Batoul M. Izzularab1* , Ehab Tousson2
, Ehab Tousson2 , Nabiha I. Abdo3
, Nabiha I. Abdo3  and Doha M. Beltagy1
and Doha M. Beltagy1
1Biochemistry Division, Chemistry Department, Faculty of Science, Damanhour University, Egypt.
2Department of Zoology, Faculty of Science, Tanta University, Egypt.
3Department of Basic Sciences, Higher Institute of Engineering and Technology, New Borg El-Arab City, Egypt.
Corresponding Author E-mail: batoul.izzularab@sci.dmu.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2350
Abstract
Lead nanoparticles (Pb-NPs) are used in different industrial aspects with potential risk in human health. The current work aimed to appreciate the therapeutic rocket seeds extract effect on against renal toxicity stimulated by (Pb-NPs) via estimation of kidney functions and electrolytes with different histological and immunological studies. Pb-NPs were synthesized by biocompatible chemical coprecipitation of Pb2+ and glucose as a reducing agent. These nanoparticles have been characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM) techniques. Pb-NPs have a relatively cubic shape with diameter about 16 nm. The study was performed on 60 male albino rats distributed into four groups (control, rocket- seeds extract, Pb-NPs, and treated) The results demonstrated the toxic effects of Pb-NPs via the destruction of the renal cell resulted in significant elevations of urea and creatinine concentrations which affected on electrolyte hemostasis. The rocket seed extract administration showed beneficial curative effects against renal toxicity induced by Pb-NPs exposure. Rocket seeds extract administration showed regression of the kidney functions and improvement of electrolytes hemostasis. These result were indicated by histopathological and immunohistochemical analysis
Keywords
Apoptosis; Electrolytes; Lead nanoparticles; Renal dysfunction; Rocket seeds extract
Download this article as:| Copy the following to cite this article: Izzularab B. M, Tousson E, Abdo N. I, Beltagy D. M. Curative Consequences of Rocket Seeds (Eruca Sativa) Extract against Lead Nanoparticles Induced Renal Dysfunction in Rats. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Izzularab B. M, Tousson E, Abdo N. I, Beltagy D. M. Curative Consequences of Rocket Seeds (Eruca Sativa) Extract against Lead Nanoparticles Induced Renal Dysfunction in Rats. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3tZaKUN |
Introduction
Many nanoparticles have important roles in different fields belong to the human health and also several nanoparticles like carbon – based particles and metallic give rise to toxicity.1-3 Lead is a heavy metal has very poisonous effect in all human organs while lead nanoparticles is synthetized to improve the lead properties to use in industry and medicinal field .Nowadays, Lead nanoparticles are used in many metal industrial activities especially ceramics industry which have influent on public health on the workers’ health and it have significant effect on several microorganisms. 4 furthermore, the exposure of lead nanoparticles has harmful effects on different system like the hematopoietic, renal, and central nervous system. This deleterious effect caused through the increasing of oxidative stress. Lead exposure cause renal functional abnormality due to morphological changes as glomerular, tubulointerstitial abnormalities, and dysfunction of tubular transport mechanism. 5
The use of alternative medicine has been a marked increase in recent decades. 6-9 The antioxidant effects of many plant output can exert many substantial roles in remediation of many diseases. 10, 11 Rocket plant (jarjeer) is the common name of Eruca sativa and used as a medicinal curative for various diseases. Traditionally, it applied in treatment of different disease like stomachic, scurvy, increase sexual desire and diuretic. 12 The seeds Rocket consist of several organic compounds like taramira oil, protein, and glucosinolate. Plant extract contains erucic acid , oleic acid and linoleic acid also it contain saturated fatty acids. Different phytochemicals are detected in rocket inclusive its seeds it included Vitamin C, carotenoids, flavonoids, phenolics and others. This Phytochemicals are widespread in boost health and restraining various diseases. The natural product and its isolated compounds has hopeful results from researchers and some clinical trials in treatment of kidney diseases. 13 These compounds have a pharmacological effect of rocket like antioxidant, cytoprotective, anticancer.14, 15This study purposed to evaluate the therapeutic effect of rocket seeds extract versus renal toxicity prompted by lead nanoparticles via estimation of kidney functions and electrolytes with different histological and immunological studies.
