Hendri Busman1* , Sutyarso1
, Sutyarso1 , Salman Farisi1
, Salman Farisi1 , Fukrapti2 , Aulia Rika Fahrumnisa2
, Fukrapti2 , Aulia Rika Fahrumnisa2
1Department of Biology, Faculty of Mathematics and Natural Sciences, University of Lampung, Indonesia
2Faculty of Medicine, University of Lampung, Indonesia
Corresponding Author E-mail: hendri.busman@fmipa.unila.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2367
Abstract
Turmeric rhizome extract has been shown to have antifertility effects as antiestrogenic and is reversible. This study aims to rate turmeric rhizome extract (Curcuma longa L.) antiestrogenic potential towards epithelium cell and endometrium layer thickness reduction on female rats. Twenty-eight female rats aged around 6-8 weeks old and weighing around 200-250 g were divided into four groups using a completely randomized design. The control group received only aquadest. Treatment groups 1, 2, and 3 received 250, 500, and 1.000 mg/kg BW turmeric rhizome extract, respectively, for five days. At the end of the examination, there was a significant decrease in the number of endometrial epithelial cells in the turmeric group (p=0,000), in line with the increase in the dose given. This research also shows the presence of antiestrogenic potential effects associated with an endometrium layer thickness (p=0.013), and there was a decrease in endometrium thickness associated between the control group and treatment group (p<0,05). Conclusions: Turmeric rhizome extract has an antiestrogenic potential and can reduce the total of epithelium cells and endometrium layer thickness on female rats.
Keywords
Antiestrogenic Potential, Endometrium Epithelium Cells, Endometrium Layer, Turmeric Rhizome Extract
Download this article as:| Copy the following to cite this article: Busman H, Sutyarso S, Farisi S, Fukrapti F, Fahrumnisa A. R. Turmeric Rhizome’s Extract Reduce Epithelium Cells and Endometrium Layer Thickness of Female Rats. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Busman H, Sutyarso S, Farisi S, Fukrapti F, Fahrumnisa A. R. Turmeric Rhizome’s Extract Reduce Epithelium Cells and Endometrium Layer Thickness of Female Rats. Biomed Pharmacol J 2022;15(1).Available from: https://bit.ly/3JzlrTn |
Introduction
Interaction between blastocyst, implantation, and endometrium stages during initiation and nidation is a complex matter. When blastocysts carry out adhesion and invasion, the endometrium will be able to respond. Structurally and molecularly, the initiation of trophoblast apposition and adhesion in the endometrium of primate species is not very well known. In rodents, exceptions occur after fertilization, the trophectoderm does not undergo adhesion, and the microfilic portion will assist the application of blastocysts. During adhesion and implantation, the gap between the trophectoderm cell membrane and the surface of the endometrium epithelium will decrease1.
It added that the situation was caused by extensive endometrium destruction. It results in amenorrhea and habitual abortion, which is believed to occur due to inadequate endometrium to support implantation of the fertilization results so that failure of implantation of embryos in the endometrium will cause abortion2,3.
Turmeric rhizome extract has been shown to have antifertility effects as antiestrogenic and is reversible. Giving turmeric rhizome extract with cumin (Carum carvi) reduces levels of the hormone Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) significantly through the inhibition of positive feedback to the pituitary, thereby inhibiting the formation of estrogen1.
In addition, turmeric rhizome extract affects the female reproductive organs, evidenced by the administration of turmeric ethanol extract using multi-level doses orally showing the higher dose given the thinner the layer and the less number of uterine endometrium glands formed4. On the other hand, it was explained that turmeric extract could reduce the number of endometrium luminal epithelium cells in LH-induced mice, and post-coitus given turmeric extract showed no implantation or fetal sites5.
Methods
Plant Materials
Turmeric rhizomes were collected from Bandar Lampung city area, Lampung, Indonesia. The collected turmeric rhizomes (Curcuma longa L.) were labeled and transferred to the laboratory, washed with tap water, air dried, and stored until further investigations.
