Neyder Contreras-Puentes , Daimer Pérez-Orozco
, Daimer Pérez-Orozco and Fernando Camacho-Díaz
and Fernando Camacho-Díaz
GINUMED, Medicine, Rafael Nuñez University Corporation, Cartagena D.T. y C.
Corresponding Author E-mail: neyder.contreras@curnvirtual.edu.co
DOI : https://dx.doi.org/10.13005/bpj/2384
Abstract
Aims: Alzheimer's disease is a disorder associated to dementia that widely affects to population. In the molecular study, key enzymes have been associated with the regulation of the amyloid pathway, which have a focus in the discovery of possible inhibitors. Likewise, the absence of specific treatments, has promoted the development of promising molecules from natural sources. Material and Methods: In this study was carried out an in-silico exploration of curcumin analogues against β-secretase, γ-secretase and GSK-3β. A virtual screening of 373 curcumin analogues against enzymes implicated in the pathology was implemented, using molecular docking simulations through Autodock-Vina based on PyRx 0.8. Followed by in-silico prediction of ADMET properties to molecules with higher affinity using SwissADME and GUSAR prediction. Results: It was obtained that the molecules of highest affinity were 92296662, 102584924, 92341226 for β-secretase, γ-secretase and GSK-3β, respectively. These were contrasted with selective inhibitors for enzymatic systems. Additionally, the predictions of the ADMET properties of the analogues showed a variability in terms of metabolism, non-permeation on blood–brain barrier and toxicity values according to reported in the literature. Thus, in-silico prediction indicated curcumin analogues as possible regulatory agents of the enzymatic activity associated to Alzheimer's disease.
Keywords
Analogues; Alzheimer Diseases; Curcumin; Molecular Docking
Download this article as:| Copy the following to cite this article: Puentes N. C, Orozco D. P, Díaz F. C. Curcumin Analogues as Promissory Compounds for Inhibition of Β-Secretase, Γ-Secretase and GSK-3β Implicated at Alzheimer Disease: In Silico Study. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Puentes N. C, Orozco D. P, Díaz F. C. Curcumin Analogues as Promissory Compounds for Inhibition of Β-Secretase, Γ-Secretase and GSK-3β Implicated at Alzheimer Disease: In Silico Study. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3LuSSHb |
Introduction
Alzheimer’s disease is the most common cause of dementia that impacts global health, with enormous repercussions on society1. This neurodegenerative disease with a slow progression affects 5-10% of the population over 65 years old, with a prevalence that increases exponentially with age. its prevalence has increased, estimating more than 45 million people diagnosed of dementia in worldwide and prevalence projections around 130 million people with this type of manifestation to 2,0502,3.
In addition, the family history of dementia is an important aspect in the development of the pathology, which has been linked to genetic component; likewise, the epigenetic and etiopathogenic environmental component has been highlighted, as contributors to indirect development of the disease4. Therefore, there has also been an increase in research that has helped to improve diagnostic methods, especially focusing on the pathophysiology of the disease and search for biomarkers in the initial stages5.
On the other hand, the presence of regulatory enzymes in progression has been considered, hence the importance in the study of β-secretase and γ-secretase, constituting the main enzyme systems that cause accumulations of β-amyloid peptide, responsible at the formation of senile plaques on brain. Likewise, has been studied other enzymes as glycogen synthase kinase 3β (GSK-3β), in which are linked to phosphorylation of proteins6–8.
Currently, the conventional treatments are based on the regulation of neurotransmitters such as acetylcholine, specifically in the inhibitory activity of acetylcholinesterase, preventing degradation of the endogenous substance and favoring stimulation, however this treatment isn´t sufficient, as well as, ineffective for prolonging the quality of life of patients9.
Based on this, possible therapeutic alternatives have been reports in regulation of the enzymes associated to proliferation of AD10,11. Some natural sources such as Curcuma longa L., and its major metabolite as curcumin, may be associated to its antioxidant potential and protective activity against neurodegenerative diseases12,13. Therefore, the search and evaluation at the in-silico level of metabolites such as curcumin and its analogues were established, promoting the study of agents as possible candidates in the inhibition of enzymes involved in Alzheimer’s disease.
