Marwa I. Hegazy1 , Aman M. Asaad1
, Aman M. Asaad1 , Lila A. Rashed2, Hanaa H. Ahmed3,4
, Lila A. Rashed2, Hanaa H. Ahmed3,4
1Zoology Department, Faculty of Science, Cairo University, Cairo, Egypt
2Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Cairo University, Cairo, Egypt
3Hormones Department, Medicine and Clinical Studies Research Institute, National Research Centre, Giza, Egypt
4Stem Cell Lab., Center of Excellence for Advanced Sciences, National Research Centre, Giza, Egypt
Corresponding Author E-mail: hanaaomr@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2346
Abstract
In spite of the enormous evolution of the novel anti-seizure medications, about one-third of epilepsy patients stay resistant to the existing therapeutic drugs. Stem cells have provoked hopeful for treating diverse neurologic diseases comprises epilepsy. The rational of this investigation was to appraise the therapeutic intervention of a combination of levetiracetam (LEV) with rodent adipose-derived mesenchymal stem cells (ADMSCs) or rodent bone marrow-derived mesenchymal stem cells (BMMSCs) in counteracting pilocarpine-induced acute epilepsy in rats. In this research, the isolation and preparation of ADMSCs and BMMSCs from male albino rats were carried out. The identification of ADMSCs and BMMSCs was performed morphologically in the culture by using the inverted microscope and by the detection of the cell surface profile by using the flow cytometry technique. The induction of acute epilepsy was achieved by intraperitoneal injection of a single dose of pilocarpine (380 mg/kg b.wt). This study was conducted on fifty six adult male albino rats which were assigned into seven equal groups (8 rats/group); Group (1): Control, Group (2): Epileptic, Group (3): Epileptic + LEV (300 mg/kg b.wt daily for 12 weeks by gastric intubation with an oral gavage), Group (4): Epileptic + ADMSCs (single dose of ADMSCs; 3 × 106 cells/rat; intravenously), Group (5): Epileptic + BMMSCs (single dose of BMMSCs 3 × 106 cells/rat; intravenously), Group (6) : Epileptic + ADMSCs + LEV and Group (7) Epileptic + BMMSCs + LEV. After the end of the experimental period (12 weeks), all rats were tested by the mean electric shock (MES) test to prove the presence of strong seizures in the epileptic group and the modulation of these seizures after treatments. Then, the rats were decapitated and the whole brain of each rat was dissected into two halves, the first half was used for the quantitative determination of GABA, glutamate, dopamine, bFGF, BDNF, IL-6 and TNF-α, while the second half was fixed in formalin saline for histological investigation. The findings of the present work demonstrated that the morphological appearance of the isolated MSCs manifests spindle-shape. The flow cytometric analysis showed that the isolated MSCs are positive for CD90 and negative for CD14 and CD45. The homing of MSCs in the brain tissue of the treated rats was verified by their staining with the fluorescent dye. The recordings of the MES indicated the presence of strong seizures in the epileptic rats which were ameliorated after treatment with LEV, ADMSCs, BMMSCs. The level of brain GABA decreased significantly in the epileptic rats, whereas the level of brain glutamate, dopamine, bFGF, BDNF, IL-6 and TNF-α increased significantly; these alterations were improved after treatment with LEV or ADMSCs or BMMSCs. The histological examination of the brain tissue of the epileptic rats showed great histopathological alterations which were amended by the different treatment options. The combined treatment of either ADMSCs or BMMSCs with LEV displayed superior advantageous effect versus the single use of each type of cell in combating the acute phase of epilepsy. In conclusion, the outcomes of the present approach disclosed that the combined treatment of either ADMSCs or BMMSCs with the antiepileptic drug LEV has synergistic effect in alleviation of the behavioral and biochemical aberrations as well as brain histological deformation during the acute phase of epilepsy.
Keywords
Acute Epilepsy; Biochemical Parameters; Brain Histology; levetiracetam; Mesenchymal Stem Cells
Download this article as:| Copy the following to cite this article: Hegazy M. I, Asaad A. M, Rashed L. A, Ahmed H. H. Combined Treatment of Levetiracetam and Mesenchymal Stem Cells Reverses Behavioral and Biochemical Aberrations in the Acute Phase of Experimental Epilepsy. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Hegazy M. I, Asaad A. M, Rashed L. A, Ahmed H. H. Combined Treatment of Levetiracetam and Mesenchymal Stem Cells Reverses Behavioral and Biochemical Aberrations in the Acute Phase of Experimental Epilepsy. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3MivvBX |
Introduction
Epilepsy is a long-standing neurologic ailment with a series of conditions involving several kinds of seizures, syndromes and comorbidities1. Seizures in epilepsy are frequent paroxysmal phenomena, manifested by stereotyped behavioral modifications that reflect the behind neural mechanisms of the disease2. Also, epileptic seizures may arise after an acute injury of the central nervous system (CNS). The acute symptomatic or prompted seizures are considered as acute manifestations of the insult3, but they may not reoccur when the implied reason has been uncovered or the acute phase has elapsed4. The epileptic seizures arise in specific areas of the brain and they may persist enclosed confined to their area of origin (“focal” or “partial” seizures), or diffused to the whole cerebral hemispheres (“generalized” seizures)5.
Epilepsy is considered as one of the most prevalent critical brain situation, impacting over 70 million individuals all over the world. Its incidence has a bimodal distribution with the highest risk in infants and children6. More and more recent studies have referred to epilepsy as one of the most prevalent neurological disease, affecting 0.5-1% of the population globally of all ages, races, social classes, and geographical locations2,7.The integrated incidence rate of epilepsy is 61.4 per 100.000 persons /year and this incidence is higher in lower middle – income nations than in higher income countries8.Commonly, the cause of epilepsy is unknown, but sometimes the origin of epilepsy may be associated with stroke, brain injury, brain tumors, or infections (such as meningitis, brain abscess, AIDS). Exposure to some particular drugs, atypical sodium/glucose levels in blood, or hyperpyrexia can be sometimes linked to impermanent cause of seizures9.
Currently, the antiepileptic drugs (AEDs) are the main treatment method for epileptic patients, and it was reported that, approximately two third of the epileptic seizures are controlled by the AEDs but about 30% of epilepsy cases are resistant to that treatment10. One of the most common second-generation anti-epileptic drugs is called levetiracetam (LEV) which was approved by FDA in 1999 as a drug for focal epilepsy in adults11. Levetiracetam acts via binding to synaptic vesicle protein 2A, which minimizes the rate of calcium-dependent vesicular neurotransmitter liberation12. Levetiracetam is characterized by rapid absorption and high bioavailability. Additionally, LEV is not metabolized by the cytochrome P450 system and it has a low protein binding rate, which indicates that there is no competition for protein binding sites with other drugs13. Kidneys excrete about two-third of the LEV dose in its native form owing to its efficiency and safety profile. However, the long use of the AEDs can lead to undesirable side effects10. The other available treatments for epilepsy like epilepsy surgery, and vagal nerve excitation are either not convenient in all patients or only slightly efficient and they may cause adverse events such as brain damage14. So, the current treatment options for epilepsy are not satisfactory, therefore there is an urgent need for replacement therapy having a therapeutic potential and minimal side effects.
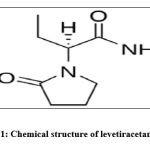 |
Scheme 1: Chemical structure of levetiracetam (LEV) |
Stem cell therapy has been proposed as a possible alternative and a potential breakthrough in the treatment of epilepsy15,16 with minimum adverse effects. Stem cells can treat both the biologic and psychiatric outcomes of epilepsy15. Mesenchymal stem cells (MSCs) have become the most employed adult stem cells in the regenerative medicine17,18 as they are able for self-renewal and multilineage differentiation19. MSCs can be derived from adipose tissue (AD), bone marrow (BM), the umbilical cord and several other adult tissues20. However, MSCs derived from adipose tissue (ADMSCs) and MSCs derived from bone marrow (BMMSCs) are the commonly MSCs used in the regenerative medicine owing to their ease of harvesting and conceivable autologous application, relatively low immunogenicity, good availability, and lack of ethical concerns16, 21. Numerous researches demonstrated that BMMSCs share with ADMSCs in the morphological characteristics and the expression of cell surface antigen, but considerable biological variations have been detected regarding their proliferation rates22,23, cytokines and chemokines receptor expression and differentiation capacities24. A growing body of evidence demonstrated the efficacy of MSCs in the management of diverse neurodegenerative diseases like Alzheimer‘s disease, multiple sclerosis, stroke, and brain injury in animal models25. Also, many investigators have considered MSCs as a hopeful curative option for the treatment of epilepsy26,27. However, little information is available about the therapeutic potential of rodent MSCs in the acute phase of experimental epilepsy. Also, the combination of anti-epileptic drug and rodent ADMSCs or BMMSCs is not clearly described and entails to be clarified. Therefore, this approach was constructed to appraise the therapeutic outcome of a combined treatment of LEV and ADMSCs or BMMSCs against pilocarpine-induced acute phase of epilepsy in rats and to explore the possible mechanisms underlying this action. Thus, a set of relevant brain biochemical indices was investigated and histomorphological features of different brain areas were examined to achieve these purposes.
