Saad Abdulrahman Hussain1*  , Zainab Saad Abdulrahman2
, Zainab Saad Abdulrahman2 , Wael Waleed Mustafa1
, Wael Waleed Mustafa1
1Department of Pharmacology and Toxicology, Faculty of Pharmacy, Al-Rafidain University College, 10052 Baghdad, Iraq.
2Department of Clinical Pharmacy, Al-Kindy Teaching Hospital, 10052 Baghdad, Iraq
Corresponding Author E-mail: saad.hussain@ruc.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2345
Abstract
The aim of pharmacotherapy for moderate cognitive impairment (MCI) is to reduce the present clinical signs and delay the progression of Alzheimer's disease (AD). There is currently no viable pharmacological therapy for the treatment of AD. Early intake of nutraceuticals, on the other hand, may help to alleviate and delay MCI. The goal of this study was to see how silibinin (SIL) supplementation affected cognitive function in older people with memory problems. A total of 85 subjects with memory impairment were randomly assigned to one of two groups: SIL (n = 42) supplied with 250 mg twice daily, or placebo (n = 43) for 16 weeks. A computerized neurocognitive function test was used to assess cognitive function (CNT). When comparing the SIL group to the placebo group, the "verbal learning test index" items of the CNT were significantly improved in the SIL arm (P < 0.05). Supplementing older people with memory impairment with SIL for 16 weeks appears to have a positive impact on their state of "verbal memory." Further studies are highly recommended.
Keywords
Cognitive impairment; Alzheimer disease; Memory function; Silibinin
Download this article as:| Copy the following to cite this article: Hussain S. A, Abdulrahman Z. A, Mustafa W. W. Silibinin Improves the Clinical Scores of Memory Function in Patients with Mild Cognitive Impairment: A Double-Blind, Placebo-Controlled Pilot Study. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Hussain S. A, Abdulrahman Z. A, Mustafa W. W. Silibinin Improves the Clinical Scores of Memory Function in Patients with Mild Cognitive Impairment: A Double-Blind, Placebo-Controlled Pilot Study. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3HcL8qA |
Introduction
Minor “cognitive impairment” (MCI) is a broad clinical disorder marked by mild loss of memory that is an etiology for Alzheimer’s dementia.1 MCI affects 10–20% of people older than 65 years. Furthermore, around 10% of MCI cases progress to AD after one year, and 30–50% of them progress to AD after five years.2 It is thought to have a substantially higher progression rate to AD than people who do not have MCI.3 AD is accompanied by pathological changes associated with neuropsychological symptoms, with no therapeutic option available. As a result, an early diagnosis at the “MCI stage” is critical for preventing and managing AD.4 The goals of MCI treatment are to alleviate current clinical manifestations, slow the deterioration of cognitive impairment, and prevent the onset of AD. Unfortunately, no effective drug therapy exists for MCI. Changing dietary habits, on the other hand, may help in the treatment of MCI. According to epidemiological studies, the Mediterranean diet can reduce the risk of Alzheimer’s disease (AD) and the progression of MCI to AD.5,6 This dietary strategy has been found to protect the brain by reducing inflammation and oxidative stress. As a result, certain dietary changes could help to reduce the risk of cognitive impairment.7,8 Silibinin is the main active component in the extracts of the Silybum marianum seeds. It is characterized as the first of a natural compound class called flavonolignans and is traditionally used as folk medicine in many countries to treat hepatic diseases of different etiologies.9 In an in vitro study, silibinin shows a concentration-dependent inhibition of the Aβ(1-42) peptide aggregation.10 Moreover, silibinin acts as a dual blocker of Aβ peptide aggregation and acetylcholinesterase enzyme, thus being suggested as a treatment choice for Alzheimer’s disease (AD) and evaluated in a mouse model of AD.11 Previous studies have shown that silibinin protects against high-fat-diet-induced liver damage via the inhibition of lipogenesis and enhances fatty acid oxidation in animal models.12 Additionally, various studies have revealed the capacity of silibinin to improve memory and learning in LPS-treated mice.13,14 In a most recent study, a nutraceutical containing silibinin improves cognitive ability in aged LDLR (+/-) golden Syrian hamsters through maintaining the integrity of the blood-brain barrier, among other mechanisms.15 The present pilot study aims to provide clinical data on the protective role of silibinin against cognitive impairment in older adults.