Preparation of the Plant Extract
The obtained turmeric rhizomes are then cleaned and then processed to dry using a 70°C oven for 15 minutes. After drying, the plants are milled using a blender to produce a powder obtained 283.9 grams. Turmeric rhizome’s which was crushed, was put into a 2.000 ml glass beaker then macerated using 96% ethanol solvent for 3 x 24 hours to obtain macerate. The filtrate obtained is concentrated or thickened using a rotary evaporator at a temperature of 500C for 1 hour.
Animal Treatment
Rats conducted acclimatization for one week under laboratory conditions in cages prepared. Appropriate 28 female white rats Sprague Dawlye’s strain age around 6-8 weeks old weighed around 200-250 g that splits into four groups and can be maintained 100 cm x 50 cm, placed in a research room, or placed in a research room rat cage. The rat cage contained a bowl filled with food, rice husks, and a place to drink. Rats are fed with small chicken feed and drink water daily. Husks are replaced every two days due to moisture due to spillage of food or drink rats to prevent bacteria or fungi growth.
Turmeric Rhizome’s Extract Treatment
Rats received an oral dose of freshly prepared turmeric rhizome extract. The Control group only received aquadest. The treatment group 1, 2, and 3 received 250, 500, and 1.000 mg/kg BW turmeric rhizome extract, respectively, for five days.
Sample Collection
After five days, all rats were sacrificed using ketamine (80-100 mg/kg BW). The endometrium was taken and fixed in 10% phosphate-buffered formalin. After being fixed for 48 hours, the endometrium was then made histopathological preparations with Mayer Hematoxylin staining, carried out following the protocol prepared by the Department of Pathology, Faculty of Medicine, University of Lampung. The number of epithelial endometrium cells was observed using a light microscope with 100x magnification in 10 visual fields. The thickness of the endometrium layer was observed using the Olympus Stream Software.
Statistical Analysis
The number of epithelial endometrium cells and the thickness of the endometrium layer data were tested using One Way Anova followed by the Least Significant Difference (LSD) test. All tests were performed at a 95% confidence level.
Ethical Clearance
This study was approved by the Health Research Ethics Committee with EC number: 3813/UN26.18/PP/05.02.00/2019.
Results
Turmeric rhizome extract decreases the number of endometrium epithelium cells.
The results showed that the group of female rats given turmeric rhizome extract had a significantly lower number of endometrial epithelial cells when compared to the control group. A decrease in the number of endometrial epithelium occurs with an increase in the dose of the extract given (Tabel 1).
Turmeric rhizome’s extract decreases the endometrium layer thickness
Similar to the number of endometrium epithelium cells, the endometrial layer thickness in the turmeric rhizome’s extract group was significantly thinner when compared to the control group. The highest dose of turmeric rhizome extract had the thinnest endometrial layer thickness (Tabel 1).
Table 1: The average number of endometrium epithelium cells and endometrium layer thickness of white rats.
| Group | Number of endometrium epithelium cells | Endometrium layer thickness | ||
| Mean±SD (cells) | P value | Mean±SD (µm) | P value | |
| C | 291,00 ± 43,9a | 0,000* | 764,74 ± 80,19a | 0,013* |
| T1 | 234,86 ± 55,7a | 615,06 ± 119,50b | ||
| T2 | 174,29 ± 24,93b | 646,17 ± 139,29b | ||
| T3 | 167,00 ± 55,5b | 566,18 ± 68,74c | ||
*indicates a significant difference based on the One Way Anova test at α = 5%. The mean followed by different letters indicates a significant difference based on the LSD test at 5%.
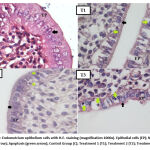
Histological observations on preparations with 1000x magnification showed a microscopic picture of endometrium epithelium cells of white rats and their cell nuclei. Endometrium epithelium cell counts are only performed on epithelium cells that have a normal appearance. Normal endometrium epithelium cells in female white rats (Rattus norvegicus) are ciliated columnar epithelium cells with oval-shaped nuclei arranged in the mucosal lining of the white rat’s uterus. The abnormal picture of endometrium epithelium cells in mice includes necrosis or lysis of endometrium epithelium cells6.