Material and methods
Preparation of ligands and receptors
From bibliographic reports, it has been obtained that the curcumin is a good candidate for its use as a promising agent for the treatment of the disease. Hence, the search for curcumin analogues was developed using the PubChem database14, performing a selection filter of 98% structural similarity. 373 molecules were found and downloaded in SDF format. The ligands were minimized energetically using the force-field mmff94; by conjugated gradients in 200 steps developed through Open Babel tools15. Then, BIOVIA Discovery Studio version 4.5 and Avogadro software were used for hydrogen addition and adjusted of charges.
On the other hand, the Protein Data Bank (PDB) database was used to search for the three-dimensional structure of the β-secretase, γ-secretase and GSK-3β proteins related to AD pathology, identified with access code: 4TRZ (Resolution: 3.25 Å, complex with 2-thiophenyl HEA-type inhibitor), 6LR4 (Resolution: 3.00 Å, complex with Semagacestat) AND 3L1S (Resolution: 2.90 Å, complex with Z92 or (4E)-4-[(4-chlorophenyl)hydrazono]-5-(3,4-dimethoxyphenyl)-2,4-dihydro-3H-pyrazol-3-one), respectively. Likewise, each protein was previously prepared by means of the extraction of associated molecules, the addition of hydrogens and eliminating of water.
Molecular Docking
Molecular docking was performed by AutoDock-Vina linked to PyRx 0.8 software16,17. A virtual screening was implemented on conformational search in center grid space of x = 26.895, y = 5.520, z = 16.585 for β-secretase; x = 168.318 y = 173.555, z = 146.790 for γ-secretase and x = 38.189, y = 35.741, z = 53.087 for GSK-3β. A size grid value of x = 18.61, y = 14.82, z = 17.98 for β-secretase; x = 17.46, y = 17.03, z = 22.15 for γ-secretase and x = 10.83, y = 17.05, z = 13.10 for GSK-3β. Additionality, the molecular docking calculation with an exhaustiveness of 8. Then, it was simulated obtaining stable conformations. Prior the structural conformation were converted to PDB format using PyMOL18. BIOVIA Discovery Studio visualizer was used in the identification of interaction force and residues19.
Pharmacokinetic, toxicity and drug-likeness prediction
The molecules with best affinity for enzymes were selected. A predictive search of the pharmacokinetic, toxicological and drug-likeness properties was performed using the SwissADME and Gusar on-line server20.
Results and Discussion
Curcumin reports antioxidant, anti-cancer, and anti-inflammatory activities. As well as, some reports of inhibitory potential for acetylcholinesterase, which has been associated to neuro-protective capacity focused on cognitive deficits and amyloid accumulation21,22. Alike, Alzheimer’s disease as a progressive and multifactorial neurodegeneration, one of the mechanisms widely studied is related to the amyloid theory; therefore, β-secretase, γ-secretase and GSK-3β are the enzymes directly related to the formation of amyloid plaques, which are considered therapeutic targets in the treatment of AD23.
Experimental bases have led to the molecular study of the role of curcumin as a possible modulator element of key enzymes in the pathogenesis of Alzheimer’s disease. Thus, experimental approaches have established that modified curcumin such as GT863/PE859 have showed an inhibitory role about in vivo aggregation of Aβ and tau protein, besides have demonstrated the regulation of cleavage by γ-secretase24. Also, Jen su et al., show through in vitro assay that curcumin analogues (TML-6) inhibit multiple pathways of pathogenesis, including the synthesis of APP and Aβ25. Likewise, the studies by Xiong et al have established that the use of curcumin contributed to the decrease of Aβ peptides (40/42a), the levels of protein and mRNA of PS1 and GSK-3β26. On the other hand, some in silico studies have evaluated the presence of curcumin and flavonoids against β-secretase, which pIC predicted between 4.52-10.27, and experimentally corroborated and demonstrating the prediction of promising bioactivities27.
