Asha Khanna1,2, Jyotsana Patel1,2, Bishal Tiwari1,2, Saurabh Pagare1,4, Daya Shankar Gautam*1,3 and Vineeta Ratoniya1,2
1AVIKA, Biological Research foundation, Jabalpur (M.P.) 482001, India.
2Department of Zoology and Biotechnology, Govt. Science College, Jabalpur (M.P.) 482001, India
3Department of Zoology, St. Aloysius’ College, Jabalpur (M.P.) 482001, India
4Department of Biotechnology, Sarojini Naidu Govt. Girls P.G. College, Bhopal (M.P.) 462016, India.
Corresponding Author E-mail: dygautam@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2393
Abstract
There is a need to increase our agricultural production of food grains and other crops to feed a continuously increasing population. To achieve this food security, use of insecticides/pesticides has become necessary. Cypermethrin is a pyrethroid insecticide used for control of pests of cereals, fruits, vegetables and cotton etc. but it has several toxic effects on human beings. Apart from being neurotoxic, it has harmful effects on lymphocytes also. Neem is also a potent insecticide of herbal and indigenous origin. In this investigation the cytotoxicity of cypermethrin (dissolved in DMSO) and aqueous extract of neem leaves to human lymphocytes was studied by MTT assay. It was found that after an exposure of two hours to LC50 concentration of cypermethrin viability of lymphocytes fell to 87.83%; however at lower concentration the viability fell further because of the increase in the DMSO concentration, proving the toxicity of DMSO. Treatment of lymphocytes with 45% of neem extract increased the viability by 196% but at lower concentrations lesser increase was noted due to the increase in concentration of PBS. Thus apart from being a safe insecticide neem extract can be used to promote viability and proliferation of cells of animal origin also.
Keywords
Cypermethrin; Cytotoxicity; Lymphocytes; MTT Assay; Neem
Download this article as:| Copy the following to cite this article: Khanna A, Patel J, Tiwari B, Pagare S, Gautam D. S, Ratoniya V. Cytotoxic Effect of Cypermethrin and Neem Extract on Human Lymphocytes. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Khanna A, Patel J, Tiwari B, Pagare S, Gautam D. S, Ratoniya V. Cytotoxic Effect of Cypermethrin and Neem Extract on Human Lymphocytes. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3LymFP0 |
Introduction
Cypermethrin is a pyrethroid of synthetic origin and is a commonly used insecticide in agricultural practices in India and globally. Natural pyrethroids are compounds derived from chrysanthemum flowers and many synthetic pyrethroids are in use as insecticides. In general the pyrethroids are considered to be less toxic to humans as compared to other classes of insecticides. Apart from its agricultural uses Cypermethrin is also used in consumer products to exterminate common domestic pests. It is used as pesticide for protecting cotton, cereals and fruits, specifically from diamond back moth, stem borer, fruit borer, Bihar hairy caterpillar in cabbage, okra, brinjal, wheat and sunflower crops. Its chemical formula is C22H10Cl2NO3, and molar mass is 416.30g/mol.
The structural formula is:
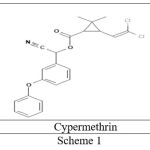 |
Scheme 1 |
IUPAC name [Cyano-(3-phenoxyphenyl]33-(2,3dichloroethylyl)-2,2-dimethylcyclopropano-1-carboxylate.
Cypermethrin has a fast acting neurotoxic effect on insects. It is very poisonous to fish, bees and aquatic insects. In humans, it is moderately toxic through skin contact or inhalation, causing irritation to skin and eyes. Pyrethroids in general adversely affect the central nervous system; Cypermethrin interacts with sodium channels in the nerve cells. These channels remain open for a longer time than normal after a signal has been transmitted.1 The effect is like transmission of repetitive impulses.2 Excessive exposure of humans engaged in spraying can cause nausea, headaches, seizures, salivation. Cypermethrin is deactivated in humans by several hydrolytic enzymes and converted into various carboxylic acid metabolites. It is easily despoiled in plants and soil but may remain for long periods on indoor surfaces. So a study of its toxicity to human peripheral blood lymphocytes was deemed useful. In this investigation its effects were compared with those of exposure of lymphocytes to aqueous neem extract which is an indigenous herbal insecticide.
