ethnopharmacol 2006; 103: 1–24.
CrossRef - Kong DG, Zhao Y, Li GH, et al. The genus Litsea in traditional Chinese medicine: An ethnomedical, phytochemical and pharmacological review. J Ethnopharmacol 2015; 164: 256–264.
CrossRef - Al-Snafi A. Fritillaria Imperialis-A Review. IOSR J Pharm 2019; 9: 47–51.
- Nhut P, An TN, Minh LV, et al. Phytochemical screening of Allium Tuberosum Rottler. ex Spreng as food spice. IOP Conf Ser Mater Sci Eng 2020; 991: 012021.
CrossRef - Eldahshan OA, Abdel-Daim MM. Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux-vomica. Cytotechnology 2015; 67: 831–844.
CrossRef - Mittal M, Gupta N, Parashar P, et al. Phytochemical evaluation and pharmacological activity of syzygium aromaticum: A comprehensive review. Int J Pharm Pharm Sci 2014; 6: 67–72.
- Hanani E, Prastiwi R, Karlina L, et al. Indonesian Mirabilis jalapa Linn.: A Pharmacognostical and Preliminary Phytochemical Investigations. Pharmacogn J 2017; 9: 683–688.
CrossRef - Khan I, AbdElsalam NM, Fouad H, et al. Application of Ethnobotanical Indices on the Use of Traditional Medicines against Common Diseases. Evid Based Complement Alternat Med 2014; 2014: 635371.
CrossRef - Shan S, Huang X, Shah M, et al. Evaluation of Polyphenolics Content and Antioxidant Activity in Edible Wild Fruits. BioMed Res Int 2019; 2019: 1–11.
CrossRef - Raghavendra MP, Satish S, Raveesha KA. Phytochemical analysis and antibacterial activity of Oxalis corniculata; a known medicinal plant. My Sci 2006; 1: 72–78.
- Kaushik P, Kaushik D, Khokra SL. Ethnobotany and phytopharmacology of Pinus roxburghii Sargent: a plant review. J Integr Med 2013; 11: 371–376.
CrossRef - Bahadori MB, Sarikurkcu C, Kocak MS, et al. Plantago lanceolata as a source of health-beneficial phytochemicals: Phenolics profile and antioxidant capacity. Food Biosci 2020; 34: 100536.
CrossRef - Karkanis A, Fernandes Â, Vaz J, et al. Chemical composition and bioactive properties of Sanguisorba minor Scop. under Mediterranean growing conditions. Food Funct 2019; 10: 1340–1351.
CrossRef - Zhao Z, He X, Zhang Q, et al. Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus Sanguisorba Am J Chin Med 2017; 45: 199–224.
CrossRef - Gao J, Sun C, Yang J, et al. Evaluation of the hepatoprotective and antioxidant activities of Rubus parvifolius L. J Zhejiang Univ Sci B 2011; 12: 135–142.
CrossRef - Cheng X, Qin J, Zeng Q, et al. Taraxasterane-Type Triterpene and Neolignans from Geum japonicum Thunb. var. chinense F. Bolle. Planta Med 2011; 77: 2061–2065.
CrossRef - Fu H, Mu X, Wang P, et al. Fruit quality and antioxidant potential of Prunus humilis Bunge accessions. PLoS One 2020; 15: e0244445.
CrossRef - Lu Q, Ma R, Yang Y, et al. Zanthoxylum nitidum (Roxb.) DC: Traditional uses, phytochemistry, pharmacological activities and toxicology. J Ethnopharmacol 2020; 260: 112946.
CrossRef - Zhang M, Wang J, Zhu L, et al. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int J Mol Sci 2017; 18: 2172.
CrossRef - Prakash B, Singh P, Mishra PK, et al. Safety assessment of Zanthoxylum alatum Roxb. essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of Piper nigrum L. fruits. Int J Food Microbiol 2012; 153: 183–191.
CrossRef - Khatun A, Rahman M, Jahan S. Preliminary phytochemical, cytotoxic, thrombolytic and antioxidant activities of the methanol extract of Murraya exotica Linn. leaves. Orient Pharm Exp Med 2014; 14: 223–229.
CrossRef - Solanke SB. Phytochemical Information and Pharmacological Activities of Eggplant (Solanum Melongena L.): A Comprehensive Review. EAS J Pharm Pharmacol 2019; 1: 103–114.
- Ohtsuki T, Miyagawa T, Koyano T, et al. Isolation and structure elucidation of flavonoid glycosides from Solanum verbascifolium. Phytochem Lett 2010; 3: 88–92.
CrossRef - Son YO, Kim J, Lim JC, et al. Ripe fruits of Solanum nigrum L. inhibits cell growth and induces apoptosis in MCF-7 cells. Food Chem Toxicol 2003; 41: 1421–1428.
