Khilola A. Rakhmanova1 , Sherzod N. Zhurakulov2
, Sherzod N. Zhurakulov2 , Firuza M. Tursunkhodjayeva1, Azizbek A. Azamatov1
, Firuza M. Tursunkhodjayeva1, Azizbek A. Azamatov1 , Dilfuza M. Saidkhodjayeva1 and Inoyat Z. Jumayev2,3*
, Dilfuza M. Saidkhodjayeva1 and Inoyat Z. Jumayev2,3*
1Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Laboratory of Pharmacology and Toxicology, Tashkent, Uzbekistan.
2Institute of Chemistry of Plant Substances named after academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Laboratory of Chemistry of Alkaloids, Tashkent, Uzbekistan.
3Institute of Biophysics and Biochemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
Corresponding Author E-mail: inoyat8585@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/2423
Abstract
It is known that natural isoquinoline alkaloids are widely used in pharmacology and have a variety of biological activities1. At the same time, synthetic analogs of isoquinoline alkaloids are of great interest, among which compounds have been identified that are promising agents that modulate the activity of the dopamine and serotonergic systems2,3, showing cardiprotective4 effect, antiproliferative5 and analgesic6 activity. Currently, in practical medicine, aspirin and anlagen are widely used as non-narcotic analgesics7. However, the low intensity of the analgesic effect, the lack of an analgesic effect in certain types of pain (thermal, mechanical, and other acute pain) and the large number of side effects caused by long-term use limits the scope of their application. The properties of 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) have not been previously described in the scientific literature. The analgesic and anti-inflammatory activity of 1-(4'-dimethylaminophenyl)-6,7- dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) was studied under conditions of thermal (hot plate test) and chemical (vinegar writhing test) irritation, anti-inflammatory activity - on the model of acute inflammatory arthritis. As a result of the studies, it was found that the compound 1-(4'-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline in various doses has an analgesic and anti-inflammatory effect. 1-(4'-Dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) showed a pronounced anti-inflammatory effect at a dose of 0.5 mg/kg, 3.3 times greater than the effect of diclofenac sodium. It has been shown that 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) has a pronounced analgesic and anti-inflammatory activity and can be used in medical practice as a non-narcotic analgesic.
Keywords
Acute Toxicity; Analgesic and Anti-Inflammatory Activity; Acetylsalicylic Acid; Diclofenac Sodium; Tetrahydroisoquinoline Hydrochloride; metamizole sodium
Download this article as:| Copy the following to cite this article: Rakhmanova K. A, Zhurakulov S. N, Tursunkhodjayeva. F. M, Azamatov A. A, Saidkhodjayeva D. M, Jumayev I. Z. Analgesic and Anti-Inflammatory Effects of 1-(4'-Dimethylaminophenyl)-6, 7-Dimethoxy-1,2,3,4-Tetrahydroisoquinoline Hydrochloride. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Rakhmanova K. A, Zhurakulov S. N, Tursunkhodjayeva. F. M, Azamatov A. A, Saidkhodjayeva D. M, Jumayev I. Z. Analgesic and Anti-Inflammatory Effects of 1-(4'-Dimethylaminophenyl)-6, 7-Dimethoxy-1,2,3,4-Tetrahydroisoquinoline Hydrochloride. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3y1wwYG |
Introduction
The understanding the mechanisms of pathological pain and the search for new highly effective pharmacological agents with minimal side effects is important in solving the problem of pain relief. Non-narcotic analgesics are most commonly used for the treatment of mild to moderate pain of various etiologies. Over the world more 30 million people daily take non-narcotic analgesics, including non-steroidal anti-inflammatory drugs (NSAIDs)8. According to the results of observations, all representatives of non-narcotic analgesics administered in equivalent doses, have relatively similar efficacy and differ mainly in individual tolerance and the ability to cause side reactions: damage gastrointestinal tract, destroy platelet aggregation, renal function, and cause negative effect on the liver and circulatory systems9.
Currently, acetylsalicylic acid and sodium metamizole are most widely used in medicine10. However, low analgesic effect, absence of an analgesic effect for some types of pain (thermal, mechanical, and other acute pains) and the large number of side effects caused limit the scope of their application.
Material and Methods
The closest analog in the chemical structure of 1-(4′-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) is the alkaloid papaverine. Papaverine is used in practical medicine as a myotropic antispasmodic agent11. Papaverine is not used as a local anesthetic and analgesic agent.The compound 1-(4”-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline hydrochloride (3) was obtained according to the well-known scheme12.