Neem extract
Being an evergreen tree, Neem (Azadirachta indica) is common in the semi deciduous forest areas of India. It is also abundant in countries having tropical/ semitropical or semiarid climate so it is common in the South Asian subcontinent as it resistant to drought and high temperatures. Neem has been regarded a popular herbal remedy and has been reported as a medicinal plant since six to twelve thousand years before the present time. It has been mentioned in ancient Indian medical texts such as that of Siddha medicine.3 Neem possesses several biochemical components and has been known to be prescribed as medicine for the treatment of many human diseases.4&5 The extracts of the various parts of the tree possess therapeutic properties such as antibacterial, antiviral, antidiabetic, hepatoprotective and antioxidant properties.6 It has been shown to cause decrease in amount of spermatozoa in rats.7 Neem leaf extract has been mentioned to have anticancer effect,8 such as proliferation inhibitory effect on prostate cancer cells, apoptosis inducing effect on breast cancer cells but it has been shown to enhance immunity through peripheral blood lymphocytes presumably by helping in their proliferation. The major components isolated from neem are triterpenoids such as Azadirachtin, Nimbin, Nimbadiol etc. These compounds and neem extracts form an essential element in traditional and complementary medicine followed by a large section of population who do not have access or means for sophisticated chaemotherapy and allopathy.9 Among the various health benefits of neem derived extracts are its use in supplements to lower inflammation and even to fight malignancies10. So it was thought worthwhile to take up this investigation to find out if the aqueous neem leaf extract does cause an increase in lymphocyte proliferation so that its use as an indigenous immune booster can be justified.
Materials and methods
The research work is approved by the Institutional ethical committee of AVIKA, Biological Research foundation, Jabalpur (M. P.). Commercially available Cypermethrin, at 10% EC (based on 50% ai) and 20% emulsifiers was used for the experiment. LD50 for cypermethrin (dissolved in DMSO) is reported to be 145 mg /Kg body weight when administered orally in the rats11. The solutions of cypermethrin were prepared in DMSO and 13.97 mg/ml was taken as the LC50 dose. This was based on the work of Suman et al (2006)12 who found that 33.6 µM proved to be the LC50 dose for cultured human lymphocytes. The 13.97mg/ml solution was used as a stock solution from which 1/5, 1/10 and 1/20 LC50 solutions were prepared by adding appropriate amounts of DMSO.
Plant material
The method followed was basically given by Agebenin and Marley (2006)13 with some modifications. Briefly, fresh neem leaves were collected from near our lab. They were cleaned with soft detergent and continuous running water for 30 minutes. The leaves were then washed thoroughly in distilled water and air dried for a short while. Then 15 g of the leaves were ground to a paste and added to 33 ml of distilled water (sterile) and left to stand in the laminar flow chamber for four hours. Whatman no.1 filter paper was used to filter slurry to give a 45% extract of fresh neem leaves. For testing the effects on lymphocytes, this neat 45% aqueous extract was used to prepare 1/5, 1/10/ and 1/20 dilutions by adding appropriate amounts of sterile PBS 1X solution.
Phytochemical analysis of the Neem Extract was conducted for Phenolic and Flavonoid content. For this aqueous extract was made from 20gm fresh neem leaves in 44ml of sterile distilled water by the method already described. The Phenolic and Flavonoid content was determined by Spectrophometric method by Folin Ciocalteu’s method and Aluminium Chloride method respectively.14
Isolation of lymphocytes from whole blood
Standard method was followed as per Khanna et al, (2014)15. Blood (2.5 ml) from young healthy female (obtained after consent of the donor) was collected in commercially available EDTA/ heparinised vials and diluted with the same volume of 1X PBS solution. Meanwhile 2.5 ml of Hi Sep TM LSM 1077 (Hi media) was transferred into a fresh tube aseptically. It was then carefully overlaid with 5 ml of diluted blood and centrifuged (400 x g) for 30 minutes. The erythrocytes were sedimented at the lowest part of the tube above which there was a Hi Sep layer. The WBCs are accumulated above the Hi Sep layer. The topmost plasma layer was discarded and then the WBC layer was aspirated into another centrifuge tube. It was given two washes with PBS 1x and centrifuged again to obtain sediment of WBC which was made into a suspension. Cells were counted in 0.5 ml of the suspension. They were diluted in TC 199 medium supplemented with foetal bovine serum to obtain a cell count of 5X105 cells/ml.