CrossRef - Tekuri SK, Pasupuleti SK, Kranthi KK, et al. Phytochemical and pharmacological activities of Solanum surattense Burm. f.–A review. J App Pharm Sci 2019; 9: 126–136.
CrossRef - Alizadeh A, Moshiri M, Alizadeh J, et al. Black henbane and its toxicity – a descriptive review. Avicenna J Phytomed 2014; 4: 297–311.
- Martínez-Valverde I, Periago MJ, Provan G, et al. A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum): Phenolics, lycopene and antioxidant activity in tomatoes. J Sci Food Agric 2002; 82: 323–330.
CrossRef - Zaidi A, Bukhari S, Khan F, et al. Ethnobotanical, phytochemical and pharmacological aspects of daphne mucronata (thymeleaceae). Trop J Pharm Res 2015; 14: 1517–1523.
CrossRef - Zheng CJ, Zhao XX, Ai HW, et al. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine 2014; 21: 838–846.
CrossRef - Usman H, Abdulrahman FI, A.A L. Phytochemical and Antimicrobial Evaluation of Tribulus terrestris L. (Zygophylaceae). Growing in Nigeria. Res J Biol Sci 2007; 2: 244–247.
- Rashid U, Khan MR, Jan S, et al. Assessment of phytochemicals, antimicrobial and cytotoxic activities of extract and fractions from Fagonia olivieri (Zygophyllaceae). BMC Complement Altern Med 2013; 13: 167.
CrossRef - Rashid U, Khan MR, Sajid M. Antioxidant, anti-inflammatory and hypoglycemic effects of Fagonia olivieri DC on STZ-nicotinamide induced diabetic rats – In vivo and in vitro study. J Ethnopharmacol 2019; 242: 112038.
CrossRef - Abbasi BH, Khan T, Khurshid R, et al. UV-C mediated accumulation of pharmacologically significant phytochemicals under light regimes in in vitro culture of Fagonia indica (L.). Sci Rep 2021; 11: 679.
CrossRef - Joshi RK. Volatile Constituents of Emilia sonchifolia from India. Nat Prod Commun 2018; 13: 1355–1356.
CrossRef - Kamboj A, Saluja AK. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int J Green Pharm 2010; 4: 129–139.
CrossRef - Ahmad H, Sehgal S, Mishra A, et al. Mimosa pudica L. (Laajvanti): An overview. Pharmacogn Rev 2012; 6: 115–124.
CrossRef - Ting YC, Ko HH, Wang HC, et al. Biological evaluation of secondary metabolites from the roots of Myrica adenophora. Phytochemistry 2014; 103: 89–98.
CrossRef - Suhagia B, Rathod I, Sindhu S. Sapindus mukorossi (areetha): An overview. Int J Pharm Sci Res 2011; 2: 1905–1913.
- Daboriya V, Kumar S, Singh S. Antibacterial activity and phytochemical investigations on nicotiana plumbaginifolia viv. (wild tobacco). Rom J Biol -Plant Biol 2010; 55: 135–142.
- Ikenaga T, Handayani R, Oyama T. Steroidal saponin production in callus cultures of Solanum aculeatissimum Jacq. Plant Cell Rep 2000; 19: 1240–1244.
CrossRef - Pandey S, Sah SP, Sah ML, et al. An antioxidant potential of hydromethanolic extract of Urtica parviflora Roxb. J Basic Clin Pharm 2010; 1: 191–195.
- Kalt FR, Cock IE. Gas chromatography-mass spectroscopy analysis of bioactive petalostigma extracts: Toxicity, antibacterial and antiviral activities. Pharmacogn Mag 2014; 10: S37–S49.
CrossRef - Singab AN, Youssef FS, Ashour ML, et al. The genus Eremophila (Scrophulariaceae): an ethnobotanical, biological and phytochemical review. J Pharm Pharmacol 2013; 65: 1239–1279.
CrossRef - Al-Abd NM, Mohamed Nor Z, Mansor M, et al. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement Altern Med 2015; 15: 385.
CrossRef - Banbury LK, Shou Q, Renshaw DE, et al. Compounds from Geijera parviflora with prostaglandin E2 inhibitory activity may explain its traditional use for pain relief. J Ethnopharmacol 2015; 163: 251–255.
CrossRef - Al-Snafi A. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J Pharm 2017; 7: 10–21.
CrossRef - Dapar MLG, Meve U, Liede-Schumann S, et al. Ethnomedicinal plants used for the treatment of cuts and wounds by the Agusan Manobo of Sibagat, Agusan del Sur, Philippines. Ethnobot Res Appl 2020; 19: 1–18.
CrossRef