In molecular docking studies, curcumin analogues have demonstrated the presence of potential structures with high affinity to enzyme complexes such as 92296662, 102584924, 92341226 for β-secretase, γ-secretase and GSK-3β, respectively. Likewise, it was contrasted with selective inhibitors related to the crystallized structures. The main interactions at the binding site are shown in Figure 1 and Table 1.
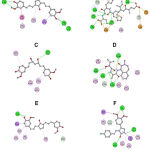 |
Figure 1: Curcumin analogues binding with enzymes implicated in AD. A. 92296662 binding β-secretase. B. 2-thiophenyl HEA-type inhibitor binding β-secretase. |
Table 1: Curcumin analogues and molecules of references of higher binding energy as possible β-secretase, γ-secretase and GSK-3β inhibitors.
| Protein | Ligands | Binding energy (Kcal/mol) | Residues | |
| Hydrogen interaction | Hydrophobic interaction and others | |||
| β-secretase | 92296662 | -10.4 | S71, T293, T390 | Y132, I171, Y259, V393 |
| 2-thiophenyl HEA-type inhibitor | -12.3 | G72, T133, Q134, D289, T292, T293, T390 | L71, Y132, I171, Y259, R296, R368, K382 | |
| γ-secretase | 102584924 | -8.9 | L432 | L268, L271, L282, A434 |
| Semagacestat | -8.3 | K380, L381, G382, D385, L432 | L268, V272, V261, A431, L425 | |
| GSK-3β | 92341226 | -9.6 | V135, R141 | F67, V70, A83, V110, L188 |
| GSK-3β Inhibitor
(3-Aryl-4-(arylhydrazono)-1H-pyrazol-5-ones) |
-8.9 | K85, V135, D133, C199 | F67, V70, A83, V110, L132, L188 | |
The complex formed between 92296662 and β-secretase, evidenced the formation of hydrogen bond with S71, T293 and T390 residues, which interacted to hydroxy and methoxy substituents of phenyl rings. Comparing with 2-thiophenyl HEA-type inhibitor, residues with a wide contact volume are shown, distinguishing G72, T133, Q134, D289, T292, T293 and T390, capable of interacting through hydrogen bonds with carbonyl groups and amines of the inhibitory peptide, which is relevant due to sequence of residues 289-292, which are key in the catalytic activity of the enzyme28,29. Likewise, it has been characterized that some residues such as R296 are conserved in the secretase sequences of vertebrates, essential in the formation of a salt bridge with negatively charged P1 residues of the APP30.
Regarding γ-secretase and 102584924, it has been identified that the majority of interactions are hydrophobic, generated through residues L268, L271, L282 and A434 with the aromatic groups of the curcumin analogue. On the other hand, the presence of hydrogen bond interactions with residues such as L432 was observed. However, contrasted with Semagacestat as a selective inhibitor of γ-secretase, was showed a high preponderance of hydrogen bonds with residues such as D385, G382, L432, L38131. Thus, in the molecular study of presenilin as a catalytic unit, the presence of aspartyl residues has been identified as transcendental in intramembranous cleavages; thus, changes in D257 and D385 are associated with decreased in vitro Aβ secretion and Notch 1 cleavage 31,32. On the other hand, some studies show that the substitution of residues such as L432 and S438 in FAD-linked mutations are important in the regulation of the γ-secretase mechanism, where it has been shown that suppressor mutants induced a decrease in Aβ42, essential for the pathophysiological progression of the disease33.
Between GSK-3β and 92341226 is evidenced a hydrophobic behavior in binding site, where residues F67, V70, A83, V110 and L188 are identified, interacting with the aromatic rings of the analogue. Hydrogen bonds are shown between the hydroxy and methoxy substituents with residues V135 and R141, respectively. In contrast, the studies of Arnost et al, corroborated that the selective inhibitors of 3-aryl-4- (arylhydrazono)-1H-pyrazol-5-one, have shown that V135 and D133 are linked to the pyrazole ring through the carbonyl and amine groups, forming hydrogen bond interactions34. Also, is shown that methoxy substituents contact K8534. Additionally, it has been distinguished that V135 plays a crucial role in the formation of the hinge region, which has been linked especially in the ATP binding pocket35. As well as, K85 has been associated to ATP binding and γ-phosphate transfer36. These approaches that were described in the control molecule demonstrate that the presence of hydrophobic interactions, in concurrence with the bonds through hydrogen bond with these residues, are fundamental for the recognition and potency of inhibitory structures.