Material and Methods
Isolation and propagation of bone marrow mesenchymal stem cells
Bone marrow mesenchymal stem cells were isolated from the bone marrow of the tibias and femurs of two-month-old (120 g) male albino rats of Wistar strain. Briefly, three rats were sacrificed by decapitation and swiped with 70% ethyl alcohol followed by sterilized dissection. The tibias and femurs were excised and cleaned from muscles. Bone marrow was flushed out of the epiphysis and suspended in Dulbecco’s modified Eagle’s medium [DMEM; Biowest, USA] supplemented with heparin (5 U/ml) to prevent clotting. Marrow in DMEM was centrifuged at 1800xg for 20 min to harvest the mononuclear cells. The cell pellet was resuspended with complete DMEM culture medium supplemented with 10% fetal bovine serum [FBS; Biowest, USA] and 1% Pencillin-Streptomycin [Biowest, USA]28. Cell counting was done after addition of trypan blue dye in an automatic cell counter (Life Technologies, USA) and was then seeded in 25 cm2 flask with a cell concentration of 1×106 cells/ml. The culture flasks were maintained in a humid condition at 37°C with 5% CO2. The culture medium was replaced every 3 days for removing of the non-adherent cells. Culture flasks were examined continuously using inverted microscope (Olympus, Japan).
When cultures approximately reached the confluence of 70-80% under the inverted optical microscope, the adherent cells were passaged using trypsin/ethylene diamine tetra acetic acid (EDTA) solution. Firstly, cultured cells were cleaned two times with phosphate buffer saline [PBS, Biowest, USA]. Then, trypsinization of the cells was done using 0.25% trypsin in 1 ml EDTA [Biowest, USA] for 5 min at 37oC. After centrifugation at 1800xg for 30 min, these cells were subcultured into 25 cm2 flasks with complete culture medium. This continued culturing step led to a removal of non-adherent cells (non-BMMSCs) and this allows for the progressive purification of the BMMSCs. These cells were nominated passage 1 which were harvested using trypsin-EDTA solution and passaged to subculture again. When the adhesive cells in passage 2 reached the confluence of 70-80%, passage 3 BMMSCs was taken on.
Isolation and propagation of adipose mesenchymal stem cells
Adult male albino Wistar rats aging three months old and weighing between 250 and 300 g were sacrificed for fat harvesting. Adipose tissue was collected surgically from rat omentum and incised with scissors and cut into small fragments and carefully washed 3-4 times with equal volume of phosphate buffer saline PBS. Then, they were immersed in a solution of 0.2% collagenase type 1 (Sigma-Aldrich, USA) in PBS (200 mg/100 ml) then incubated for 60 min. at 37oC in shaker incubator and centrifuged at 1000xg for 10 min.
10% FBS DMEM medium was used for inactivation of collagenase, then the mixture was transferred into 50 ml falcon tube and centrifuged at 1000xg for 10 min at room temperature to isolate the oil and the residual fat lobules from the stromal vascular fraction (SVF). Floating adipocytes, lipids and liquids were aspirated leaving SVF pellet which was processed with 160 mM NH4CL at room temperature for 10 min to
lyse RBCs. Then, centrifugation of this mixture was done at 400xg for 10 min and employed for a Ficoll gradient (Sigma-Aldrich, USA) to refine the mononucleated cells. Centrifugation at 1000xg for 30 min was taken place and gradient separated cells were cleaned two times with PBS and centrifuged at 400xg for 10 min between each wash. Cells pellet was resuspended in PBS, filtered and centrifuged at 400xg for 10 min. The isolated stromal vascular fraction was filtered, centrifuged and then the pellet was resuspended in DMEM containing 10% FBS and 1% pencillin-streptomycin solution29. Cell counting was done after addition of trypan blue dye in an automatic cell counter and was then seeded in 25 cm2 flask with a cell concentration of 1×106 cells/ml.
After 24 hours of culturing, the non-adherent cells in the flask were discarded by PBS washing. The cultures were preserved at sub-confluent levels at 37 oC incubator with 5% CO2 and passaged with trypsin /EDTA, when demanded as mentioned previously.
Identification of MSCs
Cell morphology
The resulting cultured cells were investigated morphologically under the inverted optical microscope (Olympus, Japan) to ensure the identity of the MSCs.
Surface markers profile
Flow cytometry analysis was used for detection of the cell surface markers of MSCs (CD90, CD14 and CD45) to assure whether the derived AD-MSCs and BM-MSCs preserve their phenotype after culture expansion30. The FITC conjugated-CD90 antibody was purchased from Immunotech SAS, (France) whereas, the PE-conjugated CD14 and PE-conjugated CD45 antibodies were obtained from R & D systems, (UK and Germany respectively). Briefly, the incubation of the cells with the antibody against each of the surface markers was done for 30 min at 4 ºC in case of CD90 and 10 min at 4 ºC for CD14 and CD45 followed by flow cytometry analysis (Beckman Coulter Elite XL, USA).
Staining of MSCs with PKH26 dye for Verification of Accommodation
The third passage of MSCs was collected and marked with PKH26 dye by using the PKH26 Fluorescent Cell Linker kits (Sigma-Aldrich, USA) following to the manufacturer’s manual to clarify their homing in acute epileptic rat brain.
Animal’s handling
Fifty-six male albino Wistar rats weighing 220-240 g were procured from the Unit of the Animal Care of the National Research Centre, Giza, Egypt and acclimated in a definite space when the temperature (25 ± 1) and humidity (55%). Rats were monitored continuously with a 12 h light/dark cycle at the National Research Centre and they had free access to water and food. Rats were cared for in accordance with the guidelines for the animal experiments which were approved by the Ethical Committee of Medical Research at the National Research Centre, Giza, Egypt (Approval No 14/018).
Experimental procedures
Animal grouping
In this study, the rats were classified into 7 equal groups (8 rats/group) as follows:
Group 1: Control group healthy normal rats.
Group 2: Epileptic group; the rats were injected intraperitoneally with methylscopolamine (1mg/kg b.wt)31 30 min prior the intraperitoneal pilocarpine injection (380 mg/kg b.wt)32 . After that, all rats received two doses of diazepam separated by one hour (each 4 mg/kg b.wt orally) 33 30 min after seizure onset. These rats were sacrificed after 24 hours from final administration34.
Group (3): Epileptic group treated with levetiracetam (Epileptic + LEV); the rats were administered orally with levetiracetam 300 mg /kg b.wt., after induction of acute phase of epilepsy, (24 hours) 35.
Group (4): Epileptic group treated with ADMSCs (Epileptic + ADMSCs); the rats were intravenously infused with a single dose of ADMSCs (3 × 106 cells/rat)36, after induction of acute phase of epilepsy.
Group (5): Epileptic group treated with BMMSCs (Epileptic + BMMSCs), the rats were intravenously infused with a single dose of BMMSCs (3 × 106 cells/rat) 37, after induction of acute phase epilepsy.
Group (6): Epileptic group treated with ADMSCs and LEV (Epileptic + ADMSCs + LEV), the rats were intravenously infused with a single dose of ADMSCs (3 × 106cells/rat) then they were orally administered with LEV 300 mg /kg b.wt./day for 12 weeks35 after induction of acute phase of epilepsy.
Group (7): Epileptic group treated with BMMSCs and LEV (Epileptic + BMMSCs + LEV), the rats were intravenously infused with a single dose of BMMSCs (3 × 106cells/rat), then they were orally administered with LEV 300 mg/kg b.wt./day for 12 weeks35 after induction of acute phase of epilepsy.
 |
Scheme 2: Graphical representation of the experimental groups |
For MSCs transplantation, the epileptic rats were deeply anesthetized via isoflurane (1–3%) inhalation and MSCs either isolated from adipose tissue or bone marrow were suspended in 100 µl PBS before infusion and then slowly infused into the tail vein in 5 min with a 27G needle. The needle was maintained in tail vein for other 5 min to prevent regurgitation and then withdrawn.
Mean Electric Shock (MES) Test
This test was principally realized according to Kitano et al.38 and Krall et al.39. All rats in the different studied groups were tested by the mean electric shock test to prove the presence of strong seizures in the epileptic group and the modulation of these seizures after treatments. An increased electric current was applied via an ear electrode, initial current of 1 mA, increment of 0.1 mA/0.2 s and at 50 Hz (Ugo Basil, ECT Unit, 57800 , Italy).Tonic extension of the hind limbs is taken as the end point. The mean convulsion threshold was considered as the mean of the maximal electric current which could be tolerated by the animal at the end point40. The obtained values represent a mean of that of eight rats (±standard error). The rats in the control group were also subjected to this test for comparison.
Brain tissue sampling
After the period of 12 weeks, the end of the experimental process, the rats were fasted for about 10 hours and then, they were submitted to anesthesia to be sacrificed by decapitation and the whole brain of each rat was quickly and carefully dissected, cleaned gently with isotonic saline and blotted dry. Then, each brain was partitioned into two longitudinally equal halves, the right half was immediately stored at -80oC pending the biochemical determinations while, the left half was fixed in formalin buffer (10%) for histomorphological investigation.
Preparation of brain homogenate
The first half of each brain was weighed and homogenized to give 10% (w/v) homogenate in ice-cold medium containing 50 mM Tris-HCl and 300 mM sucrose, pH: 7.441. The homogenates were centrifuged at 1800xg for 10 min in cooling centrifuge at 4 oC. The supernatants (10%) were stored in aliquots in -20 oC and used for the biochemical determinations [gamma aminobutyric acid (GABA), glutamate, dopamine, basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6) and tumor necrosis factor- α (TNF-α)].
Biochemical determinations
Brain gamma aminobutyric acid (GABA), glutamate, dopamine, basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were determined in brain homogenate by ELISA using kits obtained from Glory Science Co., USA, according to the recommendation of the manufacturer provided with the kits.