MTT Assay
The method followed for this is that of Mossmann (1983)16 with some modifications. MTT assay is a method based on colorimetric measurements of optical density. It measures the reduction of 3-(4,5-dimethylthiosol-2-yl)-2,4, diphenyl tetrazolium bromide (MTT) by succinic dehydrogenase of mitochondrial origin. The MTT entering the cells enters into mitochondria where it is reduced into coloured formazan crystals which are insoluble. Cells are then solubilised with DMSO and the produced formazan which is now soluble is spectophotometrically measured.
180 µl of the cell suspension (in the medium) was seeded into the wells of a 96 well plate of ELISA plate reader. Each type of treatment consisted of test run in multiple of triplicates. The first row consisted of 180 µl of cells in medium and 20 µl of distilled water. This served as a control. The successive rows consisted of 180 µl cell suspension and 20 µl of LC50, 1/5 LC50, 1/10 LC50 and 1/20 LC50 preparation of cypermethrin , all seeded in a multiple of triplicate wells. Readings of empty wells and 20 µl of LC50, 1/5 LC50, 1/10 LC50 and 1/20 LC50 were also taken for making corrective allowances in the O.D. The row containing only cells and medium served as a control. The last row consisted of cells, medium and 20 µl of DMSO to observe the harmful effect of DMSO alone. The plate was incubated for 2 hours at 370 C in a humid incubator to observe the effect of the insecticide on the number of surviving cells. After 2 hours of incubation aliquots of MTT solutions (5mg/ml) were added to each well and re-incubated for 2hours at 370 C. Hundred microliters of DMSO was then added to each well and the plate was incubated overnight. The OD of experimental plate was read at 600nm.
For Neem Extract (NE)
For Neem Extract (NE) in general the same procedure was followed. The concentrations taken for this experiment were- Neat neem extract (45% aqueous), and 1/5, 1/10,1/20 of the NE(in 1X PBS). The readings of 20 µl of each of these concentrations were also noted at the beginning of the experiment to make corrective changes. The last row consisted of cells, medium and 20 µl 1X PBS.
The amount of colour produced depends on the number of viable cells, greater number of viable cells resulting in more colour. Cell viability was calculated as the percentage of MTT absorption according to the formula:
% survival = (Mean experimental absorption/Mean control absorption) x 100
The results were subjected to student’s t-test for statistical analysis.
Results
Effect of Cypermethrin
In the case of Cypermethrin, if the viability of cells plus medium (without any drug) is taken to be 100 %, then cells containing LC50 dose of the insecticide (with a 9.86% concentration of DMSO) has a viability of 87.83%. The table 1 shows that there is a constant drop in the viability of cells as the dose of insecticide becomes weaker (from LC50 to 1/5, 1/10 and 1/20 of LC50) with a successive increase in the concentration of DMSO (from 9.86% in LC50 9.93% in 1/20 LC50). The viability falls from 87.83% (at LC50) to 68.46% at 1/20 LC50 (Fig 2). In the cells that were tested for the same aliquots of pure DMSO (10% concentration in the reaction mixture) the viability was observed to be 68.48% which is nearly the same as that found in 1/20 LC50.The differences in viability between control cells and cells treated with LC50dose were found to be significant at p<0.01 where as the viability at the rest of the concentrations as compared to the control were highly significant (p<o.001).Thus DMSO itself is quite harmful to the lymphocytes. (Table 1, Fig 1, Fig 2).
Table 1: The OD and viability of lymphocytes after treatment with different concentrations of cypermethrin
| S.No. | Concentration of Cypermethrin | Concentration of DMSO (%) | Average OD | Cell Vibility (%) |
| 1 | 0 | 0 | 0.399±0.023 | 100 |
| 2 | LC50 | 9.86 | 0.35±0.011 | 87.83** |
| 3 | 1/5 LC50 | 9.94 | 0.29±0.02 | 72.76* |
| 4 | 1/10 LC50 | 9.97 | 0.285±0.012 | 71.71* |
| 5 | 1/20 LC50 | 9.99 | 0.273±0.01 | 68.46* |
| 6 | 0 | 10 | 0.273±0.02 | 68.48* |
| * highly significant (p<o.001); ** significant at p<0.01 ( Data analysed by Student t test ) | ||||
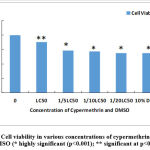 |
Figure 1: Cell viability in various concentrations of cypermethrin and 10% DMSO (* highly significant (p<0.001); ** significant at p<0.01) |
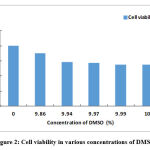 |
Figure 2: Cell viability in various concentrations of DMSO. |
Effect of Neem extract (NE)
The biochemical contents tested
The total pheonolic content was found to be 15.55 mg/100g of neem extract. This is comparable to the values reported by Hoda Salim Khamis Al-Jadidi, and Mohammad Amzad Hossain,12 (their range was from 20.80mg/100g to 107.29mg/100g). The total flavonoid content was found to be 100.75mg/100g of neem extract. This was also comparable to the values quoted by the same authors (from 136.50mg/100g to 484.50mg/100g) for crude neem extract.