Materials and Methods
Synthesis of lead nanoparticles (Pb-NPs)
Synthesis of Pb-NPs was performed by the precipitation from aqueous solutions. 16, 17 Lead acetate [Pb(CH3COO)2.3H2O] and glucose were purchased from Sigma-Aldrich. A solution was intended by dissolving 36g of glucose as reducing agent in 200 ml water then 5g of lead acetate was added. The solution put on a water bath at 80–85 oC. By time, the solution initially became cloudy and then turned into yellow color, and finally a light brown color which indicates the formation of lead nanoparticles. The solution was evaporated to three-quarters of its volume. Another 200 ml dist.H2O was mixed with the whole volume and sonicated for 10 min. Stirring was stopped and the precipitate was allowed to be settled. The supernatant was decanted, filtered, washed with Deionized water repeatedly and dried at room temperature.
Characterization of the synthesized Pb-NPs
Scanning Electron Microscopy (SEM)
The structure of powder sample of Pb-NPs were analyzed utilizing scanning electron microscopy (JEOL SEM, JSM-636OLA, Japan) at an accelerated voltage 10 kV.
X-Ray Diffraction Pattern (XRD)
XRD measurements were recorded on (Shimadzu LabX XRD-6100 X-ray diffractometer, Japan). It was carried out at 40 kV voltage and 30 mA current under excitation of CuKα radiation (λ= 1.541 Å), scan angle ranged from 5-80o and 12o/min scanning rate with 0.02o step width. For XRD measurements, Pb-NPs data obtained as powder samples.
Particle Size Analysis
The particle size average has been rated by using formula of DebyeScherrer 18
[D = 0.9 λ / β cos Ɵ]
Where ‘λ’ is wave length of XRay (0.1541 nm), ‘β’ is FWHM (full width at half maximum), ‘θ’ is the diffraction angle and ‘D’ is particle diameter (size). Lattice constant has been rated by utilizing the formula, a = d*√(h2+k2+l2) for lead nanoparticles.
Rocket samples & extraction
Eruca sativa (Rocket) seeds were purchased from Agricultural Research Center, Ministry of Agriculture and Land Reclamation, Egypt. The extract of Rocket Seeds extract was obtained by mixing 50 g of the grinding seeds (dry powder) with 500 ml of distilled water at 70°C by the ratio (1/10, W/V). The mixture was shacked usage a magnetic stirrer for 24 hours. The blend was centrifuged at 5000 rpm for 10 min. The supernatant was filtered. This step was repeated twice. The filtrated was condensed by heating in oven on at 40°C for 72 hours to gain the crude extract. At 4°C the extract was storage in a dark sterile bottle until use. 19
Determination of total phenolic content of extract
Folin-Ciocalteu method was used to determine the total phenolic content of the extract with the usage of a serial concentration of gallic acid solution as a standard. The total concentration was expressed (mg) as gallic acid equivalent per gram of extract according to Cheok et al . 20
Estimation of total flavonoids of extracts
Total flavonoids content was determined using the aluminium chloride colorimetric method with Catechin standard solution as a standard, and expressed (mg) as Catechin equivalent per gram of extract according to Zhishen et al . 21
Determination of DPPH scavenging activity of extract
Antioxidant activity of the extract was evaluated using one of the most popular techniques for radical scavenging assessment, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Trolox Used as reference solution according to procedures of Saggu et al . 22
Animals and experimental design
This study carried out on sixty male albino rats weighting about (200±10 gram) and aged around (13±1 weeks age). Rats were obtained from the accredited breeding and empirical lab, zoology departments, Tanta University, Egypt. The animal were kept and patronize accordingly to Faculty of Science, Tanta University instructor for the animal, which confirmed by the Institutional Animal Care and Use Committee (IACUC-SCI-TU-0185). The animals were housed for about two week then it divided into four groups (each groups about 15 rats). Group 1 is a negative control group, Group 2 which are giving rocket seeds extract daily (intragastrically, 30 mg/kg body weight) 15, Group 3 administrate by intraperitoneally injection with Pb-NPs (50 mg/kg body weight) for 8 weeks 23, and group 4 was intraperitoneally injected with Pb-NPs (50 mg/kg body weight) for 8 weeks and then giving rocket seeds extract (intragastrically, 30 mg/kg body weight/day) for 4 weeks.