Histomorphological procedure
Twenty-four hours after fixation of brain tissue in 10% formalin buffer, washing was done in tap water, then, serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in molten paraffin wax at 56 oC in hot air oven for twenty-four hours. Paraffin wax tissue blocks were prepared for sectioning at 4 microns thick slices by rotary microtome. The obtained tissue sections were collected on glass slides, deparaffinized, stained by hematoxylin & eosin stain for routine examination through the optical microscope (Leica DM2500 & DM2500 LED, Germany)42.
Statistics
In the current investigation, all results were expressed as Mean ± standard error (SE) of the mean. Data were analyzed by one way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) program version 20, followed by least significant difference (LSD) to compare the significance between groups. The difference was considered significant when P value was <0.05.
Results
Microscopic photographs of MSCs
The photomicrographs presented in Fig. (1) showed the morphology of cells derived from rat adipose tissue (A) and bone marrow (B) at the third passage. Images indicate that MSCs isolated from rat adipose tissue and bone marrow have obvious characteristic of spindle-shaped as fibroblast-like morphology cells.
Surface marker profile of MSCs
Flow cytometry analysis showed that the isolated ADMSCs are positive for CD90 (88.1%), negative for CD14 (4.20 %) and CD45 (6.70%) (Fig. 2A, B, C). Also, the results of flow cytometry analysis revealed that the isolated BMMSCs are positive for CD90 (86.3%), negative for CD14 (6.59%) and CD45 (7.50%).(Fig. 2D, E, F).
Homing manifestation using PKH26 Dye
Fluorescence microscope investigation of brain tissue sections of rats treated with ADMSCs (Fig. 3 (A), BMMSCs (Fig. 3 (B), ADMSCs + LEV (Fig. 3 (C) and BMMSCs + LEV (Fig. 3 (D) displayed that the MSCs are stained with PKH26 dye. They appeared in the different brain areas of ADMSCs, BMMSCs, ADMSCs + LEV and BMMSCs + LEV treated rats making sure of MSCs homing in the damaged areas of the epileptic rat brain.
Behavioral assessment
After pilocarpine injection, the rats were closely observed and the behavioral changes were recorded. During the first 5 minutes after pilocarpine administration, the rats evolved diarrhea and other manifestations of cholinergic excitation. After 15-20 minutes following pilocarpine administration, the rats displayed head bobbing, scratching, chewing, and exploratory behavior. Repeated seizures started around 40-50 minutes after pilocarpine administration, which lasted for 1-2 days, this period was described as the acute phase of epilepsy43, 44.
Mean Electric Shock (MES) recordings
The results of Table (1) visualized a significant (p˂0.05) decrease in the MES test of the epileptic rats in comparison with that of the control counterparts. Whereas, the MES test recorded significant (p˂0.05) enhancement, in the epileptic rats treated with LEV, or ADMSCs or BMMSCs, respectively, versus rats administered pilocarpine alone (epileptic rats). Also, the MES test registered significant (p˂0.05) elevation in the epileptic rats treated with either ADMSCs + LEV or BMMSCs + LEV, when compared to the results of the rats administered pilocarpine alone.
The data in Table (1) also revealed that there is a great difference in MES records between the group of the epileptic rats treated with ADMSCs and those treated with BMMSCs; the leading effect has been observed in the group of epileptic rats treated with ADMSCs. However, the groups of epileptic rats treated with of ADMSCs + LEV or with BMMSCs + LEV showed an almost similar effect in MES recordings in (Table 1). The preferable outcomes have been demonstrated in the epileptic rats treated with ADMSCs + LEV (Table 1).
Biochemical outcomes
The data in Table (2) showed that during the acute phase of epilepsy, 24 hours after the administration of pilocarpine, the level of brain GABA of the epileptic rats decreased (p˂0.05) significantly, while the levels of brain glutamate and dopamine increased (p˂0.05) significantly when compared to the findings of the control rats. Upon treatment of epileptic rats with LEV or ADMSCs or BMMSCs, the level of brain GABA increased (p˂0.05) significantly, with respect to the findings of the epileptic rats. Significant decrease in the level of brain glutamate, as well as in the level of brain dopamine were recorded in the same treated groups, in comparison with the data of the epileptic group. It could be observed that ADMSCs showed an almost similar effect on the brain neurotransmitters like that of the antiepileptic drug Levetiracetam, and the effect of BMMSCs was lower than that of the ADMSCs.
Table (2) showed also that treatment of the epileptic rats with ADMSCs + LEV or BMMSCs + LEV yields significant elevation (p˂0.05) in the level of brain GABA and, and significant decrease in the level of brain glutamate respectively), as well as, in the level of brain dopamine, relative to the results of the epileptic rats. It could be observed that the effect the combined treatment of ADMSCs + LEV or BMMSCs + LEV was close to the effect of either ADMSCs or BMMSCs alone regarding brain neurotransmitters levels, since insignificant changes were recorded in the levels of brain GABA, glutamate and dopamine after administration of either ADMSCs + LEV or BMMSCs + LEV, in comparison with the findings of either ADMSCs or BMMSCs alone. The effect of ADMSCs + LEV on brain GABA, glutamate and dopamine levels was higher than the effect of BMMSCs + LEV.
Table (3) showed that in the acute phase of epilepsy, 24 hours after pilocarpine administration (epileptic group), the level of brain bFGF and brain BDNF increased (p˂0.05) significantly, versus the result of the control group. When the three groups of epileptic rats were treated with LEV or ADMSCs or BMMSCs, a significant reduction was recorded in the levels of brain bFGF as well as in the level of brain BDNF, when compared with the results of the epileptic rats. It could be demonstrated that the effect of the ADMSCs was similar to the effect of anti-epileptic drug LEV on the levels of brain bFGF and BDNF, and the effect of ADMSCs was more pronounced than the BMMSCs. Table (3) showed also that the treatment of the epileptic rats with either ADMSCs + LEV or BMMSCs + LEV, caused a significant depletion (p˂0.05) in level of brain bFGF and BDNF, relative to the results of the epileptic rats. It could be noticed that the effect of the combined treatment with either ADMSCs + LEV or BMMSCs + LEV, on the level of brain bFGF and brain BDNF was nearly similar to the effect of each type of MSCs alone since insignificant difference (p ˃ 0.05) was recorded between the results of the combined treatment of ADMSCs + LEV or BMMSCs + LEV and the results of each type of MSCs alone. Moreover, the data of Table (3) indicated that the influence of BMMSCs + LEV, on brain bFGF and brain BDNF was lower than that of the effect of ADMSCs + LEV.
The data depicted in Table (4) revealed that in the acute phase of epilepsy, 24 hours after administration of pilocarpine (epileptic group), the levels of brain IL-6 and brain TNF-α were increased (p˂0.05) significantly, when compared to the results of the control group. When the epileptic groups were treated with either LEV, ADMSCs or BMMSCs, significant decreases (p˂0.05) were recorded in the level of brain IL-6, and also in the level of brain TNF-α versus the results of the epileptic group. It could be detected that the two types of MSCs showed roughly similar effect on the level of brain IL-6 and brain TNF-α like that of antiepileptic drug Levetiracetam, and the effect of ADMSCs on brain IL-6 and brain TNF-α was higher than that of BMMSCs.
After 24 hours of administration of pilocarpine (acute phase of epilepsy), the treatment with either ADMSCs + LEV or BMMSCs + LEV produced significant decrease (P˂0.05) in the level of brain IL-6, respectively, and also in the level of brain TNF-α, in respect with the findings of the epileptic group (Table 4). Thus, there is an insignificant difference (P˃0.05) between the effect of ADMSCs + LEV and BMMSCs + LEV on brain IL-6 and brain TNF-α levels.
Histomorphological observations
The histomorphological description of brain tissue section of rat in the control group revealed normal histological structure of the cerebral cortex and covering meanings, subiculum neuronal cells in the hippocampus as well as striatum and neurons of cerebellum (Fig. 4). While, histomorphological examination of brain tissue section of rat in the epileptic group showed degeneration and atrophy in the neuronal cells of subiculum in hippocampus, degeneration and congestion blood vessels in the neuronal cells of subiculum of hippocampus, degeneration and atrophy in the neuronal cells of fascia dentata in hippocampus, focal haemorrhage in the striatum of the cerebrum, congestion in blood vessels in the striatum of the cerebrum, focal eosinophilic plaques formation in the striatum of the cerebrum and congestion in blood vessels of the cerebellum (Fig. 5).
Histomorphological examination of brain tissue section of rat in the group treated with LEV showed focal gliosis of the cerebral cortex, congestion in blood vessels of cerebral cortex, normal histological structure of subiculum neurons in hippocampus, neuronal degeneration in the striatum and focal eosinophilic plaques formation in the striatum (Fig. 6). Furthermore, histomorphological examination of brain tissue sections of rat in the group treated with ADMSCs showed congestion in the blood vessels of cerebral cortex, congestion in the blood vessels of cerebral cortex, normal histological structure of subiculum neurons of hippocampus and normal histological structure of fascia dentata in hippocampus (Fig.7). Histomorphological examination of brain tissue section of rat in the group treated with BMMSCs showed nuclear pyknosis in neuronal cells of cerebral cortex and subiculum of hippocampus as well as fascia dentata of hippocampus. In addition to, focal eosinophilic plaques formation in the striatum (Fig. 8). Moreover, histomorphological examination of brain tissue section of rat in the group treated with ADMSCs + LEV showed nuclear pyknosis in neuronal cells of cerebral cortex, normal histological structure of subiculum neurons in hippocampus, normal histological structure of fascia dentata and hilus neurons in hippocampus and focal eosinophilic plaques formation in the striatum (Fig. 9). Finally, histomorphological examination of brain tissue section of rat in the group treated with BMMSCs + LEV showed congestion in cerebral cortical blood vessels, normal histological structure of neurons of subiculum, fascia dentata and hilus neurons in hippocampus and focal eosinophilic plaques formation in the striatum (Fig. 10).