Discussion
This study showed a decrease in the average number of epithelium cells in each treatment group, along with the increasing dose of turmeric rhizome extract given. The lowest mean number occurred in the T3 group, given the highest dose of turmeric rhizome extract at 1.000 mg/kg with mean epithelium cells 167 ± 55.5 cells. The results obtained in this study support the results of research5, which states that the administration of 500 mg/kg BW turmeric extract after coitus for five days showed the absence of implantation and fetal sites in rat endometrium.
The decrease in the number of normal endometrium epithelium cells occurs due to the antifertility mechanism of turmeric rhizome extract to the cascade of female reproductive cycles. Meanwhile, the reproductive cycle is an endometrium maturation to become a blastocyst implantation medium. It is related to maturation occurring when the endometrium is exposed to estrogen and progesterone7.
The antifertility mechanism of a turmeric extract inhibits positive feedback from the hormone estrogen. Physiologically, the hormone estrogen is formed when the ovarian follicles are stimulated by the hormones FSH and LH produced by the anterior pituitary. FSH and LH help the development of ovarian follicles, in line with this internal theca cells under the influence of LH produce androgens. On the other hand, granulosa cells under the influence of FSH will produce the aromatase enzyme, which helps convert androgens to the hormone estrogen. Estrogen is a sex hormone responsible for cellular proliferation and tissue growth in the reproductive system. Estrogen has a significant role in the endometrium proliferation phase8.
The formation of estrogen will constantly give a signal in the form of positive feedback resulting in a surge in LH that triggers ovulation. If positive feedback is inhibited, ovulation does not occur so that the corpus luteum, which produces the hormone progesterone, is not formed. In addition, FSH suppression occurs, which results in inadequate development of ovarian follicles so that de Graaf follicles are not formed4.
Estrogen will work if it binds to receptors in endometrium epithelium cells, the receptors ERα, Erβ, and G-protein-coupled estrogen receptor 1 (GPER1)9. Like estrogen, progesterone works when it binds to progesterone receptors (PR), namely PR-A and PR-B10. The receptors are scattered on the surface of the endometrium11. This regulation is regulated explicitly by epithelium cells and endometrium stroma under the influence of estrogen12. For these reasons, if the number of endometrium epithelium cells is reduced, the number indicates that fertility is also declining.
Together with the blastocyst, the endometrium expresses adhesion molecules important for the implantation process, namely integrin ß3, fibronectin, trophies, tastin, and blystin. The endometrium widely produces integrin ß3 during the menstrual cycle and pregnancy, which functions for adhesion, migration, invasion, and regulation of cellular signals. The expression integrin ß3 illustrates the value of endometrium receptivity13. It is known that if the number of endometrium epithelium cells is reduced, it can be assumed that the production of adhesion molecules is also reduced, which results in decreased endometrium receptivity.
Curcuminoid is one of the many turmeric contents consisting of curcumin, demethoxycurcumin, and bisdemethoxycurcumin14. Curcumin is an ingredient that has the main therapeutic effect of turmeric15. Curcumin inhibits the formation of luteal steroid hormones (steroidogenesis) by inhibiting the accumulation of cyclic adenosine 3′, 5′ monophosphatase (cAMP). It makes an obstacle in extracellular signal-regulated kinase (ERK) phosphorylation in the steroidogenesis pathway so that it inhibits the conversion of cholesterol into pregnenolone16.
It is known that curcuminoids can bind to the active site of enzymes that play a role in steroidogeneses, such as P450 side-chain cleavage (P450scc), CYP17A1, CYP19A1 (aromatase), and CYP21A2 so that enzymes are inhibited. The P45scc enzyme is an enzyme in charge of converting cholesterol into pregnenolone in the mitochondria. CYP17A1 functions to convert pregnenolone to 17OH-pregnenolone, whereas CYP19A1 or aromatase is an enzyme that converts androstenedione to estrogen. Thus, if ERK phosphorylation and steroidogenesis enzymes are inhibited, steroid metabolism will decrease, which causes the production of sex steroid hormones will decrease, thereby inhibiting fertility17.