Materials and Methods
Participants
After the study was approved by the local Research Ethics Committee, Faculty of Pharmacy, Al-Rafidain University College (Certificate ID: REC-23-2021), it was carried out in accordance with the Good Clinical Practice Criteria of the International Conference on Harmonization (ICH GCP). The investigation was carried out in compliance with the Helsinki Declaration’s laws and clinical trial management standards (IGCP). All participants signed an informed consent form and received a general clinical assessment before being included in the study. Eighty-five elderly adults with memory impairment were found to be qualified for the study and were randomized into 2 groups: the SIL-treated (42 patients) and the placebo-treated (43 patients) groups. The required sample size was calculated based on previous data that explained how to calculate the required sample size.16 To achieve 90% power for a 5% significance level with a two-sided test, each group needs 40 participants. Under the assumption of a 20% dropout rate, a total of 85 AD patients were required. As a consequence, 85 subjects with Alzheimer’s disease were recruited and randomized to either the SIL group (42 patients) or 43 patients included in the placebo group.
Inclusion and exclusion criteria
The current study’s inclusion criteria are as follows: subjects must be over the age of 60 at the time of screening; subjects must be able to understand both English and Arabic translation; and subjects must have memory index scores in the neuropsychological component of the “Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-NB)” that differed by more than one standard deviation (SD) compared with the normal level of each item in the test (worse). In the meantime, the individual was ruled out if any of the following were present: A three-year history of therapy for Axis I diseases (SCID) according to the Structured Clinical Interview for DSM-IV; has been abusing or relying on alcohol over the last three months; the presence of any of certain chronic co-morbidities (epilepsy, mental retardation, CNS illness, endocrine dysfunction, hematological malignancies, cardiovascular ailments, and/or Crohn’s disease); those with abnormal laboratory values such as AST, ALT, or any abnormal laboratory values that exceeded the top limit of the normal range by more than three times; those who received prescriptions or herbal medicines within two weeks after the initial day of ingestion were excluded from the study, those who had taken any OTC medication or vitamin supplements within one week of the first day of ingestion were also excluded. Moreover, those who had participated in another clinical study in the three months prior to the first day of intake, those who donated whole blood or a blood component within two weeks of the first day of consumption or a month prior to the first day of intake, and those who the research supervisor determined were unsuitable for inclusion in this clinical trial for any other reason were also excluded.
Study Design and Randomization
For 16 weeks, researchers conducted a multi-center, randomized, double-blind, placebo-controlled clinical investigation. Prior to the start of the investigation, an authorized subject manually generated a sequence of random A and B numbers for the random assignment table. Subjects were enrolled four weeks before the screening test to review the eligibility criteria for inclusion and exclusion after the first visit. Subjects were randomly assigned to the SIL group (42 patients) or the placebo group (43 patients) in a 1:1 random ratio to complete the baseline by the first visit date. Patients in the SIL group were given the test product (Silibinin 250 mg capsules, specially produced for the trial) and told to take it twice a day for 16 weeks. Patients in the placebo group were given a microcrystalline cellulose-based placebo mixture. Volunteers were requested to come to the hospital in the eighth week for an assessment of drug tolerance, vital signs, and the occurrence of side effects. Other safety-related testing was also carried out. The effects of the medication on cognitive function were measured using three different tests. During the pre-inclusion screening, the first test was performed. The second test (week 0) was conducted before the subjects started using the test product, and the third test (week 16) was conducted after the 16-week period had ended.