Table 1: Influence of treatment with rat BMMSc or rat ADMSc with or without LEV on the MES values of acute epileptic rat model.
|
Group Number |
Groups |
(MES) values |
| Group 1 | Control | 11.0±0.087 |
| Group 2 | Epileptic | 4.3±0.61*
(-60.9%)a |
| Group 3 | Epileptic + LEV | 10.8±0.40**
(-1.8)a (151%)b |
| Group 4 | Epileptic + ADMSc | 10.0±0.71**
(132.5%)b (20%)f (42.8%)g |
| Group 5 | Epileptic + BMMSc | 7.0±0.26**
(62.9%)b (-2.7%)e |
| Group 6 | Epileptic + ADMSC + LEV | 8.3±0.33**
(93%)b (-23%)c |
| Group 7 | Epileptic + BMMSc + LEV | 7.2±0.37**
(67.44%)b (-33%)c (-13%)d |
MES: Mean electric shock.
LEV: Levetiracetam
ADMSCs: Adipose tissue- derived mesenchymal stem cells
BMMSCs: Bone marrow-derived mesenchymalstem cells
Data were represented as Mean ± SE
Data were analyzed using One-way ANOVA followed by Tukey’s multiple comparisons test
Significant change at p˂ 0.05 between epileptic group and the control group*
Significant change at p˂ 0.05 between epileptic group and all the treated groups**
a Percent of change between epileptic group and the control group
b Percent of change between epileptic group and all the treated groups
c Percent of change between Levetiracetam (LEV) and ADMSCs +LEV as well as BMMSCs+ LEV
d Percent of change between ADMSCs +LEV and BMMSCs+ LEV
e Percent of change between BMMSCs+ LEV and BMMSCs
f Percent of change between ADMSCs +LEV and ADMSCs
g Percent of change between BMMSCs and ADMSCs
Table 2: Influence of treatment with rat BMMSc or rat ADMSc with or without LEV on brain GABA, glutamate and dopamine levels of acute epileptic rat model.
| Group Number | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7
|
| Group / parameters | Control | Epileptic | Epileptic + LEV | Epileptic +ADMSCs | Epileptic +BMMSCs | Epileptic +
ADMSCs + LEV |
Epileptic +
BMMSC + LEV |
| GABA µmol/g) | 4.18±0.50 | 1.26±0.21*
(-69.86%)a |
3.46±0.41**
(-17.22%)a (+174.60%)b |
3.24±0.61**
(-22.49%)a (+157.14%)b |
2.43±0.32
(-41.87%)a (+92.87%)b (-25.0%)g |
3.18±0.08**
(-23.92%)a (+152.38%)b (-8.09%)c (-1.85%)f |
2.59±0.26
(-38.04%)a (+105.55%)b (-25.14%)c (+6.58%)e (-18.59%)d |
| Glutamate (µmol/g) | 5.30±0.81 | 11.24±1.48*
(+112.07%)a |
5.71±1.14**
(+7.73%)a (-49.20%)b |
5.97±0.48**
(+12.64%)a (-46.89%)b |
7.13±0.55**
(+34.53%)a (-36.56%)b (+19.44%)g |
6.14±0.64**
(+15.85%)a (-45.37%)b (+7.53%)c (+2.45%)f |
6.69±0.47**
(+26.3%)a (-40.48%)b (+17.16%)c (+6.17%)e (+8.96%)d |
| Dopamine (ng/g) | 984±108 | 1911±238*
(+94.21%)a |
1051±159**
(+6.81%)a (-45.0%)b |
1136±89.4**
(+15.45%)a (-40.55%)b |
1248±110**
(+26.83%)a (-34.69%)b (+9.86%)g |
1163±42.3**
(+18.19%)a (-39.14%)b (+10.65%)c (+2.38%)f |
1189±90.2**
(+20.83%)a (-37.78%)b (+13.13%)c (-4.73%)e (-2.33% )d |
GABA: Gamma aminobutyric acid
LEV: Levetiracetam
ADMSCs: Adipose tissue- derived mesenchymal stem cells
BMMSCs: Bone marrow-derived mesenchymalstem cells
Data were represented as Mean±SE
Data were analyzed using One-way ANOVA followed by Tukey’s multiple comparisons test
Significant change at p˂ 0.05 between epileptic group and the control group*
Significant change at p˂ 0.05 between epileptic group and all the treated groups**
a Percent of change between epileptic group and the control group
b Percent of change between epileptic group and all the treated groups
c Percent of change between Levetiracetam (LEV) and ADMSCs +LEV as well as BMMSCs+ LEV
d Percent of change between ADMSCs +LEV and BMMSCs+ LEV
e Percent of change between BMMSCs+ LEV and BMMSCs
f Percent of change between ADMSCs +LEV and ADMSCs
g Percent of change between BMMSCs and ADMSCs
Table 3: Influence of treatment with rat BMMSc or rat ADMSc with or without LEV on brain bFGF and BDNF levels of acute epileptic rat model
| Group Number
|
Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
| Group /parameters | Control | Epileptic | Epileptic + LEV | Epileptic +
ADMSCs |
Epileptic +
BMMSCs |
Epileptic +
ADMSCs + LEV |
Epileptic +
BMMSC + LEV |
| bFGF (ng/g) | 198±17 | 462±18*
(+133.33%)a |
188±4.18**
(-5.05%)a (-59.31%)b |
177±3.26**
(-10.61%)a (-61.69%)b |
131 ±2.03
(-33.84%)a (-71.64%)b (-25.10%)g |
161±16**
(-18.69%)a (-65.15%)b (-14.36%)c (-9.04%)f |
140 ±9.48**
(-29.29%)a (-69.70%)b (-25.53%)c (+6%.87%)e (-13.04%) d |
| BDNF(pg/g) | 646±28 | 913±64*
(+41.33%)a |
539±41**
(-16.65%)a (-40.96%)b |
537±10**
(-16.87%)a (-41.18%)b |
402±23**
(-37.77%)a (-55.97%)b (-43.76%)g |
531±55**
(-17.80%)a (-41.84%)b (-1.48%)c (-1.12%)f |
419±12**
(-35.14%)a (-54.11%)b (-22.26%)c (+4.23%)e (-21.09%)d |
bFGF : basic fibroblast growth factor
BDNF: Brian deriverdneurotrophic factor
LEV: Levetiracetam
ADMSCs: Adipose tissue- derived mesenchymal stem cells
BMMSCs: Bone marrow-derived mesenchymal stem cells
Data were represented as Mean±SE
Data were analyzed using One-way ANOVA followed by Tukey’s multiple comparisons test
Significant change at p˂ 0.05 between epileptic group and the control group*
Significant change at p˂ 0.05 between epileptic group and all the treated groups**
a Percent of change between epileptic group and the control group
b Percent of change between epileptic group and all the treated groups
c Percent of change between Levetiracetam (LEV) and ADMSCs +LEV as well as BMMSCs+ LEV
d Percent of change between ADMSCs +LEV and BMMSCs+ LEV
e Percent of change between BMMSCs+ LEV and BMMSCs
f Percent of change between ADMSCs +LEV and ADMSCs
g Percent of change between BMMSCs and ADMSCs
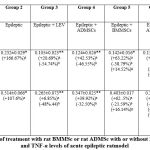 |
Table 4: Influence of treatment with rat BMMSc or rat ADMSc with or without LEV on brain IL-6 and TNF-α levels of acute epileptic ratmodel.Click here to view table |
IL-6: Interleukin -6
TNF-α: Tumor necrosis factor-α
LEV: Levetiracetam
ADMSCs: Adipose tissue- derived mesenchymal stem cells
BMMSCs: Bone marrow-derived mesenchymal stem cells
Data were represented as Mean±SE
Data were analyzed using One-way ANOVA followed by Tukey’s multiple comparisons test
*Significant change at p˂ 0.05 between epileptic group and the control group
** Significant change at p˂ 0.05 between epileptic group and all the treated groups
a Percent of change between epileptic group and the control group
b Percent of change between epileptic group and all the treated groups
c Percent of change between Levetiracetam (LEV) and ADMSCs +LEV as well as BMMSCs+ LEV
d Percent of change between ADMSCs +LEV and BMMSCs+ LEV
e Percent of change between BMMSCs+ LEV and BMMSCs
f Percent of change between ADMSCs +LEV and ADMSCs
g Percent of change between BMMSCs and ADMSCs
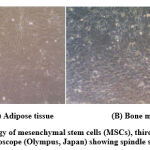 |
Figure 1: Morphology of mesenchymal stem cells (MSCs), third passage, under the inverted optical Microscope (Olympus, Japan) showing spindle shape feature. (X 100) |
 |
Figure 2: Flow cytometry analysis of CDs surface marker of ADMSCs (CD90 (A), CD14 (B) and CD45 (C)) and BMMSCs (CD90 (D), CD14 (E) and CD45 (F)). |
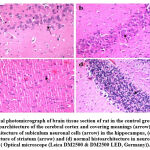 |
Figure 4: Optical photomicrograph of brain tissue section of rat in the control group showing: |
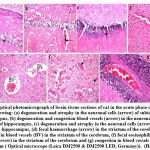 |
Figure 5: Optical photomicrograph of brain tissue sections of rat in the acute phase of epileptic group showing: |
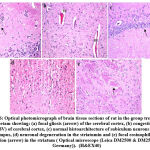 |
Figure 6: Optical photomicrograph of brain tissue sections of rat in the group treated with Levetiracetam showing: |
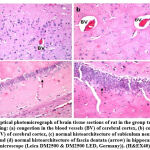 |
Figure 7: Optical photomicrograph of brain tissue sections of rat in the group treated with ADMSCs showing |
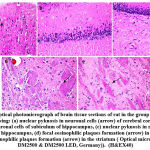 |
Figure 8: Optical photomicrograph of brain tissue sections of rat in the group treated with BMMSCs showing: |
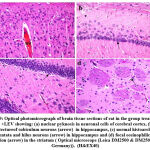 |
Figure 9: Optical photomicrograph of brain tissue sections of rat in the group treated with ADMSCs +LEV showing: |
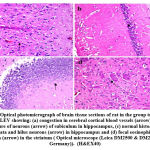 |
Figure 10: Optical photomicrograph of brain tissue sections of rat in the group treated with BMMSCs + LEV showing: |
Discussion
The current approach was undertaken to investigate the potent therapeutic effect of rat ADMSCs and BMMSCs on a pilocarpine rat model of acute epilepsy. A comparison among the effect of the antiepileptic drug, LEV, ADMSCs and BMSCs on the relevant brain biochemical markers in the acute phase of epilepsy was investigated. Also, the effect of co-administration of the two types of MSCs with LEV was evaluated. Indeed, the antiepileptic drugs fail to well-control seizures in approximately 30% of epilepsy patients and stem cell grafting has been shown to be a promising alternative approach for a better treatment of epilepsy45. There are two proposed mechanisms of stem cell transplantation in the treatment of epilepsy. In one mechanism, the transplanted stem cells may replace the cells lost by trans-differentiation. Stem cells possess self-renewal properties, and the ability to differentiate into different cell types, which may replace lost cells or specific neurons during epilepsy, such as inhibitory interneurons46. The other mechanism relies on the neuroprotective or anti-inflammatory cytokines which are released from the stem cells. These cytokines have been found to be involved in improving neurogenesis47. Thus, the utilization of stem cells to replace the cells that are lost during the course of epileptogenesis has been considered an effective strategy in the treatment of epilepsy48.