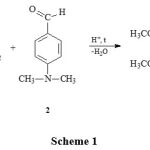 |
Scheme 1 |
Experimental Chemical Part
: IR spectra were recorded using the FTIR System 2000 spectrometer (PerkinElmer, USA) in KBr tablets. 1H NMR spectra were recorded using a Unity-400 spectrometer (Varian, USA, solvents-CDCl3, internal standard-HMDS). The Rf values were determined by the TLC method on the LS 5/40 silica gel plates using the CHCl3/MeOH (6:1) solvent system. The melting point of the synthesized substance was determined on the BOETIUS microplate.
Method of application of 1-(4’- dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquine hydrochloride (3), C17N24O2N2 ×HCl.
A mixture of 26.3 g (0.14 mol) of 3,4-dimethoxyphenylethylamine (1) with 21.67 g (0.14 mol) of 4-dimethylaminobenzaldehyde (2) was heated at 80°C in an oil bath with a contact thermometer for 2 hours with a reverse refrigerator. After cooling to room temperature, 100 ml of trifluoroacetic acid was added to the reaction mixture and boiled with a reverse refrigerator for 4 hours. The course of the reaction was controlled by TLC. Next, the reaction mixture was cooled with ice and alkalized with a 10 % aqueous NaOH solution to a pH of 9-10. The amine was exhaustively extracted with chloroform. After distilling the chloroform, the crude product was dissolved in acetone and acidified conc. HCI to pH 5-6. The precipitated hydrochloride precipitate was filtered, washed 3 times with acetone and recrystallized from ethyl alcohol. We obtained 49.04 g (92%), hydrochloride with a melting point of 246-248o C (from C2H5OH), Rf 0.72.
IR spectrum (KBr, vmax, cm-1): 3418 (NH), 2935, 2895, 2771 (Ar-H), 1616, 1519 (C=C), 1361 (CH2), 1262, 1233 (C-O) 1119 (C-N).
1H NMR (400 MHz, CDCl3, MD, J / Hz): 2.88 (6H, s, 4′-N (CH3)2), 2.93 and 3.11 (each 2H, m, H-3,4), 3.60 (3H, s, 7-OCH3), 3.81 (3H, s, 6-OCH3), 5.29 (1H, s, H-1), 6.22 (1H, s, H-8), 6.55 (1H, s, H-5), 6.61 (2H, d, J=9.0, N-2′,6′), 7.15 (2H, d, J=9.0, H-3′, 5′).
Experimental studies were conducted in accordance with the requirements of the «European Convention for the Protection of Animals Used for Experimental and Scientific Purposes»13.
The studied compounds were administered intragastrically using non-traumatic metal probe 60 minutes before the chemical or thermal irritation.
Analgesic activity of the studied compound was tested on 80 white outbred mice weighing 18-20 g in compare with acetylsalicylic acid and sodium metamizole in the acetic writhing and hot plate tests.
In the hot plate test 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) at doses of 0.1-0.5-1.0-5.0-10.0 mg/kg, acetylsalicylic acid 100.0-200.0 mg/kg, and metamizole sodium 10.0-20.0 mg/kg were administered orally, then in 60 minutes the mouse was placed on a hot plate (58°C) to determine pain sensitivity.
The acetic writhing test is used to assess visceral pain. The pain was caused by intraperitoneal administration of 1% acetic acid solution, which leads to the appearance of the “writhing” syndrome. The number of writhing in animals of the experimental and control groups was calculated within 20 minutes.
Anti-inflammatory activity of the compound was determined on white male rats weighing 200-220 g on a model of acute inflammatory edema caused by injection of 0.1 ml 2% aqueous solution of formalin into the rat’s hind paw.
The volume of the paw was measured oncometrically before the start of the experiment and at maximum development of edema in 3 hours after the injection of formalin. The studied substance was administered intragastrically in an hour before the introduction of formalin. The activity of the compound and the comparison drug diclofenac sodium was evaluated by the suppression of paw edema.