Clinical Outcomes Measurement
Changes in memory function tests were the study’s key outcome indicator. The “visual learning test (Visual LT)” and the “verbal learning test (Verbal LT)” were shown to be adequate measures for assessing cognitive performance among the CNT’s 18 sub-categories.16 Memory loss is one of the most common symptoms of MCI, and memory encoding necessitates attention. Therefore, worsening in these cognitive skills is common.17 As a result, we chose the best subtests to investigate these cognitive domains in subjects with dementia. As the study participant glanced at the touch screen display, the CNT replied by pressing the monitor by hand or pressing a button device, and the CNT tester did so using standardized phrases. The “Visual LT” test involves memorizing the first of 30 figures that are displayed on a computer monitor. By repeating the technique five times, the visual LT was computed (A1 to A5). After 20 minutes of Visual LT, the participant was shown a total of 30 values on a monitor before being reminded of the previous statistics. In the “Verbal LT,” participants listened to a list of 15 words (A list) five times through the computer speaker’s recorded voice (Trial A1A5) and recalled as many words as possible after each trial (immediate recall). A new interference list (list B) was supplied and recalled after the sixth trial (A5). The patients remembered the terms from list A after seeing list B, “Verbal LT B.” The patients were asked to recall as many original words from list A as possible after a 20-minute wait (Verbal LT A20 delayed recall). They were then instructed to choose 15 keywords from List A from a list of 50 words displayed on the computer screen (Verbal LT REC delay recognition). In the current study we utilized the following scores as outcome indicators: 1) The total number of words recalled immediately after trials A1 to A5 (A1+A2+A3+A4+A5; Verbal LT A1A5 total learning index), 2) the difference between A5 and A1 (A5-A1; Verbal LT learning Slope A5-A1), 3) the total number of words recalled after a 20-min delay (Verbal LT A20 delayed recall), and 4) the total number of words recalled after a 20-min (A5-A20; Verbal LT A5-A20 memory retention). A visual working memory test was decided upon “Visual WMT”. The “Visual WMT” was determined by having the patients say a total of 15 words with the same frequency of usage over a computer speaker, regardless of their memory order.
Statistical analysis
The SPSS version 24 software was used for all statistical analyses (IBM Co., Armonk, NY, USA). The means ± SD of continuous variables were shown, whereas the frequencies of categorical variables were shown. Chi-square tests (Fisher’s exact test) were used to determine the significance of differences in categorical variables, and the independent t-test was used to compare the means of the two groups. To see if the observed difference in verbal learning between the two groups was independent of visual working memory tasks, the researchers employed an analysis of covariance (ANCOVA) corrected for visual working memory items. The Z-scores were evaluated and compared using subgroup (Visual LT, Verbal LT, and Visual WMT). The positive Z-score of the individual indicates that they outperformed the placebo group’s average score. To achieve a composite memory score, the standardized Z-scores of the three tests were calculated and averaged. An independent t-test was used to compare the composite score and the combined composite score of visual memory, verbal learning memory, and working memory between the two groups. Less than 0.05 was chosen as the statistical significance limit.
Results
A total of 100 people who agreed to participate in this clinical investigation were chosen and given the required exam, with 85 of them meeting our inclusion and exclusion criteria. Table 1 shows the overall demographic characteristics of the participants. Male and female individuals were 71.5% and 28.5% in the SIL group, respectively, and 67.4% and 32.6% in the placebo group, respectively. The two groups did not differ significantly in terms of gender distribution, age, drinking, smoking, blood pressure, physical measurement indices, or cognitive function ratings. A total of 85 patients completed the human research protocol’s procedures in its entirety. The findings were based on data from 85% of the participants (Figure 1). After 16 weeks of treatment, the changes in the SIL group’s cognitive function scores were considerably greater than those treated with placebo formula (Table 2). The SIL group showed a statistically significant increase in the “Verbal LT delayed recognition” item score when compared to the placebo group. The “Verbal LT” score in the SIL group was significantly higher after 16 weeks of treatment than before treatment (p<0.05). There was also a statistically significant difference in “Verbal LT” ratings between the SIL and placebo groups after 16 weeks (p<0.05). The “Visual WMT” accuracy in the placebo group was significantly improved after 16 weeks of therapy, although there was no significant difference in “Visual WMT” scores between the two groups after baseline correction (p>0.05). Table 3 illustrates the results of a sub-analysis of the change in the Z-score for each memory function item in this study, removing people who were taking medications for metabolic illness (blood pressure, thyroid function). When compared to the placebo group, the Z-score of recognition and the composite z-score of the verbal memory domain of the verbal memory function significantly increased after 16 weeks of SIL treatment (p<0.05). Furthermore, the average value of the overall domain composite scores of “Visual LT”, “Visual WMT,” and “Verbal LT” was utilized to assess global cognitive function change, with the SIL group exhibiting a significant improvement in memory (z = 3.4, p = 0.016) compared to the placebo group.