In the present investigation, the morphological characteristics of ADMSCs and BMMSCs as fibroblast like cells come in line with the study of Masoud et al.49. Moreover, flow cytometry analysis revealed that ADMSCs are positive for CD90 and this finding is in conformity with that of Bourin et al. 50. Meanwhile, these cells were negative for CD14 as in the study of Mildmay-White and Khan51. Similarly, ADMSCs were negative for CD45 and this result is in harmony with that of Bourin et al.50. Regarding BMMSCs flow cytometry analysis showed that, these cells are positive for CD90 and negative for CD14. These results are in accordance with those of Dominici et al.30. Also, BMMSCs were negative for CD45 which is in concert with the report from Chaudhary and Rath52.
It has been reported that the rat pilocarpine model resembles human epilepsy53 and its use help investigators to expand knowledge in this field and to develop more effective treatments54. The presence of ADMSCs and BMMSCs cells in the brain of the epileptic rats treated with these cells confirmed by the staining of ADMSCs and BMMSCs with PKH26 in the present study. This finding indicated that the transplanted MSCs have survived and accommodated in the site of degradation. This finding converges with the studies Tamura et al.54 and Abdanipour et al.37.
The present study showed that when the pilocarpine-challenged rats were examined by the mean electric shock (MES) test, a significant drop in the MES value was recorded, indicating the existence of strong seizure, appeared as ”quick convulsions”. This finding shows parallelism with that of Kitano et al.55. On the opposite side, a significant rise in the MES values was registered in the other treated groups (epileptic + LEV, epileptic + ADMSCs, epileptic + BMMSCs, epileptic + ADMSCs + LEV and epileptic + BMMSCs + LEV) indicating that LEV, ADMSCs and BMSCs ameliorated the epileptic impact of pilocarpine and proved the anticonvulsant and antiepileptic effects of ADMSCs and BMSCs like LEV. The antiepileptic and anticonvulsant activity of LEV was previously reported56,57,58.
The findings of the present work indicated a significant reduction in the brain level of GABA and a significant elevation in the level of brain glutamate, dopamine, bFGF, BDNF, IL-6 and TNF-α, in the rats during acute phase of epilepsy, (24 hours after administration of pilocarpine) in comparison with those of the control counterparts.
The recorded decrease of brain GABA and the increase of brain glutamate levels after pilocarpine administration, agree with the results of the previous studies59,60,61,62. These investigators mentioned that pilocarpine induces a decline in the GABA level and an elevation in the level of glutamate, either in the hippocampus or in the brain cortex. McCormick and Contreas63 reported that seizures can be generated in response to the deterioration between the inhibitory and excitatory balance leading to tonic depolarization or repetitive rhythmic burst discharges. Also, Hasegawa et al.64cited that the imbalance of the inhibitory and excitatory neurotransmitters in the brain has been suggested to cause epileptic seizures. It is well known that GABA is the common inhibitory neurotransmitter in the cortical neurons65, and glutamate is the main excitatory neurotransmitter in the mammalian central nervous system59. Thus, in the present investigation, the drop in the level of brain GABA recorded in the acute phase of epilepsy, may be due to the following: (1) The alterations in the metabolism of GABA, which may have a role in the genesis and propagation of seizure activity as a result of pilocarpine administration65, (2) The damage of the GABAergic neurons in the brain cortex of the epileptic animals65, (3) Loss of GABA neurons that may represent the epileptic status in acquired epilepsy65. Loss of GABA neurons has been observed in the acquired epileptic models, including kindling, status epilepticus and traumatic brain injury models66,67 and (4) A dysfunction of glutamate transporters induced by excess release of glutamate64.
The increase in brain glutamate level, obtained in the present work, 24 hours after administration pilocarpine, may be attributed to: (1) The hyperactivity of brain neurons due to the effect of pilocarpine59, (2) A possible severe damage in the brain as a consequence of the pilocarpine administration64, (3) Activation of glutamate transporters by pilocarpine68, and/or (4) An increase in Ca++ level which was suggested to increase brain glutamate level and induce status epilepticus43.
In the current research, the level of brain dopamine increased significantly in the rats administered with pilocarpine. This result is in accordance with the previous studies of Cavalheiro et al.69 and Bozzi and Borrelli5, which explained this phenomenon by the abnormal control of dopamine levels or a deviation in the dopamine receptors expression. Pilocarpine displays an unusually elevated dopaminergic neuron drive in the form of an enhancement in the activity of the dopaminergic neuron population70. The Ventral Tegmental Area (VTA) of dopaminergic neurons activity is governed by hippocampal VTA loop which involves ventral subicular-nucleus accumbens-ventral pallidal-VTA pathway71. Therefore, the pathological hyperactivity of the hippocampal formation (including subiculum) can result in increased activity of VTA dopamine neuron. Since the phasic burst firing response of dopaminergic neurons premised on their spontaneous activity72, the abnormal high levels of dopaminergic neurons population activity would enable the phasic burst promoter to trigger burst firing in a large number of dopamine neurons, thereby laying the dopaminergic system in a hyper-responsive status in the epileptic brain71,73. Also, the specific receptors of dopamine can modify the neuromodulatory effect of dopamine on brain circuits particularly in the limbic system. The binding potential of D2/D3 receptors has been found to be reduced in the brain of epileptic patients and the decrease in D2 receptor protein expression in the temporal neocortex has been detected in epileptic patients74.
The data of the current approach recorded a significant increase in the level of brain bFGF in the pilocarpine administered rats (acute phase of epilepsy) and this finding is in conformity with the result of Simonato et al.75 who demonstrated an increased level of bFGF in the hippocampus, neocortex and hypothalamus in the rat kindling model of epilepsy. Also, Gwinn et al.76 reported a significant rise in the bFGF level in the rat cortex, which remained significantly high up to 72 hours after the electroconvulsive shock. Moreover, Paradiso et al.77 concluded that the bFGF is highly implicated in the changes of brain functions during the epileptic seizures.
In the current investigation, the level of brain BDNF increased significantly in the pilocarpine-challenged rats. This result is in harmony with that of the previous research works78,79, which mentioned a significant enhancement in BDNF level in several brain regions after administration of a convulsive dose of pilocarpine in animals. It has been proposed that the increased production of BDNF may affect the survival of the neurons and the growth of fiber, thus maintaining the neuronal networks organization in brain area involved in the epileptic activity80 and indicating active neosynthesis81,82.
The findings of this work revealed a significant rise in the level of brain IL-6 and TNF-α of the pilocarpine-challenged rats. This finding could be interpreted by the influence of epileptic seizures on the immune system to produce cytokines such as IL-6 and TNF-α 83,84. IL-6 is a multifunctional cytokine that regulates inflammatory responses and other immune reactions. It has been stated that IL-6 level is elevated in the various areas of the rat brain during the epileptic seizures85,86.