Table 2: ADME, and acute oral toxicity prediction of potential Inhibitors using SwissADME and GUSAR Server
| Parameter | Compounds | ||
| 92296662 | 102584924 | 92341226 | |
| Permeation BBB | No | No | No |
| P-gp | No | No | No |
| CYP1A2 | No | No | No |
| CYP2D6 | No | No | No |
| CYP3A4 | Yes | Yes | Yes |
| AOT in rats–mg/Kg
(OECD Class) |
2,529 (V) | 3,822 (V) | 2529 (V) |
| Lipinski | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 |
Table 2 shows the predictions of ADMET properties, these results described that curcumin analogues don’t have the ability to permeate the blood-brain barrier (BBB), as well as a variability in inhibition on the CYP450 system, toxicity values predicted greater than 2500 mg/Kg, as well as compliance of Lipinsky rules. Therefore, although it has been established that curcumin exhibits low absorption, rapid metabolism, and limited permeability on BBB37; These properties can be improved with the development of pharmaceutical formulations based on liposomes, nanoparticles or micelles that favor bioavailability and application in the modulation of neuronal disorders. So, some investigations have shown that the administration of curcumagalactomannosides in Wistar rats evidenced a better behavior of plasma bioavailability, stability and permeability of the blood-brain barrier and other organs, which was validated by monitoring free curcuminoids38. Likewise, the implementation of curcumin nanoliposomes favors the absorption and its distribution, denoting in the studies a high affinity for the Aβ1-42 peptide39. Similarly, studies with curcumin analogues against Caenorhabditis elegans models determined that it could be associated with SKN-1/Nrf activation pathways, linking protection against Aβ toxicity40. Thus, in terms of toxicity it has been described that the predictions yielded theoretical LD50 values between 2500 and 3850 mg/Kg, which is validated by experimental studies where higher doses around 5000 mg/Kg not induce toxicity, which is evidenced with the administration of curcumin in the form of extracts or pharmaceutical formulations that improve absorption. As well as, the studies in Wistar rats where the administration of repeated doses for 90 days at maximum values of 1,000 mg/Kg showed that low changes compared to control groups.
Conclusion
Virtual screening of curcumin analogues by molecular docking indicated a high energy affinity in compounds as 92296662, 102584924, 92341226 for β-secretase, γ-secretase and GSK-3β, respectively. As were compared with selective inhibitors of crystallized structures, highlighting the presence of key residues in the functioning of the catalytic activity, and involved in functional regulation as described in the reported experimental studies. Likewise, the ADMET properties prediction studies showed that curcumin and analogues presented a series of variable characteristics in terms of absorption, permeability through BBB, metabolism and toxicity values that could be consistent with In vivo studies reported. Then, it is established that the study of promising molecules such as curcumin and analogues can be tools for possible regulators of neurodegenerative functioning, specifically focused on promising molecules against these types of enzymes associated with Alzheimer’s disease.
Acknowledgment
Rafael Nuñez University Corporation
Conflict of Interest
There is no conflict of interest
Funding Sources
There is no funding Source.
Reference
- Barragán Martínez D, García Soldevilla MA, Parra Santiago A, Tejeiro Martínez J. Alzheimer’s disease. Med. 2019;12(74):4338–46.
CrossRef - Morley JE, Farr SA, Nguyen AD. Alzheimer Disease. Clin Geriatr Med [Internet]. 2018;34(4):591–601. Available from: https://doi.org/10.1016/j.nurpra.2017.10.014
CrossRef - WHO. Dementia [Internet]. 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
- Llibre Rodríguez J, Gutiérrez Herrera RF. Demencias y enfermedad de Alzheimer en América Latina y el Caribe. Rev Cuba Salud Pública. 2014;14(3):378–87.