Blood and tissue preparation
The rats in any group were anesthetized by sodium pentobarbital and were necropsied in the termination of the experiments after animal fasting for 10-12 hours. Blood samples were gathered from each rat from the vena cava in plain tube and permit the sample to clot at 37°C. The blood samples are centrifuge at 5000 rpm for 10 minutes. Serum uric acid, urea and creatinine were detected in the rat serum using commercial kits (Biomed EGY- CHEM for lab technology). Also the electrolytes in serum (Potassium K+, sodium Na+, calcium Ca+2 and chloride Cl– ions) were determined by utilized commercial kits (Sensa core electrolyte, India) .24
Histological preparation
After autopsy, the kidney were speedy removed and settled by inundation in 10% neutral buffered formalin solution for 24-48 hours. The pattern was then dehydrated, cleared and embedded in paraffin. Sequent sections of 5 µm thick were cut off by rotary microtome (Litz, Wetzlar; Germany). This section stained by haematoxylin and eosin. 25
Immunohistochemical detection P53 apoptotic protein
The destination is determination the apoptotic p53 proteins expression in the kidney sections. The process of avidin Biotin Complex (ABC) (Elite–ABC, Vector Laboratories, CA, USA) was utilized of P53 (dilution 1:200 DAKO Japan Co, Ltd, Tokyo, Japan). 26,27
Statistical Analysis
The data were statistical analyzed by utilizing SPSS statistical version 21 software package (SPSS® Inc., USA). The data were presented as mean ± Standard Error of means (S.E.M). Means were significantly differed by applying ANOVA test (Analysis of Variance) for multiple comparisons. P-values less than 0.05 were reflected as statistically significant.
Results
SEM analysis
The results of SEM morphological and nanostructure studies of the Lead nanoparticles are shown in Fig 1. It was shown that a roughly cube and relatively uniform shaped of the lead nanoparticles .28
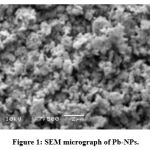 |
Figure 1: SEM micrograph of Pb-NPs. |
XRD analysis
The crystalline structure of the synthesized lead nanoparticles were confirmed by X-ray diffraction (Fig. 2). The X-ray diffraction pattern of the synthesized nanoparticles (Pb-NPs) shows diffraction peaks at 2θ= 19.44º, 24.87º, 30.30º, 34.15º and 42.77º, which can be respectively indexed to (110), (111), (200), (210) and (220) lattice planes. XRD result are presented in Table 1.
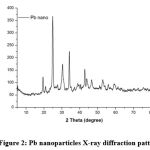 |
Figure 2: Pb nanoparticles X-ray diffraction pattern. |
Table 1: Interplanar spacing, lattice parameters, (XRD) of Pb nanoparticles.
| Diffraction angle [2Ɵ]
(degree) |
FWHM
[] (radian) |
Size
[D] (nm) |
dhkl-spacing
(Å)
|
Diffraction planes
(hkl) |
Relative intensity (%) |
| [19.44] 9.72 | 0.008 | 17.58 | 4.56 | 110 | 22 |
| [24.87] 12.44 | 0.009 | 15.77 | 3.57 | 111 | 100 |
| [30.30] 15.15 | 0.013 | 11.05 | 2.94 | 200 | 35 |
| [34.15] 17.08 | 0.007 | 20.71 | 2.62 | 210 | 55 |
| [42.77] 21.39 | 0.008 | 18.61 | 2.11 | 220 | 27 |
| Average particle size [D] = 16.74 nm | |||||
| Lattice constant [a] = 6.07 Å | |||||
Phytochemical of rocket seeds extract.