Regarding brain TNF-α level, several animal studies reported that after seizures evoked in the rat kindling model of epilepsy, TNF-α levels displayed a significant increase in the brain cortex as well as amygdala and hippocampus. TNF-α has been found to have anticonvulsive effect during the epileptic seizure77. Thus, the increased brain level of TNF-α observed in this study in the pilocarpine-challenged rats may be related to its role as an anti-inflammatory mediator and an anticonvulsive factor during the epileptic seizures in the acute phase of epilepsy84,87.
The present data revealed that LEV caused a marked improvement in the changes induced by pilocarpine in rats, since LEV recovered the behavior of the rats which was observed after pilocarpine administration. Also, a significant increase in GABA level and a significant reduction in glutamate, dopamine, bFGF, BDNF, IL-6 and TNF-α levels were recorded after treatment with LEV. These results may be explained by the antiepileptic and anticonvulsive effects of LEV as reported by previous investigators44, 61,88. Levetiracetamhas been reported to be mostly advantageous in repressing seizures in animal models of spontaneous epilepsy, acute seizure and status epilepticus89. Potschka et al.90 cited that LEV is a broad-spectrum antiepileptic drug for the treatment of partial and generalized epilepsy. Also, the anticonvulsive activity of LEV has been mentioned by Lyseng-Williamson91, where LEV retarded the outset of convulsive activity and lessened the injury of the neurons in the pilocarpine model of epilepsy. Moreover, the anticonvulsant activity of LEV includes the conservation of hippocampal noradrenalin and dopamine levels61.
The present results showed that the two types of rat MSCs (ADMSCs and BMMSCs) significantly ameliorated all the behavioral signs of pilocarpine administration which were observed in the pilocarpine-challenged rats. The beneficial effectiveness of MSCs in epilepsy has been reported in the previous investigations. Helbokazov et al.92 stated that the treatment of refractory epilepsy patients with autologous MSCs reduced the seizure frequency. Moreover, it has been suggested that MSCs have the ability to prohibit the development of chronic epilepsy when infused early after the status epilepticus (SE) and alter the disease process after the establishment of the chronic epileptic state 25. In parallel with our findings Abdanipour et al.37 noticed that the treatment of epileptic rats with BMMSCs yielded improvement in the behavioral experiment as evidenced by the decreased number of seizures in the treated groups. Also, the anticonvulsant effect of ADMSCs has been reported by Tamura et al.54 who explained this effect by the ability of ADMSCs to block the tonic component of generalized seizures. The present study also revealed that ADMSCs and BMMSCs improved the effect of pilocarpine on the brain biochemical parameters. This is documented by increasing the level of brain GABA and decreasing the level of brain glutamate, dopamine, bFGF, BDNF, lL-6 and TNF-α. It could be observed that the effect of rat ADMSCs and rat BMMSCs on these biomarkers in the present study is almost similar to that of LEV. These findings indicate that the examined types of MSCs may have antiepileptic and anticonvulsive effects.
D, Souza et. al.93 commented that the favorable action of MSCs may derive from the induction of local neurogenesis through the secretion of neuronal growth factors, such as (bFGF) and (BDNF). Salem et al.94 concluded that in an epileptic rat model, treating the rats with BMMSCs ameliorated all the tested biochemical parameters which were markedly perturbed after administration of pilocarpine. The neuroprotective effect of BMMSCs in a cell culture model has been demonstrated when the purified BMMSCs were applied in the early phase after seizure95. These authors observed the reduction in neuronal loss and the activation of astrocytes and microglia after treatment with BMMSCs. The research study conducted by Shetty et al.27 examined the effect of i.p. infusion of human BMMSCs, one hour after seizure, in epilepsy rat model; these investigators concluded considerable protection of the principal neurons, reduction of gabaergicinterneurons, loss normalization of the proinflammatory cytokines’ level, enhancement of expression of genes coding anti-inflammatory cytokines in the hippocampus and reduction of myeloperoxidase activity.
ADMSCs proved potent efficacy and efficiency in some clinical applications for both autologous and allogeneic purposes, hence becoming believed as promising tools for substituting, restoration, and regenerating dead or damaged cells96. In particular, the seizures induced in transplanted mice have been found to trigger an acute inflammatory condition; altering the pattern of the gene expression of IL1-α, IL-6, iNOS, caspase-1 and IL-4. The immunomodulatory and anti-inflammatory properties of ADMSCs have been documented, providing evidence for utilizing ADMSCs as therapeutic candidate for a wide variety of degenerative diseases. ADMSCs could affect the activity of neurons in the hippocampus by ameliorating the exaggerated and synchronous neuronal firings that yield seizures and or by producing modulatory molecules which could “turn off” the tonic activity of the generalized seizures. One possible reason for the neuroprotective effect of ADMSCs is that the implanted cells act as, a barrier prohibiting the hyperexcitability of the local circuit and preventing the spread (generalization) of seizures, represented by the lessening in the duration (tonic) and in the post-ictal period. Thus, the anticonvulsant effect of the ADMSCs on acute convulsive seizure may be due to inhibitory factors and immunomodulatory mechanisms54. Moreover, ADMSCs could transdifferentiate into neuron-like cells that have multiple neuronal characteristics like synaptic transmission, action potential, neurotrophic factors, and spontaneous postsynaptic current97.
Histomorphological investigation of brain tissue section of rat in the epileptic group showed degeneration and atrophy in the neuronal cells of fascia dentata and congestion of blood vessels in the neuronal cells of subiculum of the hippocampus. The striatum in the cerebrum showed focal hemorrhage in the epileptic group. These findings echo those observed by Scorza et al.43. Improvement in the histopathological feature of the brain tissue section of rat treated with LEV, ADMSCs, BMMSCs after 3 months has been noticed as represented by the presence of congestion in the cortical blood vessels only. These observations fit those obtained from the previous studies15, 21, 98 which documented the capability of MSCs in inducing an increase in the neurogenesis of the hippocampus and a reduction in the neuronal death.
The outcomes of the present study showed a marked difference between the effect of ADMSCs and BMMSCs on the investigated parameters in the brain of the treated rats. This difference may be related to the different tissues from which MSCs are derived as the ADMSCs are derived from the adipose tissue, and the BMMSCs are derived from the bone marrow tissue. This interpretation is concordant with the previous investigators who suggested that MSCs from various sources may have different characteristics99, 100,101. Also, historic differences between ADMSCs and BMMSCs in the secretome and chemokine receptors expression have been reported by Hsiao et al.24 and Elman et al.102. Several secreted molecules such as cytokine (interferon-α), growth factors (basic fibroblast growth factor, hepatocyte growth factor and insulin like growth factor-1), and chemokine (stem cell derived factor-1) have been shown to be more pronounced in ADMSCs than BMMSCs21.
The findings of the current investigation revealed that the combined treatment of either ADMSCs or BMMSCs with LEV has a superior effect over the single treatment of each type of the MSCs alone. These results fit similar findings mentioned by Liu et al.103 and Liu et al. 104 who stated that the co-injection of stem cells with the pharmacological therapy can enhance the migratory ability of MSCs to reinforce the damaged sites beside the action of the pharmacological drug.
Conclusion
The results revealed that rat ADMSCs and rat BMMSCs may have therapeutic potential as antiepileptic and anticonvulsive candidates in the rat model of acute phase of epilepsy. Noteworthy, the effect of the rat ADMSCs has been shown to be higher than that of the rat BMMSCs in modulation of the relevant biomarkers of epilepsy. Additionally, the combined treatment of either ADMSC or BMMSCs with the anti-epileptic drug (LEV) showed a synergistic effect as it exhibited superior beneficial effect in retrieving the biochemical alterations as well as the histological distortion of brain tissue in comparison with the single use of each type of cells during the acute phase of epilepsy. The outcomes received in this attempt pave the way for establishment of cell-based therapy in combination with the conventional therapy in the treatment of epilepsy.
Limitations of the study
Some crucial questions are needed to be answered by conducting more studies. For example; more studies are needed to confirm therapeutic effect of MSCs in epilepsy models, clarifying how to improve and achieve the long survival time, integration, and long-term functions of transplanted MSCs in the host environment and elucidating the specific differentiation of MSCs and their paracrine therapeutic effects. Also , a longer follow-up time and optimized stem cell administration to accurately test the long-term curing potency of MSCs therapy in epilepsy are demanded. Solving these questions will certainly expedite the realization of the great promise of MSCs-based therapy in epilepsy.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding Sources
References
- Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 15: 106- 115 (2016).
CrossRef - Beghi E. The Epidemiology of epilepsy. Neuroepidemiology. 54:185-1 91 (2020).
CrossRef - Beghi E, Carpio A, Forsgren L, Hesdorffer D, Molmgren K, Sander JW. Recommendation for definition of acute symptomatic seizure. Epilepsia. 51(4):671-675 (2010).
CrossRef - Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute sympatomatic seizure epilepsy? Mortality and risk recurrent seizure. Epilepsia. 50(5): 1102-1105 (2009).
CrossRef - Bozzi Y, Borrelli E. The role of dopamine signaling in epileptogenesis. i Neurol. 7(157): 1-12 (2013).
CrossRef - Thijs RD, Surges R, O, Brien TJ, Sander JW. Epilepsy in adults. Lancet. 393(10172): 689-701 (2019).
CrossRef - Sterlini B, Fruscione F, Baldassari S, Benfenatr F, Zara F., Corradi Progress of induced pluripotent stem cell technologies to understand genetic epilepsy. Int. J. Mol. Sci. 21 (2): 482 (2020).
