Hyeusoo Kim1 , Kyoung-Sun Seo2
, Kyoung-Sun Seo2  and Kyeong Won Yun3*
and Kyeong Won Yun3*
1R and D Center, ACT Company, 114-6 Central town-ro, Yeongtong-gu, Suwon16506, Republic of Korea.
2Jangheung Research Institute for Mushroom Industry, Jangheung 59338, Republic of Korea.
3Department of Oriental Medicine Resources, Sunchon National University, Suncheon 57922, Republic of Korea.
Corresponding Author E-mail: ykw@sunchon.ac.kr
DOI : https://dx.doi.org/10.13005/bpj/2412
Abstract
The fruits of Rosa multiflora Thunberg and Rosa wichuraiana Crépin are oriental medicine resources used complementary in management dropsy, edema and nocturnal enuresis in Korea. The objective of the present study was to evaluate the antioxidant activity and the content of kaempferol and quercetin of Rosa multiflora and Rosa wichuraiana fruits and flowers. Crude ethanol extracts of the species’ fruits and flowers from the two Rosa species were fractionized with hexane, ether, ethyl acetate and water, and antioxidant activities of the resulting fractions were evaluated in vitro using 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity and superoxide anion radical scavenging activity. The content of kaempferol and quercetin was quantified by high-performance liquid chromatography (HPLC) analyses. The water fraction of R. multiflora and ethyl acetate fraction of R. wichuraiana exhibited the highest DPPH free radical scavenging activity, which are generally proportionally to concentration, and the ethyl acetate fraction of fruit and ether fraction of the flower from the two Rosa species exhibited the highest superoxide anion radical scavenging activity. Meanwhile, the ethyl acetate and ether fraction of flower and fruit from the two Rosa species contained high level content of kaempferol and quercetin. These findings indicate that the antioxidant activity and the content of kaempferol and quercetin of Rosa multiflora and Rosa wichuraiana is dependent on solvent fraction. Moreover, both Rosa species fruits and flowers are promising sources of antioxidant phytochemicals, which further supports their use in complementary oriental medicine resource in Korea.
Keywords
Antioxidant; Kaempferol; Quercetin; Rosa multiflora; Rosa wichuraiana
Download this article as:| Copy the following to cite this article: Kim H, Sun K. S, Yun K. W. Antioxidant Activity and Flavonoid Estimation in Rosa multiflora and Rosa wichuraiana Fruits and Flowers. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Kim H, Sun K. S, Yun K. W. Antioxidant Activity and Flavonoid Estimation in Rosa multiflora and Rosa wichuraiana Fruits and Flowers. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3PFBLW7 |
Introduction
Species belonging to the genus Rosa (Rosaceae) are widely distributed in temperate and subtropical regions of the northern hemisphere. Numerous Rosa species are used for medical purposes or ornamentals. In Korea, the fruits of Rosa multiflora called Yeongsil are used to treat dropsy, edema, constipation, nocturnal enuresis and the species’ flowers are used to treat malaria and bleeding1-4. Rosa multiflora is a perennial shrub with thorny stems and has alternate compound leaves, generally with five to eleven sharply toothed leaflets, and tolerance for a broad range of soil, moisture and light conditions. The principal components of the species’ fruit include quercetin glycosides, kaempferol glycosides, methyl gallate, and lycopene which is red pigment of fruits5-7 and several studies have reported that Rosa multiflora exhibits antimicrobial, antioxidant, melanogenesis-inhibiting, and anti-inflammatory activities8,9. Meanwhile, the closely related species Rosa wichuraiana is native to Japan, Korea, east China and Taiwan, where it grows best in lowland thickets, near ocean. The species can be differentiated from R. multiflora by its thicker and more lustrous leaves. Interestingly, the somatic hybridization and propagation of R. wichuraiana has been studied to use the species as a novel source of disease resistance in ornamental rose breeding10-12. However, the bioactivity of the species has yet to be investigated.
