Ahmad Fauzi1,3*, Alvarino2 , Yanwirasti2
, Yanwirasti2 and Roni Eka Sahputra2
and Roni Eka Sahputra2
1Student of Doctoral Program in Biomedical Science, Universitas Andalas, Padang, Indonesia.
2Faculty of Medicine, Universitas Andalas, Padang, Indonesia.
3Faculty of Medicine, Universitas Lampung, Bandar Lampung, Indonesia.
Corresponding Author E-mail: ahmadfauzi_dr@yahoo.co.id
DOI : https://dx.doi.org/10.13005/bpj/2440
Abstract
One of the causes of nonunion is inadequate vascularization due to severe injury mechanisms that cause defective bone healing factors. Therefore, in non-union fractures, a trigger is needed for the growth factors to work properly. This study aimed to determine the effects of ALSR on VEGF and CD-34 in non-union fractures. This study used an experimental post-test only control group design that involved white rats of the Sprague-Dawley strain. The VEGF expression was assessed by anti-VEGF staining, which was done by cutting paraffin blocks into a thickness of 5 μm, then the blocks were deparaffinized in xylol and rehydrated with alcohol. The results of the analysis are displayed with their mean ranks between control groups and treatment groups. It was found that CD-34 expression was high in the ALSR group. This shows the occurrence of angiogenesis and the regeneration in the case of non-union fractures in this study.
Keywords
Amnion Lyophilization Sterile Radiation; Cluster of Differentiation 34; Nonunion fractures; Vascular Endothelial Growth Factor
Download this article as:| Copy the following to cite this article: Fauzi A, Alvarino A, Yanwirasti Y, Sahputra R. E. Effects of Amnion Lyophilization Sterile Radiation on Vascular Endothelial Growth Factor and Cluster of Differentiation 34 in Nonunion Fractures. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Fauzi A, Alvarino A, Yanwirasti Y, Sahputra R. E. Effects of Amnion Lyophilization Sterile Radiation on Vascular Endothelial Growth Factor and Cluster of Differentiation 34 in Nonunion Fractures. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3QIli3Q |
Introduction
Fractures have become a prominent issue in the health sector due to their increasing incidence and related complications that require longer treatment. One of the complications of fracture healing is non-union, which is the failure of the fracture to heal1,2. The incidence and prevalence of reported non-union vary according to the region, anatomy, and criteria used to define non-union.
One of the causes of nonunion is inadequate vascularization due to severe injury mechanisms that cause defective bone healing factors. Therefore, in non-union fractures, a trigger is needed for the growth factors to work properly. A study has found that the lower levels of the vascular endothelial growth factor (VEGF) in nonunion may be due to prolonged hypoxia.3
Research has also found a link between VEGF and hypoxia. VEGF is an important factor in stimulating the neovascularization process, which includes angiogenesis. VEGF activity is influential in every stage of fracture healing, from hematoma formation to bone turnover during the remodeling phase.4 In addition to the VEGF, the role of Cluster of Differentiation 34(CD-34) antigens in angiogenesis and vasculogenesis cannot be ignored. Antigen surface proteins of CD-34 cells are markers found on the surface of the cell that are commonly found in progenitor, hematopoietic, and endothelial cells. These progenitor endothelial cells would express CD-34 to form new blood vessels from existing vascular structures (angiogenesis) in tissues that are ischemic or stimulate angiogenic growth factors.5
Amnion lyophilization sterile radiation (ALSR) could help overcome the onset of problems in the fracture healing process, namely nonunion and its complications. However, evidence regarding the effect of ALSR in aiding the fracture healing process and increased expression of VEGF and CD-34, especially in nonunion conditions, has not been found. Therefore, a study is needed to prove this theory. This study was done to Sprague-Dawley rats instead of human.
This study aimed to determine the effects of ALSR on VEGF and CD-34 in non-union fractures.
Material and methods
This study used an experimental post-test only control group design that involved white rats of the Sprague-Dawley strain. The research began with a pilot study to determine the nonunion model. The samples used were 8 week old rats, weighing 250–350 grams of male sex. The study was conducted on five groups of rats with a total of seven rats per group, and thus the total number of animals needed for this study was 35, 21 of which were used for the preliminary study (pilot study) and 14 were for the research with ALSR.
After preliminary research, a nonunion model was obtained for further research with ALSR. In the study with ALSR, the rats were divided into 2 groups. Group 1 (control group): rats that experienced nonunion in a preliminary study (osteotomy, periosteal stripping or cauterization, and ORIF with K-wire). Group 2: non-unionized rats, which were given amnion lyophilization sterile radiation. The two groups were sacrified in the fourth and eight weeks, respectively for histological examination of the expression of VEGF and CD-34.
The VEGF expression was assessed by anti-VEGF staining, which was done by cutting paraffin blocks into a thickness of 5 μm, then the blocks were deparaffinized in xylol and rehydrated with alcohol. The endogenous peroxide was then blocked with 3% methanol and H2O2 for 30 minutes, then antigen recovery was performed by adding the antigen recovery solution (Dako) and placing the blocks ) in the microwave for 5 minutes. After the blocks were washed in a PBS solution, the blocks were added with antibodies for VEGF with a dilution of 1:400. Further detection was done using an envision kit (Dako) with a DAB substrate (diaminobenzidine). The results were then assessed by an anatomical pathology specialist11.
