Mutiara Rahmah Amari1 , Hesti Lina Wiraswati1,2,3
, Hesti Lina Wiraswati1,2,3 , Nisa Fauziah2,3
, Nisa Fauziah2,3 and Ilma Fauziah Ma’ruf4
and Ilma Fauziah Ma’ruf4
1Oncology and Stem Cells Working Group, Faculty of Medicine, Universitas Padjadjaran, Bandung.
2Infections Working Group, Faculty of Medicine, Universitas Padjadjaran, Bandung.
3Parasitology Division, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung.
4Biochemistry Research Group, Department of Chemistry, Faculty of Mathematics and Natural Sciences, Institut Teknologi Bandung. Bandung.
Corresponding Author E-mail: hesti.lina@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2369
Abstract
Plasmodium falciparum is the most common species of Plasmodium that causes malaria in Southeast Asia. Artemisinin, a drug with the mechanism of action by inducing oxidative stress in infected red blood cells (RBC) is currently used as the main therapy for malaria, after resistance to chloroquine has been found. However, evidence of artemisinin resistance was discovered in several regions in Southeast Asia. Therefore, a research is required to prove the existence of other drugs that have anti-malaria effects. A drug candidate, doxorubicin also can induce the formation of oxidative stress inside the cells. This study aims to determine the activity of doxorubicin to inhibit the development of P. falciparum in vitro. Red blood cell (RBC) infected with P. falciparum were treated with various concentrations of doxorubicin. Giemsa technique was applied to detect P. falciparum inside RBC. After 48 hours of incubation, the culture was observed to measure the number and the confluence of RBC and P. falciparum in the medium. This study revealed that doxorubicin reduced the number of RBC infected with P. falciparum lysis. The effective dose of doxorubicin-inhibit RBC cell lysis is 0.4 μM, which only reduces 81% RBC cell lysis compared to the control group that reduces 95% RBC cell lysis. At this concentration also found a decrease in the number of P. falciparum cells in the medium. The results proved that doxorubicin has an inhibitory effect on the development of P. falciparum and can decrease the lysis of RBC due to P. falciparum infection. This findings provide an insight that doxorubicin is a potential candidate for antimalarial drugs.
Keywords
Artemisinin; Antimalaria; Oxidative Stress; Giemsa
Download this article as:| Copy the following to cite this article: Amari M. R, Wiraswati H. L, Fauziah N, Ma’ruf I. F. Antimalarial Effect of Doxorubicin on Plasmodium Falciparum: An in Vitro Study in FCR-3 Strain. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Amari M. R, Wiraswati H. L, Fauziah N, Ma’ruf I. F. Antimalarial Effect of Doxorubicin on Plasmodium Falciparum: An in Vitro Study in FCR-3 Strain. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3zYY7dL |
Introduction
Plasmodium falciparum is the Plasmodium species that mainly causes malaria in Southeast Asia, including Indonesia (62.8%).1 The Annual Parasite Incidence (API) rate in Indonesia, which states the number of positive cases of malaria per 1000 population is still relatively high (High category Cumulative Incidence (HCI) II (API = 50-100)), especially in eastern Indonesia such as Papua, Nusa Tenggara Timur, and Maluku.2
Falciparum malaria caused by P. falciparum is the most severe type of malaria because it can generate high levels of parasitemia compared to other types of malaria. Falciparum malaria most often becomes severe malaria which can cause death.3,4
Recently the recommended treatment for falciparum malaria is the use of artemisinin-based combination therapy (ACT). In Indonesia, dihydroartemisinin/piperaquine been applied as a combination with artemisinin since WHO recommended it as a first-line drug for malaria.5 Artemisinin works as an antimalarial drug by inducing oxidative stress causes the reduction of parasites in the body.6,7
Resistance to both artemisinin and non-artemisinin components in artemisinin-based combination therapy emerging in Southeast Asia.8 This resistance can lead to treatment failure in malaria due to the reduced P. falciparum sensitivity toward artemisinin. This resistance condition can lead to the loss of the ability of artemisinin as an effective antimalarial drug, especially for the treatment of malaria with high severity. Hence, research and development of potential antimalarial drugs are needed to overcome the problem of P. falciparum resistance to various currently available antimalarial drug regimens. One of those is the use of doxorubicin as an example oxidative stress-inducing agent with a different mechanism of action from artemisinin.