CrossRef - Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J. The prevalence and incidence of epilepsy. A systemic review and meta-analysis of international studies. 88(3): 296-303 (2017).
CrossRef - Shakeel S, Rehman MU, Tabassum N, Amin U, Mir M Effect of naringenin (A naturally occurring flavanone against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn Mag. 13(suppl 1): S154- S160 (2017).
CrossRef - Yi ZM, Wen C, Cai T, Xu L, Zhong XL, Zhan S. Levetiracetam for epilepsy: An evidence map of efficacy, safety and economic profils. Dis. Treat. 15: 1-19 (2019).
CrossRef - Tidwell A, Swims M. Review of the newer antiepileptic drugs. J. Manag. Care. 9:253–276 (2003).
- Kumar A, Kadian R. StatPearls: Treasure Island (FL): StatPearls Publishing 414 StatPearls Publishing LLC (2018).
- Ben-Menachem E. Levetiracetam treatment in epilepsy. Expert Opin. Pharmacother. 4:2079–2088 (2003).
CrossRef - Jobst BC, Cascino GD. Respective epileptic surgery for resistant focal epilepsy review. A.M.A. 313(3): 285- 293 (2015).
CrossRef - Hammadi AA. Autologous bone marrow derived mononuclear cells for the treatment drug resistance epilepsy. J. Stem Cell Res. Ther. 5(1): 23- 25 (2019).
- Mu J, Bakrean A, Juntunen M, Korhonen P, Bakrean A, Juntunen M. Combined adipose tissue-derived mesenchymal stem cell therapy and rehabilitation in experimental stroke. i Neurol. 10: Article Number 235 (2019).
CrossRef - Deng L, Peng Q, Wang H, Pan J, Zhou Y, Pan K. Injection of allogenic bone marrow derived mesenchymal stromal cells in treatment of patients with severe ischemic stroke: Study prorocol for a randomized controlled observer-blind trial. Stroke Res. 10(2):170-17 7 (2019).
CrossRef - Pan K, Deng L, Chen P, Peng Q. Safety and feasibility of repeated intrathecalallogenic bone marrow derived mesenchymal stromal cells in patients with neurological diseases. Stem Cells 2019, Article ID 8421281: 15 pages (2019).
CrossRef - Zhao L, Hu C, Zhang P, Jiang H, Chen J. Novel preconditioning strategies for enhancing the migratory ability of mesenchymal stem cells in acute kidney injury. Stem Cell Ther. 9 (1): 225 (2018).
CrossRef - de Selvia ML, Chegastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-meta organs and tissues. J. Cell Sci. 119: 2204- 2213 (2006).
CrossRef - Li C, Wu X, Tong J, Yang X, Yang X, Zhao J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under Xeno- free conditions for all therapy. Stem Cell Res. Ther. 6 (1)-:55 2015).
CrossRef - Vishnubalaji R, Al-Nabaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone marrow and adipose–derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 347: 419- 427 (2012).
CrossRef - Chung CS, Fugita N, Kawahara N, Yui S, Nishimura R Comparison of neurosphere differentiation potential of canine bone marrow derived mesenchymal stem cells and adipose –derived mesenchymal stem cells. J. Vet. Med. Sci. 75: 879- 886 (2013).
CrossRef - Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY. Comparative analysis of paracrine after expression in human adult mesenchymal stem cells derived from bone marrow, adipose and dermal tissue. Stem Cells Dev.; 21 (12): 2189-22 03 (2012).
CrossRef - Agadi S, Shetty AK. Concise Review: Prospect of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells 33(7): 2093- 2103 (2015).
CrossRef - Long Q, Qiu B, Wang K, Yang T, JiaC, Xin W. Genetically stem cells improve functional out come in a rat model of epilepsy. Brain Res. 1532: 1-13 (2013).
CrossRef - Shetty AK, Hattiangady B, Shetty G. Intraperitoneal administration of human mesenchymal stem cells restrains status epilepticus induced neurodegeneration and inflammatory reaction in the hippocampus. Abstract of 12th Annual Meeting of International Society for Stem Cell Res. F-3145 (2014).
- Boregowda SV, Krishnappa V, Phinney DG. Isolation of mouse bone marrow mesenchymal stem cells. Mol. Biolog. 1416: 205-22 3 (2016).
CrossRef - Francis MP, Sachs PC, Elmore LW ,Holt SE. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 6 (1):11- 14 (2010).
CrossRef - Dominici M, Le Blanc K, Muller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 8 (4): 315- 317 (2006).
CrossRef - Guli X, Tokay T, Kirschstein T, Köhling R. Status epilepticus enhances depotentiation after fully established LTP in an NMDAR-Dependent but gluN2B Independent manner. Neural Plasticity 2016, Article ID 6592038 (2016).
CrossRef - He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 43:31-42 (2004).
CrossRef - Feng S, Ma S, Jia C, Su Y, Yang S, Zhou K. Sonic hedgehog is a regulator of extracellular glutamate levels and epilepsy. EMBO Rep. 17(5): 682-6 94 (2016).
CrossRef - Kim YJ, Kim JY, Ko AR, Kang TC. Reduction in heat shock protein 90 correlates to neuronal vulnerability in the rat piriform cortex following status epilepticus. Neurosci. 255: 265- 277 (2013).
CrossRef - Patel NC, Landan IR, Levin J, Szaflarski J, Wilner AN. The use of levetiracetam in refractory status epilepticus. Seizure. 5(3): 137- 141 (2006).
CrossRef - Ahmed HH, Metwally FM, Aglan HA, Sayed AH. Exploring potential mechanisms of action of mesenchymal stem cells in Parkinson’s disease: In vivo study. J. of Curr. Pharm. Rev. and Res., 7(5): 275-282 (2016).
- Abdanipour A, Tiraihi T, Mirnajafi-Zadeh J. Improvement of the pilocarpine epilepsy model in rat using bone marrow stromal cell therapy. Neurol Res. 33(6): 625– 632 (2011).
CrossRef - Kitano Y, Usui C, Takasuna K, Hirohashi M, Nomura M. Increasing-current electroshock seizure test: a new method for assessment of anti- and pro-convulsant activities of drugs in mice. J Pharmacol. Toxicol. Methods 35(1): 25–29 (1996).
CrossRef - Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 19(4): 409–428 (1978).
CrossRef - Loscher W, Nau H, Marescaux C, Vergnes M. Comparative evaluation of anticonvulsant and toxic potencies of valproic acid and 2-en-valproic acid in different animal models of epilepsy. J. Pharmcol. 99(2–3): 211–218 (1984).
CrossRef - Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+ -ATPase activities induced by the in vitro galactosaemia. Res. 49(5):475-47 9 (2004).
CrossRef - Banchroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. Fourth Ed Churchil Livingstone New York London San Francisco Tokyo; (1996).
- Scorza FA, Arida RM, Mazzacorati MGN, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: What have we learned? Anais da Academia Brasileira de Ciencias. 81(3): 345- 365 (2009).
CrossRef - Zheng Y, Mousally J, Cash S, Karnam H, Cole A . Intravenous levetiracetam in rat pilocarpine-induced status epilepticus model: Behavioral, physiological and histological studies. Neuropharmacolo. 58(4-5): 793–798 (2010).
CrossRef - French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 62: 1261–1273 (2004).
CrossRef - Roper SN, Steindler DA. Stem cells as a potential therapy for epilepsy. Neurol. 244: 59–66 (2013).
CrossRef - Shetty AK. Neural Stem Cell Therapy for Temporal Lobe Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. (Eds.), Jasper’s Basic Mechanisms of the Epilepsies, Bethesda (MD); (2012).
CrossRef - Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, Bolshakov VY, Chung S. HPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15: 559–573 (2014).
CrossRef - Masoud MA, Mohamed EG, Hassan WA, Mohamed FE. Effect of bone marrow and adipose mesenchymal stem cells on rat intestinal injury induced by methotrexate. Tissue Repair Reg. 1(2): 1-11 (2018).
CrossRef - Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Society for Cellular Therapy (ISCT). Cytotherapy 15(6): 641-648 (2013).
CrossRef - Mildmay-White A, Khan W. Cell surface markers on adipose-Derived stem cells: A systemic review. Curr Stem Cell Res. Ther. 12(6): 484- 492 (2017).
CrossRef - Chaudhary JK, Rath PC. A simple method for isolation, propagation, characterization and differentiation of adult mouse bone marrow- derived multipotent mesenchymal stem cells. Cell Sci. Ther. 8(1): 1-10 (2017).
- Leroy C, Roch C, Koning E, Namer IJ, Nehlig A. In the lithium-pilocarpine model of epilepsy, brain lesions are not linked to changes in blood –brain barrier permeability: An autoradiographic study and developing rats. Neuro. 182: 361- 372 (2003).
CrossRef - Tamura, B.P., Almeida, D., Felizardo, R.J., Olanda, G.C., Bocca, P., Alves-de-Moraes, Convulsive seizure protection after hippocampal transplantation of mesenchymal cells from adipose tissue in mice. J. Stem Cell Res., 4(4): 1-7 (2014).
- Kitano Y, Usui C, Takasuna K, Hirohashi M, Nomura M. Increasing-current electroshock seizure test: A new method for assessment of anti- and pro-convulsant activities of drugs in mice. Pharmacol. Toxicol. Meth. 35: 25- 29 (1996).
CrossRef - Klitgaard H, Matagne A, Gobert J. Evidence for a unique profile of levetiracetam in a rodent models of seizures and epilepsy. J. Pharm. 253(2-3): 191- 206 (1998).