In case of neem (Table 2) the cells treated with neat 45% aqueous extract showed a 196% increase in viability as compared to the control cells. This trend of increase of viability continues with 1/5 and 1/10 of NE, the cells showed an increase of 43% and 13.7% in viability respectively. However, the cells treated with 1/20 of NE showed a decrease of viability of 1.83% whereas an addition of the same aliquot of pure PBS to the culture mixture created a decrease in viability of 3.4%. The decrease in viability found after treatment with 1/10 and 1/20 of NE and that of pure PBS were found to be statistically non significant as compared to the control, whereas the increase in viability after treatment with neat 45%NE and 1/5NE were found to be significant (p<0.001). Thus neem extract was found to be very effective in causing an increase in proliferative activity of the cells at these two concentrations. This may be put to several uses where cell proliferation is desired.
Table 2: The effect of concentration of NE and PBS on cell viability.
| S. No. | Concentration of neem extract (N.E) | Concentration of PBS 1x | Average OD± SD | Cell viability % |
| 1 | 0(control cells) | 0 | 0.4329±0.043 | 100 |
| 2 | Neat neem extract (NE) 45% | 0 | 1.2840±0.109 | 296 |
| 3 | 1/5 NE | 8% | 0.6238±0.023 | 143 |
| 4 | 1/10 NE | 9% | 0.5571±0.078 | 113.7 |
| 5 | 1/20 NE | 9.1% | 0.5009±0.036 | 98.17 |
| 6 | – | 10% | 0.4182±0.019 | 96.60 |
Discussion
Assessment of cytotoxicity of cypermethrin on human lymphocytes by MTT assay was worked out by Suman G, Naravaneni and Kaiser Jamil12. Their results reported steady dose dependent decrese in viablity starting from 21.6µM to 33.6µM of the insecticide. Similar trends were also observed regarding comet tail lenghts at LC50 (33.6µM) and chromosome aberrations.The cytotoxic effect of cypermethrin on human lymphocytes were also studied by Chakravarthi et al17. They studied the chromosome abberrations after exposure of lymphocytes for 24 hours to various concentrations of cypermethrin (prepared from 1% solution in DMSO) and they found a dose depended increase (from 3.6 to 7.6 µM ) in chromosome aberrations
In vitro genotoxic effects of α cypermethrin on human peripheral blood lymphocytes were also studied by Kocaman and Topaktas (2009)18. In their work lymphocytes were treated with 5,10,15 and 20 mg/ml of α cypermethrin for 24 and 48 hours and a dose dependent increse in SCE and CA were found. In addition, the insecticide was found to decrese the MI. This agrees with the reduction of proliferation of lymphoctes found in the present investigation. The immunotoxic effects of cypermethrin on chicken lymphocytes was studied by Ambwani et al19(2018).They used a NOEL/103 dose of the commercial preparation of the insecticide for in vitro exposure of mitogen stimulated chicken lymphocytes for two hours for MTT assay.They found that the blastogenic activity of T and B lymphocytes was reduced by 48% and 40% respectively. The toxic effects of cypermethrin on PBL was aso studied by Gautam et al20 by using MTT assay after 2 and 18 hours exposure. They also reported a fall in viabliity with increse in insecticide concentration. However, they reported that exposure for 18 hours resulted in less damage to cells in terms of viability probably because the cells were able to recover.
This trend of decrese in viability with (LC50) dose as compared to untreated cells is also seen in the present investigation, but it was found that the viability fell further with dilution of the insectiside with DMSO, thus demonstarting the hamful effect of the solvent (DMSO). The present investigation brings to light the positive correlation of loss of viability after treatment with the drug. It also showes that loss of viability is inked at the same time to increse in concentration of DMSO in the culture medium.