The production of free radicals in the human body can induce oxidative stress, which can damage DNA, proteins, and lipids, ultimately causing various chronic illnesses (e.g., cancer and cardiovascular disease)13-15. However, even though antioxidants can be used to reduce the incidence of reactive oxygen species (ROS; e.g., superoxide anion radicals, hydroxyl radicals, non-freeradical species, and single oxygen)16,17, the use of synthetic antioxidants has been associated with negative side effects18. Thus, the bioactive constituents and antioxidant activities of natural sources have received increasing interest.
Flavonoids, like kaempferol and quercetin, are plant secondary metabolites with polyphenolic structure and various biological activities19. Furthermore, kaempferol and quercetin have been identified in multiple plant species used in traditional medicine20. The most important characteristic of flavonoid compounds is their antioxidative activity, and many studies have reported linear relationships between flavonoid content and antioxidant activity21-23. This is the first study to compare the antioxidant activities and flavonoid contents of R. multiflora and R. wichuraiana.
Materials and Methods
Chemicals and solvents used for this study were purchased from Sigma Chemicals (St Louis, MO, USA) and Difco (Detroit, MI, USA).
Rosa multiflora fruits and flowers were collected from Suncheon, Korea (34°54ʹ27ʹʹN, 127°34ʹ52ʹʹE), in September 2015 and May 2016, and R. wichuraiana fruits and flowers were collected from Goheung, Korea (34°43ʹ65ʹʹN, 127°49ʹ47ʹʹE), in September 2015 and June 2016. The plant was authenticated by one of the authors (K. W. Yun), and voucher specimens (SCNU 2015 201 and SCNU 2016 53, respectively) were also collected and were deposited in the herbarium of Sunchon National University. The collected flowers and fruits were air-dried for 14 d.
The air-dried fruit and flower of the two Rosa species was ground into powder. The samples (100 g) were soaked in 1,000 mL of ethanol and kept at room temperature for 24 hr and then filtered through filter paper (Whatman No.2). The crude ethanol extract was fractionized with 500 mL of hexane and then the top layer was concentrated (comprising the hexane fraction). The remaining layer was successively fractionized with 500 mL of diethyl ether and then ethyl acetate (forming the ether and ethyl acetate fraction). The remaining residue was the water fraction. Finally, each fraction was concentrated (in vacuo, 30 °C ) to 30 mL for the subsequent measurement of antioxidant activity and flavonoid contents.
The 1,1-diphenyl-2-picrylhydrazyl (DPPH)of each fraction was measured using a modified version of the method described by Blois24. Briefly, 140 µL DPPH (0.075 mM, in methanol) was added to fraction aliquots to reach final concentrations of 3.13–100.00 µg/mL, gently mixed, and incubated in the dark at 25 °C for 30 min. Butylhydroxy toluene (BHT; 100 µg/mL) and After incubation, the optical density at 517 nm (OD517) of each reaction mixture was measured using an ELISA Reader (Color techno system Co., Tokyo, Japan), and DPPH free radical scavenging activity was calculated as follows:
Scavenging activity (%) = (1 – [absorbance of sample] / [absorbance of control]) × 100%.
The superoxide anion radical scavenging activity of each fraction was measured according to Fridovich25. Superoxide radicals were generated in 0.4 mL potassium phosphate buffer (0.1M, pH 7.5) that contained 1 mL xanthine (0.4 mM), 1 mL nitro blue tetrazolium chloride (NBT, 0.24 mM) solution, 1 mL xanthine oxidase (0.2 unit/mL) and 0.1 mL Rosa extract fraction. The reaction mixtures were incubated at 37 °C for 20 min. After incubation, the optical density at 560 nm (OD560) of each reaction mixture was measured using an ELISA Reader. Lower the absorbance value, higher the superoxide radical scavenging activity was observed. The IC50 value was inversely correlated with antioxidant activity of the tested fractions; lower IC50 value indicated higher antioxidant activity 26
For quantitative estimation of kaempferol and quercetin, each of the four extract fractions of two Rosa species’ fruits and flowers was lyophilized using lyophilizer (Ilsin Co, Korea), and the lyophilized powders were stored in airtight bottles at −5°C until used. Kaempferol and quercetin standards (30 µg/mL) were prepared using methanol. High- performance liquid chromatographic (HPLC) was performed using Aglient 1200 series system (Agilent Technologies Inc., Santa Clara, CA, USA) that was equipped with a ZORBAX SB-C18 column (4.6 mm × 150 mm, 3.5 µm; Agilent). Ultra-distilled water and acetonitrile were used as mobile phases A and B, respectively. The flow rate and injection volume were 1.0 mL/min and the 5 µL, respectively, and the elution profile was as follows: 15% B for 0–1.5 min; 17% B for 3–4 min; 20-35% B for 7–14 min. The elution was monitored in the UV range, and the data for quantitative analysis were acquired at 360 nm. The retention time of quercetin and kaempferol was 7.63 min and 10.98 min, respectively. The kaempferol and quercetin contents were quantified using the external standard calibration method27.