Table 2: Total phenols, total flavonoids and DPPH scavenging activity of the extract of rocket seeds
| Test | Total phenols
(mg GAE/g) |
Total flavonoids
(mg CE/g) |
DPPH
(mg TE/g) |
| rocket seeds | 2.792 | 0.805 | 3.488 |
Kidney function and electrolytes level
The results showed that concentration of both urea and creatinine were significantly increase in G3 (rats injected with Pb-NPs) in comparison with both G1 (control) and G2 (RS extract administered rats). After treatment with rocket seeds (G4) the results showed significant decrease in comparison with G3. There were no significant differences in concentration of uric acid in all groups (Table 2). The results also indicated that there were increases in both K+ and Cl– ions concentrations in G3 if compared to both G1 and G2. Moreover, there were decreases in the concentrations of Na+ and Ca+2 ions in G3 if compared with G1 and G2. By RS extract treatment (G4), there were decreases in both K+ and Cl– ions and increases in both Na+ and Ca+2 ions compared to PB-NPs injected rats or G3 (Table 2).
Table 3: Kidney functions and electrolytes levels
| Parameter | G1 | G2 | G3 | G4 |
| Urea (mg/dl) | 28.3±1.70 | 30.2±1.49 | 39.1a±1.37 | 34.2a,b±1.62 |
| Creatinine (mg/dl) | 0.41±0.027 | 0.40±0.034 | 0.88a±0.063 | 0.52a,b±0.036 |
| Uric acid (mg/dl) | 3.26±0.011 | 3.17±0.042 | 3.25±0.015 | 3.24±0.023 |
| K+ (mEq/L) | 4.26±0.27 | 3.99±0.15 | 5.08a±0.33 | 4.75a,b±0.29 |
| Na+ (mEq/L) | 135.5±6.2 | 135.1±8.54 | 129.0 ±7.52 | 133.9 ±8.75 |
| Ca++ (mEq/L) | 1.23±0.043 | 1.24±0.049 | 1.19 ±0.050 | 1.23 ±0.032 |
| Cl– (mEq/L)) | 100.6±7.55 | 100.2±5.40 | 112.8 ±4.20 | 103.2 ±5.66 |
Data are presented as mean ± S.E.M. Wherever, G1, control group; G2, extract of seeds rocket group; G3, Pb-NPs group; G4, PbNPs+RS group. (a) Significant difference in comparison with control group. (b) Significant difference in comparison with the Pb-NPs group.
Influence of PbNPs and rocket seeds on kidney histopathology
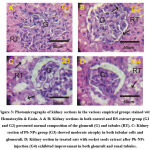
Both kidney sections in (G1) and (G2) groups displayed normal composition of the glomruli and renal tubules in the cortical and medullary parts (Fig. 3A&3B). Kidney section in the patronized rats with Pb-NPs (G3) exposed moderate atrophy in both tubular cells and glomeruli (Fig. 3C). Furthermore; kidney sections in the patronized PbNPs with rocket seeds extract (PbNPs+RS) revealed good amelioration in the glomruli and renal tubules (Fig. 3D).
Impact of rocket seeds on expression of P53
Kidney tissues of the control and rocket seeds rats gave faint positive reactions for P53 expressions in glomruli and renal tubules (Fig. 4A&4B) while; strong positive response for P53 were observed in glomruli and renal tubules in the treated rats with PbNPs group (Fig. 4C). Moreover, mild positive response for expressions of P53 were looked in kidney sections in post-treated PbNPs with rocket seeds group (Fig. 4D).