Table 1: Demographic features of the study participants.
| Parameter | SIL group
(n=42) |
Placebo group (n=43) | p-value |
| Age (year) | 69.5±6.2 | 71.2±5.8 | 0.06(1) |
| Gender (male/female)% | 30(71.5)/12(28.5) | 29(67.4)/14(32.6) | 0.07(2) |
| Alcohol consumption n(%) | 2(4.8) | 3(6.9) | 0.81(2) |
| Smokers n(%) | 5(11.9) | 4(9.3) | 0.9(2) |
| Blood pressure (mmHg) | |||
| SBP | 132.7±12.2 | 134.1±11.7 | 0.67(1) |
| DBP | 79.2±8.3 | 78.8±9.1 | 0.53(1) |
| Pulse (beats/min) | 72.0±8.3 | 71.2±7.1 | 0.64(1) |
| Academic ability (years) | 8.12±2.2 | 9.2±2.1 | 0.24(1) |
| BMI (kg/m2) | 24.8±1.7 | 25.2±1.9 | 0.71(1) |
| CERAD-NB word list memory | 12.9±2.5 | 13.1±2.6 | 0.5(1) |
| CERAD-NB word list recall | 4.1±0.9 | 3.9±1.1 | 0.31(1) |
| CERAD-NB word list recognition | 7.4±1.4 | 7.3±1.6 | 0.28(1) |
Values are expressed as means±SD or number (percentage); (1) unpaired t-test; (2) Chi-square test; BMI: body mass index; CERAD-NB: The Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery.
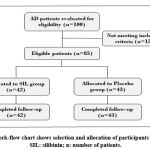 |
Figure 1: Work-flow chart shows selection and allocation of participants in the study. SIL: silibinin; n: number of patients. |
Table 2: Changes in Neurocognitive function test (NCT) pre-and 16-week post-SIL consumption by AD patients compared with placebo.
| Variable | SIL group (n=42) | Placebo group (n=43) | p (1) | ||
| Baseline | 16 week | Baseline | 16 week | ||
| Visual LT (recognition) | 9.8±1.2 | 12.1±1.4*a | 10.6±1.5 | 10.0±2.0b | 0.26 |
| Visual WMT (accuracy) | 35.7±18.2 | 33.1±16.3a | 28.3±13.2 | 32.4±14.1*a | 0.09 |
| Visual WMT (reaction time) | 614.1±82.3 | 598±78.82a | 624.2±80.6 | 610.5±76.8a | 0.51 |
| Verbal LT A20 (delayed recall) (2) | 5.27±1.8 | 9.2±2.1*a | 6.21±2.3 | 7.1±3.1*b | 0.41 |
| Verbal LT REC (delayed recognition) (3) | 11.5±1.2 | 14.3±1.4*a | 10.5±2.0 | 11.3±1.7b | 0.03 |
| Verbal LT A1A5 (Total) (4) | 36.4±5.1 | 44.3±6.2*a | 39.7±7.5 | 40.8±8.2a | 0.4 |
| Verbal LT A1A5 (average) | 7.4±1.3 | 9.4±1.4*a | 7.6±1.6 | 7.9±1.8b | 0.31 |
| Verbal LT (Learning slope A5-A1) (5) | 3.6±1.1 | 5.2±0.9*a | 3.9±1.2 | 3.6±0.8b | 0.05 |
| Verbal LT A5-A20 Memory retention (6) | 3.6±1.1 | 2.1±0.8a | 2.5±1.4 | 2.1±1.3a | 0.6 |
Values are expressed as mean±SD;* significant compared with baseline within the same group (p<0.05); values with non-identical superscripts (a,b) among post-treatment of different groups are significantly different (p<0.05); SIL: silibinin; Visual LT: visual learning test; Visual WMT: visual working memory test; Verbal LT: verbal learning test; (1) ANCOVA (baseline values included as covariates; visual working memory test p=0.03); (2) The total words recalled after 20 min; (3) Total words correctly selected from the 50-word list; (4) The total words recalled immediately after trials A1-A5; (5) The difference between A5 and A1; (6) The difference between A5 and A20 delayed recall.