The effect of neem (Azadirachta indica) leaf extract was observerd on human T-lymphocytes by Pedroza – Escobar David et al 9 . They used dried 100g neem leaves and made the extract in 1000 ml water,filtered the extract and dried it for seven days. A saturarated solution of the dried powder was used for studying. They treated the RPMI cultures of lymphocytes with 1µl,10 µl and 100 µl, of the extract and incubated for 72 hours. They found that 10 µl of the extract incresed the viability to 417.89% (compared to 100% at 1µl). According to these workers this increse is due to the presence of lectins in the extract. In this investigation also 20µl of NE/200µl of culture produced 216.3% increse in the viability. However the viability showed a progressive decrese as the neem extract was diluted in PBS to 1/5,1/10 and 1/20 of the NE(45%) and the decrese was maximum when a similar amount of PBS only was added to the culture. The addition of 1/10 of NE showed a net increse whereas 1/20 NE showed a decrese of 1.83% in the viability.
All parts of the neem tree have been described as useful in the siddha system of indian medicine as well as the Chinese system in the prehistoric times by Kumar and Navratnam4. Neem bark and neem leaf extracts (aqueous) have been therapeutically used as rural medicine as a part of treatment for leprosy, helminth infection, breathing disorders and slow bowel movement and have been used as a genral promoter for good health21 as the presence of alkoloids, saponins, tannins, glycocides, flavonoids and reducing sugars have been reported in aqueous neem extract.
The work done by Hashemi and Hossain23 showed that the flavnoids present in the neem extract possess antioxidant avtivity which helps in boosting viability of cells. Moreover according to Alzohairy24 ,who studied the therapeutic role of neem , polyphenolic flavinoids from fresh leaves of neem are known to have antifungal and antibacterial activites. This is a contributary factor for creating a favourable enviornment for an increasing trend in proliferation.
The hepatosomatic index (HSI) of mice treated with 14mg NE/kgBW/ day in mice showed a significant increse of 6.91 (compared to 4.91 in controls, as worked out by Janika Sitasiwi et al,22. This supports the proliferating activity of the neem extract on animal cells. Seriana O et al.7also reported increses in lymphocyte counts (p<0.05) in male rats after treatment with NE for 10 weeks.
The enhancement of immunity by neem leaf extract through action on peripheral blood mononuclear cells (macrophages etc.) was reported by experiments on melanoma cells by Fang Hao et al 8. This property is seen to be reflected in this study as the NE 45% was to cause a 196% increse in viability of lymphocytes.
Conclusion
Thus this investigation confirms the harmful effect of Cypermethrin on lymphocytes, as evident in the decrese of viability perccentage of the cells. At the same time the harmful effects of DMSO (being used as a carrier/solvent for the insecticide) were also evident because the viability percentage dropped with the rise in concentration of DMSO. That aqueous neem leaf extract has a boosting effect on proliferation of lymphocytes was also demonstrated by a sharp increse in viabiliy when the neat extract was used. Obviously this supports the view that neem extract has immunoboosting effects.
Acknowledgements
The authors are grateful to the Principal and Department of Zoology and Biotechnology, Government Science College, Jabalpur, M.P, for permitting the students to carry out this investigation. The authors are also grateful to Dr. Mamta Gokhale, Asstt. Prof, Botany, St. Aloysius College Jabalpur, for help in the conduction of phytochemical tests of neem extract.The authors wish to acknowledge the assistance provided by AVIKA Biologial Research Foundation, Jabalpur in the form of research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Sources
The authors declare no funding sources
References
- Clark JM and Brooks MW. Neurotoxicology of pyrethoroids:Single or multiple mechanisim of action? Environ Toxicol Chem, 1989, 8:361-372.
CrossRef - Abbassy MA, Eldefrawi ME and Eldefrawi AT. Pyrethroid action on the nicotinic acetylcholine receptor/channel,Pesticide Biochem Physio, 1983, 19: 299-308.
CrossRef - Kumar VS and Navaratanam V. Neem (Azardirachta indica): Prehistory to contemporary medicinal uses to human kind. Asian Pac J Trop Biomed, 2013, 3(7): 505-514
CrossRef - Veitch GE, Boyer A and Ley SV. The Azardirachtin story. Agnew chem. Int. Ed, Eng, 2008, 47: 9402-9429
CrossRef - Puri HS. Neem: The divine tree Azardirachta indica. 1989, 1st Ed, CRC Press.