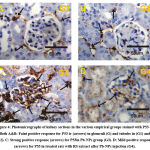 |
Figure 4: Photomicrographs of kidney sections in the various empirical groups stained with P53-ir. Both A&B: Faint positive response for P53-ir (arrows) in glomruli (G) and tubules in (G1) and (G2). |
Discussion
The lead nanoparticles contain high atom density facets (111) which recognized to be highly reactive .29 The high intensity for FCC materials is generally (111) reflection, which is showed in the sample from the most intense peak at 2θ= 24.87º. This confirmed the lattice structure to be FCC (face centered cubic). The intensity of peaks in the Pb-NPs XRD presented that the prepared lead nanoparticles are crystalline. In addition, the broad diffraction peaks elucidated the small size crystallite. The size of the Pb nanoparticles determined from Debye–Scherrer formula is 16.74 nm.
For the bcc texture, the first two sets of principal diffracting planes are (110) and (200) and for the fcc texture are (111) and (200). Based on that and from the XRD of the prepared Pb nanoparticles (Figure 2) there is a mixture of low percent bcc (22%) and the main percent fcc (100%).
Average of the value of unit cell edge (a) calculated from the value of d as obtained from the data for the peaks are found to be 6.07 Å. The lattice constant value (6.07Å) is higher than that of standard Pb nanoparticles (4.92Å) .30 It may be lattice expansion due to this bcc structure at lower angle (19.44) with fcc main structure.
Many different metals including lead are used in the industry. Lead is described as the most beneficial elements in industry but it is the most harmful element on human health. It causes major public health problem. Nowadays, Lead nanoparticles are commonly used in different beneficial uses as ceramic industry. Many negative consequences appear on the workers in the field of ceramic industry .4 This study objected to assess the effects of Pb-NPs toxicity on kidney and evaluate the curative effect of rocket seeds extract against the induced toxicity via estimation of kidney functions and electrolytes with different histological and immunological studies.
Kidneys can be considered as the climacteric organ upon long term vocational or environmental lead exposure. The major route of lead elimination by globular filtration and tubular secretion. Kidneys also is the minor storage site of lead according to extend excessive absorption. Both acute and chronic nephrotoxic results from excessive exposure of lead. This accumulation caused alteration in mitochondrial composition and function which causes disturbance of production of energy deteriorate energy dependent processes as tubular transport results of defecting in proximal tubular reabsorption .31 Renal dysfunction is consequence of excessive exposure of lead. It recognized by glomerular and tubulointerstitial alteration causing renal failure, hypertension and hyperuricemia. 32 Lead induces nephrotoxic by oxidative stress which involved increasing of reactive oxygen species (ROS) which in turn cause membrane, DNA and protein destruction. 33
The result obtained in this study indicated the significant increase in urea and creatinine concentrations in Pb-NPs group (G3) if compared to both control group (G1) and rocket seeds extract administered group (G2). These increases in G3 in comparison with G1 in urea and creatinine concentrations reached to about 1.3 and 2.1 fold increases, respectively. After treatment with RS extract (G4), the results showed significant decrease in both urea and creatinine concentration compared with both G3. These results confirmed the improvement of kidney function caused by rocket seed extract administration. This improvement due to antioxidant, cytoprotective character of rocket seeds that includes phenolic and flavonoid compound, which have highly antioxidant character determined by DPPH analysis. This antioxidant character play against ROS caused by lead accumulation in kidney tissues.13 This ROS antagonist effect of rocket seed can be explained by the presence of Vitamin C, carotenoids, flavonoids and phenolics in RS extract. 14, 33 This DNA damage indicated in the immunohistopathological studies in the form of highly expressed of the tumor suppressor protein (P53) in G3. This was previously reported by different studies .34 The treated group with RS extract (G4) showed relatively mild expression of P53 due its antioxidant and cytoprotictive effect against the toxicity of lead on the kidney tissue.