Data were expressed as mean ± standard deviation values (n = 3). Statistical analysis was performed using SPSS software (version 24.0; SPSS Inc., Chicago, IL, USA). The significance of differences between means was evaluated using Duncan’s test.
Results and Discussion
Table 1 shows the yield of the four fractions of ethanol extract from R. multiflora and R. wichuraiana. Solvents of increasing polarity were used to fractionate the crude ethanol extracts. The determination of stable DPPH radicals scavenging is a widely used and common method for the relatively rapid evaluation of antioxidant activity28,29. The DPPH free radical scavenging activity of the two Rosa species is shown in Table 2. The DPPH radical scavenging activities of hexane and water fraction of R. multiflora fruit extract are 82.93% and 79.10% at 50 µg/mL DPPH, whereas that of ether fraction is 69.57% at 12.5 µg/mL DPPH. The DPPH radical scavenging activity of the ethyl acetate fraction of R. multiflora fruit was greater than that of BHT. The DPPH radical scavenging activities of the hexane, ether, ethyl acetate, and water fraction of the R. multiflora flower were 70.07%, 79.27%, 82.54%, and 95.28%, respectively, at DPPH concentration of 100 µg/mL. The activities of hexane, ether, ethyl acetate, and water fractions of R. wichuraiana flower were 37.15%, 47.42%, 83.04%, and 77.58%, respectively, at DPPH concentration of 100 µg/mL. The DPPH radical scavenging activity of the ether fraction of the R. wichuraiana flower was 69.70% at a DPPH concentration of 50 µg/mL, and the DPPH radical scavenging activities of ethyl acetate and water fractions of the R. wichuraiana flower were greater than that of BHT, regardless of DPPH concentration. Park et al.4 reported that the DPPH radical scavenging activities of ethanol, methanol, and acetone extracts of R. multiflora roots were 80% at a DPPH concentration of 100 µg/mL and that the scavenging activity of an aqueous extract was 40% at a DPPH concentration of 50 µg/mL. The DPPH radical scavenging activities of aqueous and ethanol extracts of Potentilla supina (Rosaceae) were 25.2% and 35.97%, respectively, at 25 µg/mL DPPH, whereas those of methanol extracts of three Rosa species were 64.5%, 51.8%, and 43.6%, respectively, at 100 µg/mL DPPH30-32. In the present study, the ethyl acetate and water fractions exhibited greater DPPH free radical scavenging activities than the other two fractions, and the R. multiflora extract fractions exhibited greater DPPH free radical scavenging activity than the R. wichuraiana extract fractions.
Table 1: Yield of each fraction from Rosa multiflora and R. wichuraiana.
| Plant | Plant organ | Extract fraction | Yield (%) |
| Rosa multiflora | Fruit | Hexane | 0.63±0.12 d |
| Ether | 1.24±0.22 c | ||
| Ethyl acetate | 2.35±0.26 b | ||
| Water | 6.43±0.31 a | ||
| Flower | Hexane | 0.94±0.26 d | |
| Ether | 1.87±0.32 c | ||
| Ethyl acetate | 3.91±0.34 b | ||
| Water | 10.18±0.69 a | ||
| Rosa wichuraiana | Fruit | Hexane | 0.4±0.04 cd |
| Ether | 0.7±0.07 c | ||
| Ethyl acetate | 1.46±0.18 b | ||
| Water | 4.78±0.52 a | ||
| Flower | Hexane | 0.78±0.13 d | |
| Ether | 1.71±0.17 c | ||
| Ethyl acetate | 4.01±0.39 b | ||
| Water | 9.08±0.72 a |
a Values with different letters in the same column were significantly (p < 0.05) different.