Results
In the hot plate test, 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) showed high analgesic activity by increasing the threshold of pain sensitivity in thermal pain syndrome, significantly exceeding the comparison drugs. Metamizole sodium at a dose of 10.0 mg/kg in 60 minutes after administration depressed the threshold of pain irritation by 71.4% and at a dose of 20.0 mg/kg – by 50.7%; acetylsalicylic acid at a dose of 100.0 mg/kg – by 58.0%, 200.0 mg/ kg-71.1%; 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) at a dose of 0.1 mg/kg – by 51.8%, 0.5 mg/kg – 147.1%, 1.0 mg/kg – 116.0%, 5.0 mg/kg -76.9%, 10.0 mg/kg – 74.3%. Thus, the isoquinoline compound at a dose of 0.5 mg / kg showed analgesic activity two times higher compared to metamizole sodium and acetylsalicylic acid.
The results of the studies are presented in Table 1.
Table 1: Comparative analgesic activity of the studied substances in thermal irritation (hot plate test), n=10.
| № | Research of substance | Doze mg/kg | The latent period of the pain reaction, sec. | ||||
| Before injection | After injection through min | ||||||
| 60 | 90 | 120 | 150 | ||||
| 1 | Control | 0.2 ml | 12.6±4.1
|
11.3±3.8
|
11.7±4,0 | 13.7±4,1 | 14.5±4.3 |
| 2 | 1-(4 – dimethylamino-phenyl)-6,7- dimethoxy -1,2,3,4-tetrahydroisoquinoline hydrochloride (3)
|
0.1 | 13.7±2.88 | 15.4±4,3 | 17.6±4, 8 | 19.0±5,1 | 20.8±5.52 |
| 0.5 | 14.0±3.8 | 21.6±4.8 | 22.5±5.8 | 26.0±6.0 | 34.6±6.7 | ||
| 1.0 | 12.5±3.12 | 15.0± 3.8 | 17.2±4.3 | 21.0±4.5 | 27.3±6.2 | ||
| 5.0 | 11.3±2.6 | 13.5±3.4 | 15.2±3.9 | 16.6±4.4 | 20.3±5.3 | ||
| 10.0 | 13.4±3.6 | 15.6±4.1 | 15.8±4.2 | 19.7±5.1 | 31.2±6.5 | ||
| 3 | Metamizole sodium | 10.0 | 11.2±3.2 | 18.8±4.9 | 16.0 ±3.3 | 15.6±4.1 | 15.8±4.2 |
| 20.0 | 13.6±3.5 | 18.5 ±4.7 | 16.2 ±3.5 | 16.0±3.3 | 15.9±4.0 | ||
| 4 | Acetylsalicylic acid | 100.0 | 12.4 ±3.11 | 17.2 ±4.3 | 16.7±3.8 | 16.4 ±3.4 | 15.2 ±3.9 |
| 200.0 | 11.8±2.86 | 18.5±4.7 | 17.8±4.6 | 16.2±3.5 | 16.0 ±3.3 | ||
It was found that the studied compound exhibits a pronounced analgesic effect on the model of acetic writhing. The average effective dose (ED50) for suppressing the number of writhing caused by intraperitoneal administration of acetic acid was 0.5 mg/kg, and for metamizole sodium-180.5 mg/kg (Table 2).
Table 2: Comparative analgesic activity in chemical irritation (acetic writhing test), n=10.
| № | Substance | LD50 mg/kg | ED50 mg/kg | Index of the breadth of analgesic action LD50 / ED50 |
| 1 | Research of substance | 1250 | 5.0 | 250.0 |
| 2 | Metamizole sodium | 3500 | 180.5 | 19.4 |
Note: LD50 is an average lethal dose, ED50 is an average effective dose.
Thus, the studied compound in the acetic writhing test is 36 times more effective (ED50) and 13 times more safe (LD50/ED50) than metamizole sodium. A higher analgesic index of the investigated indicates on its greater selectivity and safety compared to metamizole sodium.
The anti-inflammatory activity of 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) was studied on a model of formalin arthritis in comparison to diclofenac sodium (Table 3).