Prostaglandin E2 (PGE2) is the key to the de Graaf follicle ovulation. Follicles produce the cyclooxygenase COX-1 and COX-2 enzymes in the event of an LH surge that triggers the expression of PLA2G4A in granulosa cells. PLA2G4A is a phospholipase that cleaves arachidonic acid from the phospholipid membrane at the time of pre-ovulation. PGE2 helps in the process of cumulus expansion, oocyte maturation, ruptured ovarian follicles, and oocyte release in the fallopian tubes18. The antifertility effect of curcumin in turmeric acts on the COX enzyme. Curcumin works to inhibit COX-2 downregulation. Therefore, the process of converting arachidonic acid to prostaglandins is inhibited19. After uterine examination in the treatment group of white rats that were given turmeric extract (Curcuma longa L.) for five days at a dose level of 250 mg/kg BW, 500 mg/kg BW, 1.000 mg/kg BW, it was found that there were differences in the average thickness of the endometrium layer of the strain rat Sprague Dawley in each group. The highest average endometrium thickness was found in the control group, the only group given feed and aquadest. The lowest mean endometrium thickness was obtained in Treatment group 3. Namely, the group was given turmeric extract (Curcuma longa L.) at a dose of 1.000 mg/kg BW. The control group showed an average endometrium thickness of 764.74 ± 80.19 µm. Treatment Group 1 (T1), namely the administration of turmeric extract (Curcuma longa L.) at a dose of 250 mg/kg BW, showed an average thickness of the endometrium layer of 615.06 ± 119.50 µm. Treatment Group 2 (T2), namely the administration of turmeric extract (Curcuma longa L.) at a dose of 500 mg/kg BW, showed an average thickness of the endometrium layer of 646.17 ± 139.29 µm. Treatment Group 3 (T3), namely the administration of turmeric extract at a dose of 1000 mg/kg BW showed the average thickness of the endometrium layer 566.18 ± 68.74 µm.
In this study, the results obtained were lower endometrium thickness in the treatment group compared with the control group. Curcumin has antifertility and antiovulation effects in the presence of antiestrogenic activity that inhibits the pituitary hypothalamus, causing estrogen receptor obstruction or decreased estrogen synthesis due to reduced cholesterol metabolism or both. Curcumin can reduce estrogen, namely estradiol 17-β, which plays a role in tissue proliferation, making up the endometrium layer1. The addition of turmeric extract (Curcuma longa L.) in the treatment group caused a decrease in the thickness of the endometrium layer. It illustrates the antifertility effect of turmeric extract (Curcuma longa L.).
In the One-way Anova test, the value of P= 0.013 (P<0.05) means that there is an antifertility effect of turmeric extract (Curcuma longa L.) on the thickness of the endometrium layer of Sprague Dawley rats. The antifertility effect is caused by turmeric (Curcuma longa L.) extract, which is weak in estrogen, causing changes in the usual biochemical environment of reproduction5. Turmeric extract (Curcuma longa L.) has antiovulation, antiimplantation, antiestrogenic effects that inhibit pregnancy. Antiestrogenic activity causes hypothalamic-pituitary inhibitors, which can inhibit ovulation and implantation20. Adhesion molecules such as integrin β3 play a role in the process of implantation. If there is interference or damage to the endometrium can inhibit the expression of integrins β3, which can affect the window/implantation window or implantation window13.
The Post hoc LSD test showed that administration of turmeric extract (Curcuma longa L.) could significantly reduce the thickness of the endometrium layer between the control group and treatment group 1 (T1) with the value of P = 0.014 (P> 0.05), Treatment 2 (T2 ) with a value of P = 0.047 (P> 0.05). Treatment 3 (T3) with a value of P = 0.002 (P<0.05).
Conclusion
Turmeric rhizome extract has an antiestrogenic potential, reducing the female rat’s total epithelium cells and endometrium layer thickness. It showed that turmeric has a potential effect as an antifertility agent. It can be made as an evaluation for embryo implantation to the endometrium mechanism onto the following research.