Duncan’s test should be compared within each part of a plant.
 |
Table 2: DPPH free radical scavenging activity of solvent fractions from two Rosa species. |
Superoxides are radicals that contain an oxygen atom with unpaired electrons. Despite having low chemical reactivity, superoxides can generate highly reactive species, such as hydroxyl radicals and the protonated form of superoxide. The superoxide anion radical scavenging activity of the two Rosa species are shown in Table3. In the present study, IC50 was calculated as the concentration that caused a 50% reduction in the superoxide anion radical concentration. The superoxide anion radical scavenging activities of ether fraction from R. multiflora and R. wichuraiana flower were 0.06 and 0.09 mg/mL, respectively, whereas those of the ethyl acetate fractions of the R. multiflora and R. wichuraiana fruit extracts were 0.14 and 0.08 mg/mL.. The superoxide anion radical scavenging activities of the ethyl acetate fractions of the fruit extracts and the ether fractions of flower extracts were greater than those of the other fractions, regardless of species. The superoxide anion radical scavenging activity of ethyl acetate fraction of Sanguisorba officinalis (Rosaceae) extract was 40% at a concentration of 1,000 µg/mL, and the scavenging activity of water extract of Prunus sargentii was 40 % at a concentration of 500 ppm33,34. Superoxide radicals are powerful oxidizing agents that can react with biological membranes and induce tissue damage. Moreover, these radicals decompose to singlet oxygen, hydroxyl radical, or hydrogen peroxide molecules and may be associated with the onset of a various pathological conditions, including rheumatoid arthritis and cancer35,36.
Table 3: The superoxide anion radical scavenging activity of solvent fractions from two Rosa species.
| Plant | Plant organ | Extract fraction | IC50 (±SD, mg/ml)a |
| Rosa multiflora | Fruit | Hexane | 0.31±0.05a |
| Ether | 0.24±0.02b | ||
| Ethyl acetate | 0.14±0.04b | ||
| Water | 0.34±0.02a | ||
| Flower | Hexane | 0.36±0.01a | |
| Ether | 0.06±0.00c | ||
| Ethyl acetate | 0.20±0.00b | ||
| Water | – | ||
| Rosa wichuraiana | Fruit | Hexane | 0.24±0.01b |
| Ether | 0.18±0.00c | ||
| Ethyl acetate | 0.08±0.00d | ||
| Water | 0.70±0.00a | ||
| Flower | Hexane | 0.16±0.01b | |
| Ether | 0.09±0.01b | ||
| Ethyl acetate | 0.69±0.04a | ||
| Water | 0.14±0.02b |
a Values with different letters in the same column were significantly (p < 0.05) different.
Duncan’s test should be compared within each part of a plant.
Kaempferol and quercetin are present in many plant species that are commonly used in traditional medicine20. The compounds have been associated with reduced risk of pancreatic cancer, and quercetin, in particular, is effective against prostate cancer37. Kaempferol is a markedly active inhibitor of COX-2 transcriptional activation and exhibits antimicrobial activity against Propionibacterium acnes38,39. One of the goals of the present study was to measure the kaempferol and quercetin contents of fractions of two Rosa species fruit and flower extracts (Table 4; Fig. 1; Fig.2). Unlike the observed for extraction yield, the flavonoids content did not show dependence on the yield. The content of kaemferol was 10.35 mg% and 6.21 mg% in ethyl acetate fraction of R. multiflora and R. wichuraiana flower, these are the highest contents of kaempferol. Among the fruit fractions of the two Rosa species, quercetin was detected only in the ether fraction of R. multiflora. The water and ethyl acetate fractions of the R. multiflora and R. wichuraiana flower extracts contained more quercetin than the other two fractions, and no quercetin was detected in fractions of the R. wichuraiana fruit extract. The ether fractions of both the R. multiflora and R. wichuraiana fruit extracts contained more quercetin than the other fractions, and more quercetin was detected in the ethyl acetate fractions of the two Rosa species flower extracts. In contrast to the previous studies21-23,40, the findings of the present study did not show strong correlation between flavonoid contents and antioxidant activity. Previous studies41,42 have reported that the kaempferol and quercetin contents of Rubus idaeus (Rosaceae) and Prunus cerasus leaves are 2.38 and 5.05 mg/kg, respectively. Liaudanskas et al.43 reported a strong correlation between total phenolic contents and radical scavenging and reducing activities of the Rosa fruits grown in Lithuania, and another study44 reported that the ethyl acetate fraction of Rosa multiflora flower extract had greater phenolic content and antioxidant activity than other tested fractions.