Our results elucidated that K+ ion concentration significantly increased by about 1.19 folds in rats injected with Pb-NPs (G3) if compared to the control rats (G1). This K+ ion increase was detected in parallel with decreases in Na+ ion concentrations by about 0.95 fold in G3 if comparison to G1. Hyperkalemia and hyponetrenima is the main indicator of lead toxic impact on cell membrane Na-K-ATPase pump activity which lead to decrease in Na concentration and increase in K concentration. The defect of sodium gradient causes dysfunction of the kidney filtration of the blood waste product. Also kidney is unable to reabsorbed amino acid and glucose as well as dysregulating of electrolyte level in the blood .35
From the results, there were decreases in Ca+2 ion concentrations in Pb-NPs injected rats (G3) reached to about 0.96 fold if compared to the control group (G1). This calcium decrease in blood resulted from the failure of the distal tubular cells of the kidney to reabsorb Ca+2 under parathyroid hormone stimulation. Another direct cause was the inhibition of 1-α-hydroxylase (CYP27B1) that is accountable for the conversion of 25-hydroxyvitamin D (25D) to 1,25D. The leading role of 1,25D rectify the low serum ionized calcium by increasing intestinal calcium absorption .36 The decrease of Na+ concentration it lead to indirect inhibition of Na+/Ca+2 exchange, thus, causes increase of intracellular Ca+2 and decrease Ca+2 extracellular .37
Current results revealed that; Pb-NPs induced elevation in P53 and moderate atrophy in glomeruli and renal tubule cells especially in Proximal convoluted tubules and the treatment with rocket seeds have the ability to improve this changes. Our results agree with Tousson et al. who reported that; rockect seed (Eruca sativa) extract have protective role of against monosodium glutamate induceed hepato-renal toxicity in male rats. Monosodium glutamate might have either interfered with creatinine metabolism leading to increased synthesis or the tissues might have compromised all or part of its functional capacity of tubular excretion. 38
Renal dysfunction has several abnormalities and failure among of these is metabolic acidosis. In nephrotoxicity, There is a direct relationship between decline of globular filtration rate (GFR) and decrease in serum bicarbonate due the failure of kidney to synthesis ammonia, absorbed bicarbonate, and excrete hydrogen ions. 39 This failure is due to distal renal tubular. hyperchloremic metabolic acidosis is correlated with a decrease in HCO3 levels in the blood .40 Our results indicated that there were increases in Cl– ion concentrations in blood in G3 reached to about 1.12 fold if compared to G1. These Cl– concentrations increases regressed to its nearly normal values after RS extract treatment in G4.
Conclusion
Lead nanoparticles had very toxic effects on the kidney tissues. It caused destruction of the renal cell results of elevation of urea and creatinine concentration. Pb-NPs in consequence affected on electrolyte hemostasis. The rocket seed extract administration showed beneficial curative effects against renal toxicity induced by Pb-NPs exposure. RS extract administration showed regression of the kidney functions and improvement of electrolytes hemostasis.
Conflict of interest
There is no conflict of interest.
Funding
There is no any received funding
References
- El-Masry T. A, Altwaijry N, Alotaibi B, Tousson E, Alboghdadly A and Saleh A. Chicory (Cichorium intybus L.) extract ameliorates hydroxyapatite nanoparticles induced kidney damage in rats. Pak. J. Pharm. Sci., 2020; 33 (3) :1251-1260 .
- Alotaibi B, Tousson E, El‐Masry T. A, Altwaijry N and Saleh A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environmental Toxicology, 2021; 36 (1):105-113.
CrossRef - Altwaijry N, El-Masry T. A, Alotaibi B. S, Tousson E, Alodhayani A. A, El-Morshedy K, Elmaghed N. A, Sayed A. E and Saleh A. Potential therapeutic effects of avenanthramide-C against lung toxicity caused by silver nanoparticles injection in rats. Pak. J. Pharm. Sci., 2021; 34 (1):337-343.
- Elbossaty W. F. Toxicology, Biological Activity, Synthesis, and Anti-Microbial Effects of Lead Nanoparticles. , 2017; 2: 2-4. DOI: 10.21767/2574-285X.100011
- Flora G, Gupta D and Tiwari A. Toxicity of lead: A review with recent updates Interdisciplinary Toxicology, 2012; 5(2):47–58.