Table 3: Comparative anti-inflammatory activity of the studied compound and diclofenac sodium on a model of formalin arthritis in rats, n=10.
| № | Research of substance | Doze
mg/kg |
Average foot volume | The increase in the volume of the foot in relation to the original one
|
Anti–inflammatory effect, %
|
||
| In normal | After
3 hours |
||||||
| ml | % | ||||||
| 1 | Control (formalin 1%) | 0.2 мл | 0.8± 0.23 | 1.4 ±0.288 | 0.6±0.216 | 55.6% | – |
| 2 | 1-(4 – dimethylamino-phenyl)-6,7- dimethoxy -1,2,3,4-tetrahydroisoquinoline hydrochloride (3) | 0.1 | 0.7± 0.17 | 1.3± 0.144 | 0.6 ±0.216 | 85.7% | 54.0% |
| 0.5 | 0.8± 0.23 | 1.1 ±0.12 | 0.3 ±0.310 | 37.5% | 67.4% | ||
| 1.0 | 0.7 ±0.17 | 1.1± 0.12 | 0.4 ±0.312 | 57.1% | 2.7% | ||
| 5.0 | 0.9±0.312 | 1.3 ±0.144 | 0.4 ±0.312 | 44.4% | 20.0% | ||
| 10.0 | 0.8 ±0.23 | 1.3 ±0.144 | 0.5 ±0.210 | 62.5% | 12.4% | ||
|
3 |
Diclofenac sodium | 50.0
|
0.9± 0.312 | 1.3 ±0.144 | 0.4 ±0.312 | 44.4% |
20.0% |
Table 3 shows that anti-inflammatory effect of the investigated compound at a dose of 0.5 mg/kg is 3.3 times higher than the reference drug diclofenac sodium.
Conclusion
Thus, the results of the studies indicate that 1-(4’-dimethylaminophenyl)- 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) has a pronounced analgesic and anti-inflammatory activity and can be used as a perspective non-narcotic analgesic in medical practice.
In contrast to the widely used in medical practice metamizole sodium, diclofenac sodium and acetylsalicylic acid, 1-(4’-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (3) has a high analgesic and anti-inflammatory activity and a greater therapeutic index
Acknowledgement
The work was financially supported by the Ministry of Innovative Development under the Cabinet of Ministers of the Republic of Uzbekistan (grant No. MUK-2021-42).
Conflict of Interest
The authors have declared that no conflict of interest exists.
Funding Sources
There is no funding sources.
References
- Semenov A.A. Essay on the chemistry of natural compounds / A.A. Semenov. -Novosibirsk, “Science”. Siberian publishing company RAS. – 2000. – p. 478 -510.
- Mi, G. Levo-Tetrahydroberberrubine Produces Anxiolytic-Like Effects in Mice through the 5-HT1A Receptor /G. Mi, S. Liu, J. Zhang // PLoS One. – 2017. -№12(1)
CrossRef - Shonberg, J. Structure-activity study of N-((trans)-4-(2-(7-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)-cyclohexyl)-1H-indole-2-carboxamide (SB269652), a bitopic ligand that acts as a negative allosteric modulator of the dopamine D2 receptor/ J. Shonberg, C. Draper-Joyce, S.Mistry // J. Med. Chem.– -№58(13). –Р.5287-5307.
CrossRef - Inoyat Jumayev., Pulat Usmanov., Shavkat Rustamov., Sherzod Zhurakulov. Comparative inotropic effects of some isoquinoline alkaloids.// Biomedical & Pharmacology Journal (India). – – V.13(1), – P. 325-333.
CrossRef - Gao, F. Design, synthesis and testing of an isoquinoline-3-carboxylic-based novel anti-tumor lead / F. Gao, H. Liu, L. Li [et al.] // Bioorg. Med. Chem. Lett. – 2015. – №25(20). – Р.4434-4436.
CrossRef - Voight E. Discovery of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl) urea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy / E.Voight, Gomtsyan, J. Daanen // J. Med. Chem. – 2014. -№57(17). –Р.7412-7424
CrossRef - Mashkovsky M. D. Medicinal products. Tashkent, Medicine, 1998, vol. 1, pp. 159-179.
- Sokolov A. S., Nikonov V. V., Feskov A. E. Analgin-effective, Cheap, Safe, What is the alternative. Emergency medicine. 2017. No. 2 (81). pp. 75-80.
- Belyakov V. A., Solovyov I. K. Analgesics (non-narcotic). N. Novgorod, 2001
- Mashkovsky M. D. Medicinal products. Medicine, 2002, Volume-1.
- Zhurakulov Sh. N., Vinogradova V. I., Djumaev I. Z., Usmanov P. B. Synthesis of 1-aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines and a number of derivatives / / Reports of the Academy of Sciences of the Republic of Uzbekistan-Tashkent. – №. 3, pp. 51-53.
CrossRef - European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, ETS №123, Strasbourg (1986).