Acknowledgment
We wish to thank the Pathology and Histology laboratory of the Faculty of Medicine, the University of Lampung, for their cooperation and pathological analyses.
Conflict of interest
The authors declare no conflict of interest.
Funding source
This research was fully funded by the authors.
References
- Gosh AK, Das AK, Patra KK. Studies on antifertility effect of rhizome of curcuma longa Asian journal of pharmacy and life science, 1(4):349-53 (2011).
- Stovall TG. Early pregnancy loss and ectopic pregnancy. In: Berek JS, ed. Novak’s gynecology. 13th Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 507-9.
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Bilstrap LC, Wenstrom KD. William Obstetrics. 22nd ed. USA: McGraw-Hill Professional; 2005. pp. 231-47.
- Yadav R, Jain GC. Post-coital contraceptive efficacy of aqueous extract of Curcuma longa rhizome in female albino rats. Pharmacologyonline, 1(91):507–17 (2010).
- Yadav R, Jain GC, 2011. Effect of contragestative dose of aqueous extract of Curcuma longa rhizome on the uterine biochemical milieu of female rats. Indian journal of fundamental and applied life sciences, 1(3):183–87 (2011).
- Al-Qudsi F, Linjawi S. Histological and hormonal changes in rat endometrium under the effect of camphor. Life science journal, 9(2):348-55 (2012).
- The WT, Mc Bain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? Journal of assisted reproduction and genetics, 33(11):1419-30 (2016).
CrossRef - Vrtačnik P, Ostanek B, Mencej BS, Marc J. The many faces of estrogen signaling. Biochemia medica, 24(3):329-42 (2014).
CrossRef - Hapangama DK, Kamal AM, Bulmer JN. Estrogen receptor ß: the guardian of the endometrium. Human reproduction update, 21(2):174-93 (2015).
CrossRef - Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Human reproduction update, 21(2):155-73 (2015).
- Griffiths M, Sinderen MV, Rainczuk K, Dimitriadis E. Mir-29c overexpression and col4a1 downregulation in infertile human endometrium reduces endometrial epithelial cell adhesive capacity in vitro, implying roles in receptivity. Scientific reports, 9(8644):1-10 (2019).
CrossRef - Marquadt RM, Kim TH, Shin JH, Jeong JW. Progesterone and estrogen signaling in the endometrium: what goes wrong in the endometriosis? International journal of molecular science, 20(15):1-28 (2019).
CrossRef - Busman H, Sutyarso, Farisi S, Yulianty. Regulation of integrin ß3 protein secretion on implantation embryo of mouse (mus musculus ) Induced by oil atsiri of purple nutsedge tubers (cyperus rotundus l.). Annual research & review in biology, 33(2):1-5 (2019).
CrossRef - Pushpakumari KN, Vaghese N, Kottol K. Enhancing the absorption of curcuminoids from formulated turmeric extracts. International journal of pharmaceutical sciences research, 6(6):2468-76 (2015)
- Mohebbati R, Anaeigoudari A, Khazdair MR. The effects of curcuma longa and curcumin on reproductive systems. Endocr regul, 51(4):220-28 (2017).
CrossRef - Purwaningsih E, Soejono SK, Dasuki D, Meiyanto E. Curcumin inhibits luteal cell steroidogenesis by suppression of the extracellular signal-regulated kinase. Universa medicina, 31(2):73-80 (2012).
- Castano PR, Parween S, Pandey AV. Bioactivity of curcumin on the cytochrome p450 enzymes of the steroidogenesis pathway. International journal of molecular science, 20(4604):1-26 (2019).
CrossRef - Duffy DM. Novel contraceptives targeted to inhibit ovulation: the prostaglandin e2 pathway. Human reproduction updates, 21(5):652-70 (2015).
CrossRef - Zhou H, Beevers CS, Huang S. Targets of curcumin. Current drug targets, 12(3):332-47 (2012).
CrossRef - Niazi J, Poonia P, Gupta V, Kaur N. Pharmacotherapeutics of Curcuma longa – a potent patent. International journal of pharma professional’s research, 1(1):24-33 (2018).