- Blennow K, Zetterberg H. Biomarkers for Alzheimer disease – current status and prospects for the future. J Intern Med. 2018;284(6):643–63.
CrossRef - Basi GS, Hemphill S, Brigham EF, Liao A, Aubele DL, Baker J, et al. Amyloid precursor protein selective gamma-secretase inhibitors for treatment of Alzheimer’s disease. Alzheimer’s Res Ther. 2010;2(6):1–21.
CrossRef - He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467(7311):95–8.
CrossRef - Folch J, Ettcheto M, Petrov D, Abad S, Pedrós I, Marin M, et al. Review of the advances in treatment for Alzheimer disease: strategies for combating β-amyloid protein. Neurol (English Ed. 2018;33(1):47–58.
CrossRef - Frozza RL, Lourenco M V, Felice FG De. Challenges for Alzheimer ’ s Disease Therapy : Insights from Novel Mechanisms Beyond Memory Defects. Front Neurosci. 2018;12:37.
CrossRef - Syamima A, Manap A, Cheng A, Tan W, Leong WH, Yoke A. Synergistic Effects of Curcumin and Piperine as Potent Acetylcholine and Amyloidogenic Inhibitors With Significant Neuroprotective Activity in SH-SY5Y Cells via Computational Molecular Modeling and in vitro Assay. Front Aging Neurosci. 2019;11:206.
CrossRef - Akram M, Nawaz A. Effects of medicinal plants on alzheimer’s disease and memory deficits. Neural Regen Res. 2017;12(4):660–70.
CrossRef - Voulgaropoulou SD, Amelsvoort TAMJ Van, Prickaerts J, Vingerhoets C. The e ff ect of curcumin on cognition in Alzheimer ’ s disease and healthy aging : A systematic review of pre-clinical and clinical studies. Brain Res [Internet]. 2019;1725:146476. Available from: https://doi.org/10.1016/j.brainres.2019.146476
CrossRef - Chainoglou E, Hadjipavlou-Litina D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int J Mol Sci. 2020;21(6):1975.
CrossRef - Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44(D1):D1202-1213.
CrossRef - Aviz-Amador A, Contreras-Puentes N, Mercado-Camargo J. Virtual screening using docking and molecular dynamics of cannabinoid analogs against CB 1 and CB 2 receptors. Comput Biol Chem [Internet]. 2021;95:107590. Available from: https://doi.org/10.1016/j.compbiolchem.2021.107590
CrossRef - Trott O, Olson AJ. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem. 2010;31(2):455–461.
CrossRef - Sargis D, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–50.
CrossRef - DeLano WL. PyMOL. Schrödinger; 2019.
- Osimertinib DA De. Virtual screening of Osimertinib and Dacomitinib Analogues with potential activity on EGFR ( T790M and l858R Mutations ) for non-small cell lung cancer treatment. Rev Cinc Biomed. 2021;10(4):234–45.
CrossRef - Daina A, Michielin O, Zoete V. SwissADME : a free web tool to evaluate pharmacokinetics , drug- likeness and medicinal chemistry friendliness of small molecules. Sci Rep [Internet]. 2017;7:42717. Available from: http://dx.doi.org/10.1038/srep42717
CrossRef - Kramer T, Schmidt B, Lo Monte F. Small-molecule inhibitors of GSK-3: Structural insights and their application to Alzheimer’s disease models. Int J Alzheimers Dis. 2012;2012:381029.
CrossRef - Singh DB, Gupta MK, Kesharwani RK, Misra K. Comparative docking and ADMET study of some curcumin derivatives and herbal congeners targeting β-amyloid. Netw Model Anal Heal Informatics Bioinforma. 2013;2:13–27.
CrossRef - Lee J, Jun M. Dual BACE1 and cholinesterase inhibitory effects of phlorotannins from ecklonia cava-an in vitro and in silico study. Mar Drugs. 2019;17(2):91.