Table 3: Differences in composite Z-score of each cognitive domain and combine cognitive function between the SIL and placebo groups.
| Variable | SIL group | Placebo group | Z** | p-value* |
| 16 Week | 16 Week | |||
| Visual memory function | ||||
| Immediate recall | 0.35±0.9 | 0.39±0.42 | – 0.12 | 0.8 |
| Recognition | 0.14±0.8 | – 0.22±0.68 | 1.4 | 0.31 |
| Domain composite score | 0.28±0.62 | 0.19±0.44 | 0.8 | 0.51 |
| Verbal memory function | ||||
| Immediate recall | 0.69±0.32 | 0.17±0.82 | 2.1 | 0.05 |
| Delayed recall | 0.96±0.41 | 0.51±0.76 | 1.5 | 0.06 |
| Recognition | 0.72±0.22 | 0.06±0.32 | 4.1 | 0.001 |
| Domain composite score | 0.84±0.28 | 0.11±0.43 | 2.7 | 0.011 |
| Working memory function | ||||
| Visual WMT (adjusted accuracy) | 0.45±0.32 | 0.12±0.71 | 1.12 | 0.2 |
| Visual WMT Reaction time | – 0.62±0.51 | – 0.44±0.9 | – 0.51 | 0.31 |
| Domain composite score | – 0.14±0.23 | – 0.21±0.66 | 0.48 | 0.32 |
| Combined cognitive function | ||||
| Domain composite score | 0.52±0.21 | 0.032±0.4 | 3.4 | 0.016 |
Values are expressed as mean z score ± SD; * unpaired t-test; ** Z-test between the two groups; WMT: working memory test.
Discussion
This is the first randomized clinical study conducted in Iraq to evaluate the efficacy of SIL supplementation in avoiding mild cognitive impairment in Alzheimer’s disease patients. Other polyphenols, such as resveratrol,18 curcumin,19 and berry polyphenols20 have previously been studied and shown promising results. In the current study, a dose of 250 mg of SIL twice a day improved verbal memory in the SIL group compared to the placebo group. Many in vitro and animal investigations have shown that silymarin, a plant extract that includes SIL, enhances memory function in animal models of cognitive impairment.21,22 The Aβ peptide has been suggested to disrupt anti-oxidative defenses in the brain, which may contribute to the etiology of Alzheimer’s disease.23 Reduced glutathione (GSH) is one of the most common non-protein thiols in the central nervous system, where it serves an important anti-oxidant role in both neurons and non-neuronal cells. Aβ25–35 lowered GSH levels in the hippocampus in our investigation, which is consistent with studies of GSH depletion in the brains of Alzheimer’s patients.24 Furthermore, silibinin administration reduced the Aβ25–35-induced drop in GSH levels, showing that silibinin’s protective impact on Aβ25–35-induced cognitive impairment includes the activation of anti-oxidative defenses.25 In terms of the mechanisms of action, silibinin has free radical scavenging properties, increases intracellular GSH and superoxide dismutase (SOD) levels, and protects neuroblastoma cells from Aβ-induced toxicity,10 while in vivo it prevents neurodegeneration in mice fed a high fat diet26 and reduces memory impairment in mice given intracerebral injection of Aβ.27 Silibinin has antioxidant and anti-inflammatory effects, as well as inhibiting amyloid aggregation and neuronal apoptosis.28 Pretreatment with silibinin protects PC12 cells against Aβ-induced toxicity via modulating the AKT/MDM2/p53 pathway and may provide a new therapeutic strategy for treating Alzheimer’s disease.29 Similarly, Lu et al. discovered that silibinin protects against memory loss and oxidative damage caused by Aβ25–35, suggesting that it could be used to treat Alzheimer’s disease.26 The poor oral bioavailability of SIL due to poor absorption and fast hepatic biotransformation may limit its therapeutic usage,30 despite its acceptable safety profile. This previously published research demonstrates that the SIL protects neurons from Aβ-induced apoptosis. SIL supplementation thereby protects nerve cells by scavenging oxidative radicals and altering MAPK signaling.31 In this study, computerized neurocognitive function tests (CNTs) were used to detect minor degrees of cognitive impairment, and they can also be used to assess treatment efficacy for cognitive impairment. The Verbal Learning and Memory Test (Verbal LT) is a clinical evaluation of verbal learning and memory that can be used to investigate the effects of a range of neurological illnesses, including MCI. The “Verbal LT” score of the SIL group was significantly higher than that of the placebo group. The SIL group confirmed a boost in their “Verbal LT” index after 16 weeks of SIL supplementation. A higher “Verbal LT” score indicates that following related information has less of an impact on the recall of previously learned content (List B). During “Verbal LT” scoring in the SIL group, the short-term store competes with new information (List B) that is unrelated to previous knowledge (List A). Memory retrieval and cognitive control are both aided by this method. In this study, SIL supplementation may have aided memory recollection. Furthermore, patients in the SIL group performed better on the “Verbal LT REC delay recognition” index.