Table 4: The contents of kaempferol and quercetin in solvent fractions from two Rosa species.
| Plant | Plant organ | Extract fraction | Kaempferol
(±SD, mg%)a |
Quercetin
(±SD, mg%)a |
| Rosa multiflora | Fruit | Hexane | – | – |
| Ether | 0.47±0.03a | 5.78±0.79a | ||
| Ethyl acetate | – | 0.93±0.14b | ||
| Water | – | 0.20±0.14b | ||
| Flower | Hexane | – | – | |
| Ether | 1.50±0.41b | 1.53±0.61b | ||
| Ethyl acetate | 10.35±0.77ab | 4.53±0.54bc | ||
| Water | 12.13±0.89a | 9.16±0.82a | ||
| Rosa wichuraiana | Fruit | Hexane | – | – |
| Ether | – | 2.73±0.00a | ||
| Ethyl acetate | – | 0.72±0.35b | ||
| Water | – | 0.82±0.44b | ||
| Flower | Hexane | – | – | |
| Ether | 1.73±0.52b | 2.01±0.69b | ||
| Ethyl acetate | 6.21±0.61a | 11.69±1.01a | ||
| Water | 1.18±0.46b | 2.26±0.99b |
a Values with different letters in the same column were significantly (p < 0.05) different
 |
Figure 1: The structure of kaempferol and quercetin. |
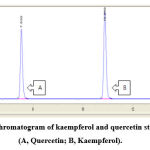 |
Figure 2: The chromatogram of kaempferol and quercetin standard by HPLC (A, Quercetin; B, Kaempferol). |
Conclusion
The antioxidant activity and flavonoid contents of various fractions (hexane, ether, ethyl acetate, and water) of ethanol extract from R. multiflora and R. wichuraiana used complementary in Korea were evaluated. The greater antioxidant activity by DPPH assay exhibited in the ethyl acetate or water fraction of two Rosa species extracts, whereas greater superoxide anion radical scavenging activity was shown in the ether or ethyl acetate fraction. The content of flavonoid, such as kaempferol and quercetin in the four fractions is not reflected clearly in the DPPH and superoxide anion radical scavenging activity. However, the findings of the study still suggest that the two Rosa species could be useful as natural antioxidants or food additives.
Acknowledgment
This work was supported by a Research promotion program of SCNU.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding Sources
There is no funding source.
References
- Matthews V. A. Rosa Linaaeus, The European garden flora, a manual for the identification of plants cultivated in Europe, both out-of-doors and under glass. Cambridge University Press, Cambridge, USA, 1995; pp. 359-379
- Yü T.-T, Lu L.-T, Ku T.-C, Li C.-L and Chen S.-X. Rosaceae (3) Prunoideae. In: Yu, T. (Eds.). Flora Reipublicae Popularis Sinicae, vol. 38. Science Press, Beijing, China, 1986; pp. 1-133
- Park G. H. Verification of biological activity and special formulation on Rose multiflora M.Sc. thesis, Daegu Haany University. Daegu, Korea, 2009.
- Park G. H, Lee J. Y, Kim D. H, Cho Y. J and An B. J. Anti-oxidant and anti-inflammatory effects of Rosa multiflora J. Life Sci., 2011; 21: 1120-1126.
CrossRef - Kwon M. W. Phytochemical constituents from the fruits of Rosa multiflora and their antioxidant activities. M.Sc. thesis, Chung-Ang University. Seoul, Korea, 2005.