CrossRef - Ji-qun C, Ishihara K, Nagayama T, Serikawa T, Sasa M. Long-lasting antiepileptic effects of levetiracetam against epileptic seizures in the spontaneously epileptic rats (SER): Differentiation of levetiracetam from convential antiepileptic drug. Epilepsia 46(9): 1362- 1370 (2005).
CrossRef - Sugaya Y, Maru E, Kudo K, Shibasaki T, Kato N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kinate-induced status epilepticus. Brain Res., 1352: 187-1 99 (2010).
CrossRef - Costa M.S. Rocha JB, Perosa SR, Cavalheiro EA, Mazzacorati MG. Pilocarpine-induced status epilepticus increases glutamate release in rat hippocampal synaptosomes. N Lett., 356 (1): 41- 44 (2004).
CrossRef - Dos-Santos PS, Campelo LM, Freitas CM, Saldanha GB, Freitas RM. Drug in models of partial and generalized epilepsy in mice and rats. J. Pharmaco. l232:147–158 (2011).
CrossRef - Al-Shorbagy MY, El Sayeh BM, Abdallah DM. Additional antiepileptic mechanisms of levetiracetam in lithium- pilocarpine treated rats. PLOS ONE 8(10): e 76735 (2013).
CrossRef - Barker-Haliski M, White HS. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 5(8): a022863 (2015).
CrossRef - McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Rev. Physiol.63: 815- 846 (2001).
CrossRef - Hasegawa D, Matsuki N, Fujita M, Ono K, Orima H. Kinetics of glutamate and γ-aminobutyric acid in cerebrospinal fluid in a canine model of complex partial status epilepticus induced by kainic acid. Vet. Med. Sci. 66(12): 1555-1559 (2004).
CrossRef - Ribak C, Yan Y. GABA neurons in the neocortex. GABA In The Nervous System. Martin DL, Olsen RW, Editors. The view at fifty years. Philadelphia: Lippincott Williams & Walkins, pp357- 368 (2000).
- Buckmaster PS, Dudek Neurons loss granule cell axon reorganization, functional changes in the dentate gyrus of epileptic kainate –treated rats. J. Comp. Neurol. 385(3):385- 404 (1997).
CrossRef - Gorter JA, VanVliet EA, Aronica E, Lopes de Silva FH. Progression of spontaneous seizure after status epilepticus is associated with mossy fiber sprouting and extensive bilateral loss of hilarpara- albumin and somatostatin- immunoreactive neurons. J. Neurosci. 13(4): 657- 669 (2001).
CrossRef - Chapman AG. Glutamate and epilepsy. Nutr.130: 1043S- 1045S (2000).
CrossRef - Cavalheiro EA, Fernandes MJ, Turski L, Mazzacorati MGN. Neurochemical changes in the hippocampus of rats with spontaneous recurrent seizures. Neurobiol. Epilepsy 9: 239- 248 (1992).
- Cifelli P, Grace AA. Pilocarpine-induced temporal lobe epilepsy in the rat is associated with increased dopamine neuron activity. J. Neuropsychopharmacol. 15: 957- 964 (2012).
CrossRef - Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Neurosci. 6: 968– 973 (2003).
CrossRef - Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. 31: 1356-1361 (2006).
CrossRef - Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry information into long-term memory. Neuron 46: 703- 713 (2005).
CrossRef - Rocha L, Alonso-Vanegas M, Villeda- Hernandez J, MuJica M, Cisnero-Franco J, Lopez- Gomez M. Dopamine abnormalities in the neocortex of patients with temporal lobe epilepsy. Dis. 45(1):499- 507 (2012).
CrossRef - Simonato M, Molteni R, Bregola G, Muzzolini A, Piffanelli M, Beani Differential patterns of induction of FGF-1and BDNF mRNA during kindling epileptogenesis in the rat. Eur. J. Neurosci. 10 (3): 955- 963 (1998).
CrossRef - Gwinn RP, Kondratyev A, Gale K. Time-dependent increase in basic fibroblast growth factor protein in limbic regions following electroshock seizures. 114: 403–409 (2002).
CrossRef - Paradiso B, Zucchini S, Simonato M. Implication of fibroblast growth factors in epileptogenesis associated circuit rearrengements. Cell. Neurosci. 7(152):1-9 (2013).
CrossRef - Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: Too much of a good thing? Trends Neurosci. 24:47– 53 (2001).
CrossRef - Biagini GA, Avoli M, Marcinkiewicz M. Brain derived neurotrophic factor superinduction parallels anti-epileptic neuroprotective treatment in the pilocarpine epilepsy model. Neurochem. 76 (6): 1814- 1822 (2001).
CrossRef - Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 17(11): 490-496 (1994).
CrossRef - Nawa H, Carnhan J, Gall C. BDNF protein measured by a novel immunoassay in a normal brain and after seizure: Partial disagreement with mRNA levels. J. Neurosci. 7(7): 1527-15 35 (1995).
CrossRef - Vezzani A, Ravizza T, Moneta D. Brain derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. , 90(4): 1445-1461 (1999).
CrossRef - Peltola J, Laaksonen J, Haapala AM, Hurme M, Rainesalo S, Keranen T. Indicators of inflammation after recent tonic-clonic epileptic seizures correlate with plasma interleukin-6 levels. Seizure 11: 44- 46 (2002).
CrossRef - Li G, Bauer S, Nowak M. Cytochromes and epilepsy. Seizure 20: 249-256 (2011).
CrossRef - Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T. Functional role of inflammatory cytokines and anti-inflammatory molecules in seizures and epileptogenesis. Epilepsia 43: 30-35 (2002).
CrossRef - Lehtimaki KA, Peltola J, Koskikallio E, Keranen T, Honkaneimi J. Expression of cytokines and cytokines receptors in the rat brain after kainic acid induced seizures. Brain Res. 110(2): 253-26 0 (2003).
CrossRef - Vezzani A, French J, Bartfai T, Baraun TZ. The role of inflammation in epilepsy. Nat. Neurol. 7(1): 31-40 (2011).
CrossRef - Ulloa CM, Towfigh A, Saldieh J. Review of levetiracetam, with a focus on the extended release formulation as adjuvant therapy in controlling partial- onset seizures. Dis. Treat., 5: 467-476 (2009).
CrossRef - Shetty AK. Prospects of levetiracetam as a neuro-protective drug against status epilepticus, traumatic brain injury and stroke. Neurol. 4 (172): 1-6 (2013).
CrossRef - Potschka H, Balts H, Loscher W. Inhibition of multidrug transporters by verapamil or probenecid does not alter blood-brain barrier penetration of levetiracetam in rats. Epilepsy Res. 58: 85-91 (2004).
CrossRef - Lyseng-Williamson KA. Levetiracetam : A review of its use in epilepsy. Drugs 71(4): 489-114 (2011).
CrossRef - Helbokazov F, Dokuukina T, Ihnatsenko S, Kosmacheva S, Potapnev M, Shakhbazou Treatment of refractory epilepsy patients with autologous MSCs reduces seizure frequency: An open label study. Adv. Med. Sci. 62(2): 273-277 (2017).
CrossRef - D’ Souza N, Rossignali F, Golinells G, Grisendi G, Spano C, Candini O. Mesenchymal stem/stromal cells as adelivery platform in cell and gene therapies. BMC Med. 13 (186): 1-15 (2015).
CrossRef - Salem N, El-Shamarka M, Khadrawy Y, ElSherbiny S. New prospects of mesenchymal stem cells for ameliorating temporal lobe epilepsy. Inflammpharmacol., 26 (4): 963-972 (2018).
CrossRef - Voulgari-Kokota A, Fairless R, Karamita M, Kyrargyri V, Tseveleki V, Evangelidou M. Mesenchymal stem cells protects CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Neurol. 236 (1): 161-170 (2012).
CrossRef - Mazini L, Rochette L, Amine M ,Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymalstem cells (MSCs). J. Mol. Sci. 20(10), 2523 (2019).
CrossRef - Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. Transl. Med. 7:29 (2009).
CrossRef - Deshpande SL, DeLorenzo JR. Mechanism of levetiracetam in the control of status epilepticus and epilepsy. Neurol. 5 (11):1-5 (2014).
CrossRef - Kwon A, Kim Y, Kim H, Kim J, Choi H, Jekarl DW, et al. Tissue specific differentiation potency of mesenchymal stromal cells from peritoneal tissues. Rep. 6: Article Number: 23544 (2016).
CrossRef - Davies JE, Walker JT, Keating A. Concise review: Warton’s jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Trans. Med. 6(7): 1620-16 30 (2017).
CrossRef - Elahi KG, Klein G, Arci-Adali M, Sievert KD, Mac-Neil S, Aicher W. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. Article ID5646384 (2016).
CrossRef - Elman JS, Li M, Wang F, Gimble JM, Parekkadan B A. Comparison of adipose and bone marrow-derived mesenchymal stromal cells secreted factors in the treatment of systemic inflammation. Inflamm. 11 Article Number: 1 (2014).
CrossRef - Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow derived mesenchymal stem cells to the acute kidney injury microenvironment. Cell Res. 319: 2019-2027 (2013).
CrossRef - Liu P, Feng Y, Dang C, Yang D, Li B, Chen X, et al. Administration of BMSCs with muscone in rats with gentamicin–induced AKI improves their therapeutic efficacy. PLOS ONE 9(5): e97123 (2014).
CrossRef