CrossRef - Beltagy D. M, Mohamed T. M, El Said A. S and Tousson E. Beneficial role of ascorbic and folic acids antioxidants against thyroxin-induced testicular dysfunction in hyperthyroid rats. Environmental Science and Pollution Research, 2016; Sep 1; 23(17):17246-17254.
CrossRef - Tousson E, El‐Atrsh A, Mansour M and Abdallah A. Modulatory effects of Saussurea lappa root aqueous extract against ethephon‐induced kidney toxicity in male rats. Environmental Toxicology, 2019; 34(12):1277-1284.
CrossRef - Tousson E, Hafez E, Zaki S, Gad A and Elgharabawy R. M. Evaluation of the testicular protection conferred by damiana (Turnera diffusa Willd.) against amitriptyline-induced testicular toxicity, DNA damage and apoptosis in rats. Biomedicine & Pharmacotherapy, 2020; 132:110819.
CrossRef - Izzularab B. M, Megeed M and Yehia M. Propolis nanoparticles modulate the inflammatory and apoptotic pathways in carbon tetrachloride-induced liver fibrosis and nephropathy in rats. Environmental Toxicology, 2021; 36(1):55-66. DOI: 10.1002/tox.23010
CrossRef - Tousson E, Bayomy M. F and Ahmed A. A. Rosemary extract modulates fertility potential, DNA fragmentation, injury, KI67 and P53 alterations induced by etoposide in rat testes. Biomedicine & Pharmacotherapy, 2018; 98:769-774.
CrossRef - Mutar T. F, Tousson E, Hafez E, Gazia M. A and Salem S. B. Ameliorative effects of vitamin B17 on the kidney against Ehrlich ascites carcinoma induced renal toxicity in mice. Environmental Toxicology, 2020; 35(4):528-537.
CrossRef - Alotaibi B, El‐Masry T. A, Tousson E, Alarfaj S. J and Saleh A. Therapeutic effect of rocket seeds (Eruca sativa ) against hydroxyapatite nanoparticles injection induced cardiac toxicity in rats. Pak. J. Pharm. Sci., 2020; 33, No.4 (Suppl):1839-1845.
- Mohany M, Ahmed M. M and Al-Rejaie S. S. Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants., 2022; 11, 15. https://doi.org/10.3390/antiox11010015
CrossRef - Jaafar N. S and Jaafar I. S. Eruca Sativa Linn.: Pharmacognostical and pharmacological properties and pharmaceutical preparations. Asian J Pharm Clin Res., 2019; 12(3):39-45.
CrossRef - Altwaijry N, El‐Masry T. A, Alotaibi B, Tousson E and Saleh A. Therapeutic effects of rocket seeds (Eruca sativa L.) against testicular toxicity and oxidative stress caused by silver nanoparticles injection in rats. Environmental Toxicology, 2020; 35(9):952-960.
CrossRef - Abdo N. I, Abobakr S. M, Abd El-Wahab A. E, El-Deeb N. M. Superparamagnetic iron oxide nanoparticles with antimicrobial activities: synthesis and characterization of stable dispersion of Fe3O4 in DMSO/ citric acid. Advanced Science, Engineering and Medicine. 2019; 11: 783-788.
CrossRef - Beltagy D.M, Tousson E, Abdo N.I, Izzularab B.M. Protective Effect of Chicory (Chichorium intybus L.) Extract against Renal Toxicity Induced by Magnetite Silver Nanoparticles in Male Rats. Online Journal of Biological Sciences. 2021; 21: 251-260.
CrossRef
- Nath S. S, Chakdar D, Gope G and Avasthi D. K. Journal of Nanoelectronics and Optoelectronics, 2008; 3: 1 4.
CrossRef - Zeheng-Mu M, Sakai I, Ose Y, Sato T, Nagase H, Kito H, Mizue M, One K and Nakane H. Antimutagenic activity by medicinal plants in traditional Chinese medicines. Shoy.Akugaku.Zasshi, 1990; 44(3): 225-229.
- Cheok C.Y, Chin N. L, Yusof Y. A. Law Extraction of total phenolic content from Garcinia mangostana Linn. Hull. I. Effects of solvents and UV–Vis spectrophotometer absorbance method, Food Bioprocess Technol 2012; 5(7): 2928-2933.