- Hubue G, Wray V and Nahrstedt A. Flavonol oligosaccharides from the seeds of Aesculus hippocastanum. Planta Med., 1999; 65: 638-640
- Masakazu A. Components of the flower petals of Rosa multiflora and Rubus hirsutus. Yakugaku Zasshi, 1962; 82: 771-773
CrossRef - Ha S. E, Kim H. D, Park J. K, Chung Y. O, Kim H. J and Park N. B. Melanogenesis inhibition effect of Rosa multiflora extracts in B16 melanoma cell. Kor. J. Plant Res., 2009; 22: 317-322
- Park G. H. The study on anti-wrinkle, anti-inflammatory skin mechanism of active ingredients in root of Rosa multiflora and its stabilization of cosmetic medical activation in W/O/W dosage form. Ph.D thesis, Daegu Hanny University. Daegu, Korea, 2012.
- Ohwi J. Flora of Japan. Smithon Institution, Washington, D.C. USA, 1965.
- Ma Y, Byrne D. H and Chen J. Propagation of rose species in vitro. In Vitro Cell Dev. Biol., 1996; 32: 103-108
CrossRef - Schum A, Hofmann K and Fulten R. Fundamentals for integration of somatic hybridization in rose breeding. Acta Hortic., 2002; 572: 29-36
CrossRef - Gyamfi M. A, Yonamine M and Aniya Y. Free radical scavenging action of medicinal herbs from Ghana Thonningia sanguineaon experimentally induced liver injuries. Pharmacol., 2002; 32: 661-667.
CrossRef - Osawa T. Novel neutral antioxidant foutilization in food and biological systems. Soci. Pres., 1994; pp. 241-251.
- Noda Y, Anzai-Kmori A, Kohono M, Shimnei M and Packer K. Hydroxyl and superoxide anion radical scaveinging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system Molecul. Biol. Int., 1997; 42: 35-44.
CrossRef - Mccall M. R and Frei R. Can antioxidant vitamins maternally reduce oxidative damage in humans? Free Radic. Biol. Med., 1999; 26: 1034-1053
CrossRef - Ismail A and Hong T. Antioxidant activity of selected commercial seaweeds. J. Nutr., 2002; 8: 167-177.
- Pourmorad F, Hosseinimehr S and Shahabimajd N. Antioxidant activity, phenol and flavonoid content of some selected Iranian medicinal plants. J. Biotechnol., 2006; 5: 1142-1145.
- Rice-Evans C. A and Miller N. J. Antioxidant activities of flavonoids as bioactive components of food. Soc. Trans., 1996; 24: 790-795.
CrossRef - Calderón-Montaño J. M, Burgos-Morón E, Pérez-Guerrero C and López-Lázaro M. A. Review on the dietary flavonoid kaemperol. Mini Rev. Med. Chem., 2011; 11: 298-344
- Khan R. A, Khan M. R, Sahreen S and Ahmed M. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Cent. J., 2012; 6: 43-48.
CrossRef - Diaz P, Jeong S. C, Lee S, Khoo C and Koyyalamudi S. R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Med., 2012; 7: 26-34.
CrossRef - Yakubu O. E, Nwodo O. F. C, Joshua P. E, Ugwu M. N, Odu A. D and Okwo F. Fractionation and determination of total antioxidant capacity, total phenolic and total flavonoids contents of aqueous, ethanol and n-hexane extracts of Vitex doniana Afr. J. Biotechnol., 2014; 13: 693-698.
CrossRef - Blois M. S. Antioxidant determination by the use of a stable free radical. Nature, 1958; 26: 1199-1200.
CrossRef - Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. Biol. Chem., 1970; 245: 4053-4057.
CrossRef - Balkan I. A, Taskin T, Dogan H. T, Deniz I and Akaydin G. A comparative on the in vitro anti-inflammatory, antioxidant and antimicrobial potential of subextracts from the aerial parts of Daphne oleoides subsp. oleoides. Ind. Crop Prod., 2017; 95: 695-703.