Study limitations
There are certain limitations to the research. The results cannot be extended due to the limited number of participants, and the test does not test attention, which is the primary ability for encoding information. The likelihood of interfering with numerous polyphenols and other natural dietary products that may be ingested during daily food consumption, as most of these constituents have potential antioxidant and cyto-protective capabilities, is one of the main constraints. To address the constraints, it will be required to conduct comprehensive research with a large number of volunteers. However, major findings from this study imply that SIL supplementation in older people with memory loss is safe and may aid in the prevention and management of Alzheimer’s disease.
Conclusion
In older people with memory impairment, sixteen weeks of silibinin treatment improves overall cognitive function, particularly verbal learning and memory function, when compared to placebo.
Acknowledgement
The authors thank Al-Rafidain University College for supporting the project.
Conflict of Interest
There is no conflict of interest.
Funding source
There is no funding source.
Ethical Committee Approval
Study protocol has been evaluated and approved by Institutional Research Ethics Committee, Faculty of Pharmacy, Al-Rafidain University College after taking informed consent from study participants.
References
- Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Re, 2008; 7: F1000.
CrossRef - Petersen RC. Mild cognitive impairment as a diagnostic entity. Intern. Med., 2004; 256(3): 183-194.
CrossRef - Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectr., 2019; 24(1): 78-87.
CrossRef - Cornelis E, Gorus E, Beyer I, Bautmans I, De Vriendt P. Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool. PLoS Med., 2017; 14(3): e1002250.
CrossRef - Abbatecola AM, Russo M, Barbieri M. Dietary patterns and cognition in older persons. Opin. Clin. Nutr. Metab. Care, 2018; 21(1): 10-13.
CrossRef - Chen X, Maguire B, Brodaty H, O’Leary F. Dietary patterns and cognitive health in older adults: A systematic review. Alzheimers Dis., 2019; 67(2): 583-619.
CrossRef - Butler M, Nelson VA, Davila H, Ratner E, Fink HA, Hemmy LS, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Intern. Med., 2018; 168(1): 52-62.
CrossRef - Solfrizzi V, Agosti P, Lozupone M, Custodero C, Schilardi A, Valiani V, et al. Nutritional intervention as a preventive approach for cognitive-related outcomes in cognitively healthy older adults: A systematic review. Alzheimers Dis., 2018; 64(s1): S229-S254.
CrossRef - Biedermann D, Vavříková E, Cvak L, Křen V. Chemistry of silybin. Prod. Rep., 2014; 31(9): 1138-1157.
CrossRef - Yin F., Liu J., Ji X., Wang Y., Zidichouski J., Zhang J. Silibinin: A novel inhibitor of Aβ aggregation. Int., 2011; 58:399-403.
CrossRef - Duan S, Guan X, Lin R, Liu X, Yan Y, Lin R, et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Aging, 2015; 36: 1792-1807.