- Yadav DK, Bhartikar YP, Chatterjee K, Ghosh M, Mondal NB, and Swarnaka S. Importance of Neem leaf: An insight into its role in combating diseases. Indian J Exp Biol, 2016, 54(11): 708-18
- SerianaI, Akmal M, Darusmanand Wahyuni S. Neem leaves extract (Azadirachta indica A. Juss) on male reproductive system: a mini-review 2019 IOP Conf. Ser.: Earth Environ. Sci. 399 012106
CrossRef - Fang Hao, Sandeep Kumar and Dhyan Chandra. Neem components as potential agents for cancer prevention and treatment. Biochimica et Biophysica acta, 2014, 1846(1): 247-257.
- Pedroza- Escobar David et al. Effect of Neem Azardirachta indica leaf extract in human T lymphocytes. Indian Journal of Traditional Knowledge, 2016, 15(2): 219-222.
- Jose Francisco Islas and Moreno-Cuevas. An overview of Neem (Azardirachta indica) and its potential impact on health. Journal of Functional Foods, 2020, 74: 104171.
CrossRef - Manna S, Bhattacharyya D, Mandal TK, Das S, Repeated dose toxicity of alfa-cypermethrin in rats, J Vet Sci,5(3)(2004)241-5.
CrossRef - Suman G, Naravaneni Rambabu and Kaiser Jamil. In vitro cytogenetic studies of cypermethrin on human lymphocytes, Indian J Exp Biol, 2006, 44(3): 233-239.
- Agbenin ON and Marley PS. In- Vitro Assay of some plant extracts against Fusarium oxysporum, sp Lycopersici causal agent of tomato wilt. Journal of Plant Protection Research, 2006, 46(3): 215-220.
Protection Research, 2006, 46(3): 215-220. - Stankovic Milan S. Total phenolic content, flavonoid concenteration and antioxidant activity of Marrubium peregrinumL.extracts. Kragujevac J.Sci., 2011, 33:63-72.
- Khanna A, Gautam DS and Gokhale M. Tobacco dust induced genotoxicity as an occupational hazard in workers of bidi making cottage industry of central India. Toxicol Int, 2014, 21(1):18-23.
CrossRef - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immun Methods, 1983, 65: 55-63.
CrossRef - Chakravarthi K, Naravaneni R and Philip GH. Study of cypermethrin cytogenesis effects on human lymphocytes using in-vitro techniques. Appl. Sci. Enviorn. Mage., 2007, 11(2): 77-81.
- Kocaman AY and Topkatas M. The in-vito getotoxic effects of a commercial formulation of cypermethrin in human peripheral blood lymphocytes. Enviormental and Molecular Mutagenesis, 2008, 50(1): 27-36.
CrossRef - Ambwani S, Ambwani TK and Chauhan RS. Immunotoxic effects of cypermethrin in mitogen stimulated chicken lymphocytes due to oxidative stress and apoptosis. Journal of Entomology and Zoology Studies, 2018, 6(2):37-42.
- Gautam D S, Chauhan R, Patel A, Lodhi N, An assessment of in-vitro cytotoxicity of cypermethrin on human peripheral blood lymphocytes using MTT assay, World Journal of Pharmaceutical Research, 6(8) (2017), 1373-1378.
CrossRef - Biswas K, Chattopadhyay I, Banerjee RK and Bandhopadhyay U. Biological activities and medicinal properties of Neem (Azardirachta indica). Curr Sci, 2002, 82(11):1336-1345
- Sitasiwi AJ, et al. Effect of ethanolic neem (Azardirachta indica) leaf extract as an herb contraceptive on hepatosomatic index of the male mice (Mus musculus). J Phys: Conf. Ser., 2018, 1025 012043, 1-6.
CrossRef - Al-Hashemi & Hossain MA, Biological activity of different neem leaf crude extract used locally in Ayurvedic Medicine, Pacific Science Review A:Natural Science and Engineering, (2)18(2016) 128-131.
CrossRef - Alzohairy A.M. ,Therapeutics role of Azadirachta indica (Neem) and their Constituents in Diseases Prevention and Treatment , Hindawi , Evid Based Complement Alternat Med.2016;2016:7382506 , Published online (2016)
CrossRef