In this study, VEGF expression was assessed by screening three to five fields of view at 100x magnification to look for hotspot areas. Unlike homogeneous solid tumors where the field of view is taken at random, this study sought hotspot areas or areas with intense vascularization, then performed blood vessel calculations at an enlargement of 400x. This follows the study method which found that each collection of endothelial cells was positive for the immunostaining body.12 CD34 and anti-VEGF are also counted as individual blood vessels for those that can be morphologically identified as the lumen of blood vessels. Then the results of the calculation were averaged and would then be referred to as VEGF expression in this study.
The results of the analysis are shown with their mean ranks between the control groups and the treatment groups13. Analysis of VEGF expression was calculated using the area of positive and negative areas in VEGF staining so that the area of proportion could be obtained as a percentage.
In addition, VEGF expression was assessed by the CD-34 staining on the fracture fragments. This analysis was carried out by calculating the wide proportion of positive and negative areas in CD-34 coloring so that the proportional area in percent could be obtained.
Results and Discussion
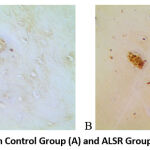 |
Figure 1: VEGF Expression in Control Group (A) and ALSR Group (B) with anti-VEGF Staining. |
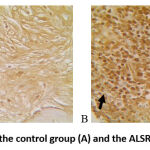 |
Figure 2: VEGF expression in the control group (A) and the ALSR group (B) With CD-34 staining. |
The results of the analysis of VEGF expressions with VEGF and CD-34 coloring showed a significant difference, where the group with ALSR had a significant. VEGF can induce the occurrence of angiogenesis and osteogenesis14,15. This growth factor increases endothelial cell differentiation and proliferation, as well as the formation, mobilization, and recruitment of endothelial progenitor cells.16 First, VEGF induces angiogenic processes in endothelial cells, and this helps bone-forming cells migrate along with blood vessels to the fracture callus. Second, through the angiocrine mechanism, the VEGF will stimulate endothelial cells to produce osteogenic cytokines that encourage the differentiation of progenitor cells into osteoblasts. Third, VEGF would directly affect osteoblast function. Similarly, osteoblasts would produce VEGF and also respond to VEGF itself by regulating osteoblast chemotaxis, proliferation, and differentiation15.
Table 1: VEGF analysis with VEGF staining in the ALSR study.
| Group | N | VEGF
(Mean± SD) |
p-value | CI 95% |
| Control | 7 | 10.69± 1.17 | <0.001* | 9.06 (6.89-11.24) |
| ALSR | 7 | 19.75± 2.37 |
*Independent T-test
Table 2: Results of VEGF analysis with CD-34 staining in the ALSR study.
| Group | N | VEGF
(Mean± SD) |
p-value | CI 95% |
| Control | 7 | 121.04± 5.33 | <0.001* | 257.67 (178.46-336.87) |
| ALSR | 7 | 378.71± 85.63 |
*Independent T-test
It appears that there is a significant difference in VEGF expression in the fracture healing process between the control group and the ALSR group. Based on the normality test, the VEGF data are distributed normally and thus the independent T-test was conducted.
There is a significant difference in VEGF expression between the control group and the ALSR group, both with VEGF and CD-34 coloring. Based on the normality test, VEGF and CD-34 data are distributed normally, therefore the Independent T-test was conducted.
As the fracture process is complex, the formation of blood vessels is controlled by growth factors and locally produced cytokines. The vascular system also plays an important role by providing oxygen, nutrients, and sending osteoprogenitors to the fracture site, resulting in bone formation cells. This study showed that the group administered with ALSR treatment showed increased VEGF expression. Furthermore, the coloring of Hematoxylin-Eosin also showed an increase in osteoblasts. Thus, the treatment of nonunion fractures with ALSR has been found to help heal the bone due to the increased VEGF expression.
The results of this study follow the research, which stated that for fracture healing, pro-angiogenic factors such as VEGF greatly increase at the beginning of post-fracture and were shown to increase along with osteogenesis.15 The VEGF in this context is likely produced by inflammatory cells as well as mesenchymal progenitors recruited to the site of bone injury. Immediately after injury, the volume of vascular tissue would increase due to vasodilation. During the healing process, the VEGF fractures play a direct role in the vascularization of calluses. Hypoxia pathway inhibitory factor (HIF) is activated in areas with low oxygen pressure, such as the avascular part of the cartilage callus17 HIF is a transcription factor that directly improves VEGF gene expression. VEGF expression can be secondarily driven to hypoxia because VEGF represents the target gene of hypoxia-induced factors.18 Rats with enhanced HIF1-alpha transcription activity have been found to show a very large increase in bone mass and the bone regeneration distraction model showed accelerated intramembranous bone formation.