CrossRef - Cui CX, Deng JN, Yan L, Liu YY, Fan JY, Mu HN, et al. Silibinin capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J Ethnopharmacol., 2017; 208: 24-35.
CrossRef - Jangra A, Kasbe P, Pandey SN, Dwivedi S, Gurjar SS, Kwatra M, et al. Hesperidin and silibinin ameliorate Aluminum-induced neurotoxicity: Modulation of antioxidants and inflammatory cytokines level in mice hippocampus. Trace Elem. Res., 2015; 168(2): 462-471.
CrossRef - Jin G, Bai D, Yin S, Yang Z, Zou D, Zhang Z, et al. Silibinin rescues learning and memory deficits by attenuating microglial activation and preventing neuroinflammatory reactions in SAMP8 mice. Lett., 2016; 629:256-2561.
CrossRef - Gu YY, Huang P, Li Q, Liu YY, Liu G, Wang YH, et al. YangXue QingNao Wan and silibinin capsules, the two Chinese medicines, attenuate cognitive impairment in aged LDLR (+/-) golden Syrian hamsters involving the protection of blood-brain barrier. Physiol., 2018; 9: 658.
CrossRef - Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Jong I, Park JI, et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Funct. Foods, 2014; 10:465-474.
CrossRef - Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and Safety of Lactobacillus PlantarumC29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 2019; 11(2): 305.
CrossRef - Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. J. Clin. Nutr., 2010; 91: 1590-1597.
CrossRef - Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer’s disease. Clin. Psychopharmacol., 2008; 28:110-113.
CrossRef - Bensalema J, Dal-Pane A, Gillarde E, Calone F, Pallet V. Protective effects of berry polyphenols against age-related cognitive impairment. Aging, 2015; 3:89-106.
CrossRef - Shokouhi G, Kosari-Nasab M, Salari AA. Silymarin sex-dependently improves cognitive functions and alters TNF-α, BDNF, and glutamate in the hippocampus of mice with mild traumatic brain injury. Life Sci., 2020; 257:118049.
CrossRef - Yön B, Belviranlı M, Okudan N. The effect of silymarin supplementation on cognitive impairment induced by diabetes in rats. Basic Clin. Physiol. Pharmacol., 2019; 30(4): 1-9.
CrossRef - Butterfield DA, Swomley AM, Sultana R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Redox Signal., 2013; 19(8):823-835.
CrossRef - Shukla D, Mandal PK, Mishra R, Punjabi K, Dwivedi D, Tripathi M, et al. Hippocampal glutathione depletion and pH increment in Alzheimer’s disease: An in vivo MRS study. Alzheimers Dis., 2021; 84(3): 1139-1152.
CrossRef - Haddadia R, Shahidib Z, Eyvari-Brooshghalan S. Silymarin and neurodegenerative diseases: Therapeutic potential and basic molecular mechanisms. Phytomedicine, 2020; 79: 153320.
CrossRef - Lu P, Mamiya T, Lu LL, Mouri A, Zou L, Nagai T, et al. Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. J. Pharmacol., 2009; 157(7): 1270-1277.
CrossRef - Nuzzo D, Amato A, Picone P, Terzo S, Galizzi G, Bonina FP, et al. A natural dietary supplement with a combination of nutrients prevents neurodegeneration induced by a high fat diet in mice. Nutrients, 2018; 10(9):1130.
CrossRef - Borah A, Paul R, Choudhury S, Choudhury A, Bhuyan B, Das Talukdar A, et al. Neuroprotective potential of silymarin against CNS disorders: insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther., 2013; 19(11): 847-853.
CrossRef - Meng J, Li Y, Zhang M, Li W, Zhou L, Wang Q, et al. A combination of curcumin, vorinostat and silibinin reverses Aβ-induced nerve cell toxicity via activation of AKT-MDM2-p53 pathway. , 2019; 7: e6716.
CrossRef - Jurcau A. The role of natural antioxidants in the prevention of dementia-Where do we stand and future perspectives. Nutrients, 2021; 13(2):282.
CrossRef - Camini FC, Costa DC. Silymarin: not just another antioxidant. Basic Clin. Physiol. Pharmacol., 2020; 31(4): 20190206.
CrossRef