CrossRef - Zhishen J, Mengcheng T, Jianming W. The determination of ûavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chemistry 1999; 64: 555-559.
CrossRef - Saggu S, Sakeran M. I, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food and chemical toxicology. 2014 Oct 1; 72:138-146.
CrossRef - Mailafiya M. M, Abubakar K, Chiroma S. M, Danmaigoro A, Abdul Rahim E. B, Moklas M. A. M and Zakaria Z. A. Curcumin-loaded cockle shell-derived calcium carbonate nanoparticles:A novel strategy for the treatment of lead-induced hepato-renal toxicityin rats. Saudi Journal of Biological Sciences, 2020; 27:1538–1552.
CrossRef - Abd Eldaim M. A, Tousson E, El Sayed I. E, Abd El A. E and Elsharkawy H N. Grape seeds proanthocyanidin extract ameliorates Ehrlich solid tumor induced renal tissue and DNA damage in mice. Biomedicine & Pharmacotherapy, 2019; Jul 1; 115:108908.
CrossRef - Tousson E. Histopathological alterations after a growth promoter boldenone injection in rabbits. Toxicology and industrial health, 2016; 32(2):299-305.
CrossRef - Tousson E , Hafez E, Zaki S, Gad A. P53, Bcl-2 and CD68 expression in response to amethopterin-induced lung injury and ameliorating role of l-carnitine. Biomedicine & Pharmacotherapy, 2014; 68(5): 631-639.
CrossRef - Tousson E, Hafez E, Zaki S and Gad A. The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environmental Science and Pollution Research, 2016; 23(20):20600-20608.
CrossRef - Akimov D.V, Andrienko O. S, Egorov N. B, Zherin I.I, Usov V. F. Synthesis and properties of lead nanoparticles. Russian Chemical Bulletin. 2012; 61: 225-229.
CrossRef - Ruparelia J. P, Chatterjee A. K, Duttagupta S. P and Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia, 2008; 4(3):707-716.
CrossRef - Davey W. P. Precision Measurements of the Lattice Constants of Twelve Common Metals. Phys. Rev., 1925; 25:753-761.
CrossRef - Alasia D. D. Lead nephropathy: revisiting an overlooked cause of kidney disease. Nephrology Reviews, 2010; 2:e8:35-41. DOI: 10.4081/nr.2010.e8 ·
CrossRef - Rastogi S. K. Renal effects of environmental and occupational lead exposure. Indian J Occup Environ Med., 2008; 12(3):103–106. doi: 10.4103/0019-5278.44689
CrossRef - Patra R. C, Rautray A. K and Swarup D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Veterinary Medicine International, 2011:1-9. doi:10.4061/2011/457327
CrossRef - Xu J, Lian L, Wu C, Wang X, Fu W and Xu L. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol., 2008; May; 46(5):1488-1494.
CrossRef - Gennari F. J and Segal A. S. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney International, 2002; 62:1–9.
CrossRef - Hill Gallant K. M and Spiegel DM. Calcium Balance in Chronic Kidney Disease. Curr Osteoporos Rep., 2017; 15(3):214–221.
CrossRef - Pirahanchi Y, Jessu R and Aeddula N. R. Physiology, Sodium Potassium Pump StatPearls Publishing: Treasure Island (FL). 2019.
- Tousson E, El-Atrash A, Karson Y. Protective role of rockect seed (Eruca sativa) extract against monosodium glutamate-induced hepato-renal toxicity in male rats. Asian Journal of Research in Medical and Pharmaceutical Sciences. 2019:1-0.
CrossRef - Dobre M, Rahman M and Hostetter T. H. Current Status of Bicarbonate in CKD. J Am Soc Nephrol., 2015; 26:515–523.
CrossRef - Sharma S, Hashmi M. F and Aggarwal S. Hyperchloremic Acidosis. StatPearls Publishing LLC. ;2020.