In the fracture healing process, pro-angiogenic factors such as VEGF greatly increase at the beginning of post-fracture and have been shown to increase with the extraction process of osteogenesis. In this case, the VEGF may be produced by inflammatory cells as well as mesenchymal progenitors recruited to the site of bone injury. Hypoxia conditions can improve VEGF expression because VEGF represents the canonical target gene of a factor that can be induced by hypoxia condition. A previous study found that rats with enhanced HIF1-alpha transcription activity showed a very large increase in bone mass. In addition, the bone regeneration distraction model also shows an acceleration of intramembranous bone formation.
In this study, it was found that CD-34 expression was high in the ALSR group. This shows the occurrence of angiogenesis and the regeneration process in the case of non-union fractures in this study. This is in line with the findings of a previous study that stated that CD-34 is considered a mediator of tissue regeneration of endothelial, epithelial, and mesenchymal cells. VEGF and CD-34 findings based on immunohistochemical examination showed that the appearance of angiogenesis in tissues around the fracture and callus tissue occurred more frequently in the ALSR group.8,15 This suggests that blood supply to the fracture area in the ALSR group was better compared to the control group.
Conclusion
In this study, it was found that CD-34 expression was high in the ALSR group. This shows the occurrence of angiogenesis and the regeneration process in the case of non-union fractures in this study.
Acknowledgement
The author would like to thank to Universitas Andalas dan Universitas Lampung, for their support and facilitation during the research.
Ethics Committee Resolution
This study was ethically approved by the research ethics board of Medical Faculty of Lampung University and obtained approval number 187/UN26.18/PP.05.02.00/2022.
Conflict of Interest
No conflict of interest is declared.
Funding Source
The study was conducted at the expense of the authors.
References
- Salter RB. Textbook of Musculoskeletal System Disorders and Injuries of the Musculoskeletal System. Ed. 2. J Bone Jt Surg. 1984;66(1):157.
CrossRef - Phieffer LS, Goulet JA. Delayed Unions of the Tibia. J Bone Jt Surg [Internet]. 2006;88(1):206–16. Available from: https://pubmed.ncbi.nlm.nih.gov/16425471/
CrossRef - Lienau J, Schmidt-Bleek K, Peters A, Haschke F, Duda GN, Perka C, et al. Differential regulation of blood vessel formation between standard and delayed bone healing. J Orthop Res. 2009;27(9):1133–40.
CrossRef - Street J, Bao M, DeGuzman L, Bunting S, Peale F V., Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61.
CrossRef - Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–36.
CrossRef - Duong T, Rodan GA. Regulation of osteoclast formation and function. Rev Endocr Metab Disord. 2001;2(1):95–104.
CrossRef - Ochman S, Frey S, Raschke MJ, Deventer JN, Meffert RH. Local application of VEGF compensates callus deficiency after acute soft tissue trauma–results using a limb-shortening distraction procedure in rabbit tibia. J Orthop Res2. 2011;29(7):1093–8.
CrossRef - Luo G, Sun S., Weng T., Zhang B, Li X., Wang Z. Effect of osteoclasts on murine osteoblastic differentiation in early stage of co-culture. Int J Clin Exp Med. 2016;9(2):1062–72.
- Kerimoğlu S, Livaoğlu M, Sönmez B, Yuluğ E, Aynaci O, Topbas M, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281–7.
CrossRef - Starecki M, Schwartz JA, Grande DA. Evaluation of Amniotic-Derived Membrane Biomaterial as an Adjunct for Repair of Critical Sized Bone Defects. Adv Orthop Surg. 2014;2014:1–4.
CrossRef - Weidner N. Measuring Intratumoral Microvessel Density. Methods Enzymol. 2008;(444):305–23.
CrossRef - Stanek J, Abdaljaleel M. CD34 immunostain increases the sensitivity of placental diagnosis of fetal vascular malperfusion in stillbirth. Placenta. 2019;
CrossRef - Garcia-Altés A, Pérez K. The economic cost of road traffic crashes in an urban setting. Inj Prev. 2007;13(1):65–8.
CrossRef - Ogilvie CM, Lu C, Marcucio R, Lee M, Thompson Z, Hu D, et al. Vascular endothelial growth factor improves bone repair in a murine nonunion model. Iowa Orthop J. 2012;32(January):90–4.
- Stegen S, Gastel N van, Carmeliet G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27.
CrossRef - Dreyer CH, Jørgensen NR, Overgaard S, Qin L, Ding M. Vascular Endothelial Growth Factor and Mesenchymal Stem Cells Revealed Similar Bone Formation to Allograft in a Sheep Model. Biomed Res Int. 2021;2021.
CrossRef - Komatsu D, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34(4):680–8.
CrossRef - Cramer T, Schipani E, Johnson RS, Swoboda B, Pfander D. Expression of VEGF isoforms by epiphyseal chondrocytes during low-oxygen tension is HIF-1α dependent. Osteoarthr Cartil. 2004;12(6):433–9.
CrossRef - Al-hussaniy HA, Altalebi RR, Alburagheef A, Abdul-Amir AG. The Use of PCR for Respiratory Virus Detection on the Diagnosis and Treatment Decision of Respiratory Tract Infections in Iraq. J Pure Appl Microbiol. 2022.
CrossRef