CrossRef - Kulevanova S, Stefova M, Panovska T. K and Stafilov T. HPLC identification and determination of myricetin, quercetin, kaempferol and total flavonoids in herbal drugs. Pharmac. Bull., 2002; 48: 25-30.
CrossRef - Abeer T and Walid H. E. T. Characterization of antioxidant activity of extract from Artemisia vulgaris. J. Pharm. Sci., 2008; 21: 321-326.
- Mukhia R, Basistha B and Chheri D. R. Variation in antioxidant activity of a Rattan species, Plectocomia himalayana Griff. by DPPH assay based on two different methods of methanol extraction. Res. J. Pharmacog. Phytochem., 2018; 10: 175-178.
CrossRef - Kumar N, Bhandari P, Singh B and Bari S. S. Antioxidant activity and ultra-perormance LC-electrosparay ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascene, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol., 2009; 47: 361-367
CrossRef - Dehghan K. A, Rasooli I, Sharai S. M, Rezaee M. B, Jalali N. M. R and Owlia R. Phytobiological characteristics of Rosa hemisphaerica extract. J. Med. Pl., 2010; 9: 97-106
- Molan A. L, Fara J. A. M and Mahdy A. D. Antioxidant activity and phenolic content of some medicinal plants traditionally used in Northern Iraq. , 2012; 2: 224-233.
- Kim H. Y, Yeo S. I and Lee J. T. Antioxidant effects of solvent fraction from Sanguisorbae officinalis with acetone. J. Appl. Biol. Chem., 2011; 54: 89-93.
CrossRef - Park J.-M, Lee J.-Y, Park T.-S, Park G.-H, Park K. S, Kim T.-H, Cho Y.-J, Kwon O.-J and Choi K.-I. An BJ. Biological activity investigation, and phenol compounds isolation from barks of Prunus sargentii Kor. J. Med. Crop Sci., 2008; 16: 173-182.
- Siriwardhana S. S. K. W and Shahidi F. Anti-radical activity of extracts of almond and its by-products. Am. Oil Chem. Soc., 2002; 79: 903-908.
CrossRef - Sfahlan A. J, Mahmoodzadeh A, Hasanzadeh A, Heidari R and Jamei R. Antioxidant and antiradicals in almond hull and shell (Amygdalus communis) as a function of genotype. Food Chem., 2009; 115: 529-533.
CrossRef - Nöthlings U, Murphy S. P, Wilkens L. R, Henderson B. E and Kolonel L. N. Flavonols and pancreatic cancer risk: the multiethnic cohort study. J. Epidemiol., 2007; 166: 924-931.
CrossRef - Liang Y. C, Huang Y. T, Tasi S. H, Lin-Shiau S. Y, Chen C. F and Lin J. K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. , 1999; 20: 1945-1952.
CrossRef - Lim Y. H, Kim I. H and Seo J. J. In vitro activity of kaempferol isolated from the Impariens balsamona alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. Microbiol., 2007; 45: 473-477.
- Lahmadi S, Belhamra M, Karoune S, Kechebar M. S. A, Bensouici C, Kashi I and Ksouri R. In vitro antioxidant capacity of Euphorbia retusa from Algerian desert. J. Pharm. & Pharmacog. Res., 2019; 7: 356-66.
- Gudej J. Kaempferol and quercetin glycosides from Rubus ideaus L. leaves. Acta Poliniae Pharma., 2003; 60: 313-316.
- Jakobek L, Seruga M, Novak I and Medvidovic-Kosanovic M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensmitt. Rundsch., 2007; 103: 369-378.
- Liaudanskas M, Noreikiene I, Zymone K, Juodyt R, Žvikas V, and Janulis V. Composition and antioxidant activity of phenolic compounds in fruit of the Genus Rosa L.. Antioxidants, 2021; 10: 545.
CrossRef - Kwak C. S, Choi H. I and Yang J. Antioxidant activity of Rosa multiflora Thunb. flower extract and suppressive activity on proinflammatory mediator production in lipopolysaccharide-stimulated RAW 264.7 macrophages. Funct. Foods Health Dis., 2016; 6: 265-278.
CrossRef