CrossRef - Urano Y, Takahachi M, Higashiura R, Fujiwara H, Funamoto S, Imai S, et al. Curcumin Derivative GT863 Inhibits Amyloid-Beta Production via Inhibition of Protein N-Glycosylation. Cells. 2020;9(2):349.
CrossRef - Su IJ, Chang HY, Wang HC, Tsai KJ. A curcumin analog exhibits multiple biologic effects on the pathogenesis of Alzheimer’s disease and improves behavior, inflammation, and β-amyloid accumulation in a mouse model. Int J Mol Sci. 2020;21(15):1–20.
CrossRef - Xiong Z, Hongmei Z, Lu S, Yu L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacol Reports. 2011;63(5):1101–8.
CrossRef - Tran T, Le M, Tran T, Tran T. with Acetylcholinesterase and Beta-Secretase. :1–21.
- Menting KW, Claassen JAHR. β-secretase inhibitor; a promising novel therapeutic drug in Alzheimer’s Disease. Front Aging Neurosci. 2014;6:1–20.
CrossRef - Kandalepas P, Vassar R. The Normal and Pathologic Roles of the Alzheimer’s βsecretase, BACE1. Curr Alzheimer Res. 2014;11(5):441–9.
CrossRef - Sauder JM, Arthur JW, Dunbrack RL. Modeling of substrate specificity of the Alzheimer’s disease amyloid precursor protein β-secretase. J Mol Biol. 2000;300(2):241–8.
CrossRef - Yang G, Zhou R, Guo X, Yan C, Lei J, Shi Y. Structural basis of γ-secretase inhibition and modulation by small molecule drugs. Cell [Internet]. 2021;184(2):521-533.e14. Available from: https://www.sciencedirect.com/science/article/pii/S0092867420316214
CrossRef - Price DL, Savonenko A V., Albert M, Troncoso JC, Wong PC. Aging of the Brain and Alzheimer’s Disease. Encycl Neurosci. 2009;187–95.
CrossRef - Futai E, Osawa S, Cai T, Fujisawa T, Ishiura S, Tomita T. Suppressor mutations for presenilin 1 familial Alzheimer disease mutants modulate γ-secretase activities. J Biol Chem. 2016;291(1):435–46.
CrossRef - Arnost M, Pierce A, Haar E ter, Lauffer D, Madden J, Tanner K, et al. 3-Aryl-4-(arylhydrazono)-1H-pyrazol-5-ones: Highly ligand efficient and potent inhibitors of GSK3β. Bioorganic Med Chem Lett [Internet]. 2010;20(5):1661–4. Available from: http://dx.doi.org/10.1016/j.bmcl.2010.01.072
CrossRef - Pandey MK, DeGrado TR. Glycogen synthase kinase-3 (GSK-3)-targeted therapy and imaging. Theranostics. 2016;6(4):571–93.
CrossRef - Hoffmeister L, Diekmann M, Brand K, Huber R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells. 2020;9(4):820.
CrossRef - Salehi B, Calina D, Docea A, Koirala N, Aryal S, Lombardo D, et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J Clin Med. 2020;9(2):430.
CrossRef - Krishnakumar IM, Maliakel A, Gopakumar G, Kumar D, Maliakel B, Kuttan R. Improved blood-brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J Funct Foods [Internet]. 2015;14:215–25. Available from: http://dx.doi.org/10.1016/j.jff.2015.01.049
CrossRef - Mourtas S, Canovi M, Zona C, Aurilia D, Niarakis A, La Ferla B, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials [Internet]. 2011;32(6):1635–45. Available from: http://dx.doi.org/10.1016/j.biomaterials.2010.10.027
CrossRef - Lee EHC, Lim SSC, Yuen KH, Lee CY. Curcumin and a hemi-analogue with improved blood–brain barrier permeability protect against amyloid-beta toxicity in Caenorhabditis elegans via SKN-1/Nrf activation. J Pharm Pharmacol. 2019;71(5):860–8.
CrossRef