Doxorubicin is an antibiotic derivative with similar effect with artemisinin by inducing oxidative stress. In carrying out its action, doxorubicin is involved in the inhibition of DNA and RNA synthesis.9–12 In contrast to artemisinin which requires interaction with heme first to form free radicals, doxorubicin can be oxidized directly into a radical (semiquinone). Therefore, doxorubicin is a promising potential alternative drug candidate to replace artemisinin. The purpose of this study is to show the effect of doxorubicin administration on the inhibition of P. falciparum growth in vitro.
Materials and Method
Materials and Tools.
In vitro study was conducted at the Parasitology Laboratory, Faculty of Medicine, Universitas Padjadjaran, Bandung. Stock culture of chloroquine-resistant P. falciparum strain FCR-3/Gambia with registration number ATCC30932 obtained from the Parasitology Laboratory, Faculty of Medicine, Universitas Padjadjaran was used. The materials used in this study included RPMI medium (1640 R8578, Sigma Aldrich), RBC and serum from type O human blood, Phosphate Buffered Saline (PBS, Sigma Aldrich), doxorubicin (44583, Sigma Aldrich), and dye (Giemsa). Cell culture was carried out using a 25 cm2 flask, while the treatment with doxorubicin was carried out using 6 wells plate. Cell culture incubation was carried out at 37o C using a CO2 incubator.
Plasmodium falciparum cell culture preparation
The medium used to grow P. falciparum was complete medium and red blood. Complete medium was prepared by adding RPMI 1640 with serum from blood type O in a ratio of 9:1.13. Serum was obtained from venous blood taken using a vacutainer. Donor blood was centrifuged at 1,600 rpm for 10 minutes to extract the serum that presents in the supernatant. Serum was inactivated first at 56oC for 30 minutes before being added with RPMI 1640.
RBC was prepared by centrifuging the type O blood for 10 minutes at 1,600 rpm. The supernatant was discarded, and the precipitated pellet was washed with RPMI 1640 twice. Then the pellet was resuspended with RPMI 1640 in a ratio of 1:1. This suspension was stored at 4oC as a stock of RBC.
falciparum stock from -20oC was heated in a water bath at 37oC for 10 minutes. The stock was centrifuged for 10 minutes at 1,600 rpm. The pellet was washed with RPMI 1640 twice and then resuspended with 1 ml of complete medium. This suspension is ready to be used for further culture.
Plasmodium falciparum cell culture
RBC were counted using a hemocytometer so that it can reach 1 million blood cells. P. falciparum stock was added to complete medium and RBC in 50 ml Falcon tubes. This suspension was put into a 25 cm2 flask with a volume of 5 ml. The flask containing cell culture was incubated in a CO2 incubator. Medium is regularly replaced twice a week. After 1 week of maintenance, the cells were ready to be used for the next treatment.
Preparation of doxorubicin Solution
Doxorubicin was dissolved in distilled water to obtain an initial stock with a concentration of 8.6 mM. This stock was then diluted with RPMI 1640 to reach concentrations of 0.1µM, 0.2 µM, and 0.4µM. Fresh doxorubicin solution was used for cell treatment.
Doxorubicin treatment in P. falciparum culture
P. falciparum cells were cultured in a flask for 24 h, with a predetermined number of RBCs (1 million RBC per well in a 6-well plate). On day 1, the cells were harvested for further treatment with menadione. Parallel to this, the presence of parasites in RBC was detected using the Giemsa staining technique. Cell harvesting was done by transferring the culture medium into a falcon tube. Then the cells were washed twice with PBS, and the liquid was transferred to the same falcon. After that trypsin was added into the flask and incubate for 5-10 minutes in a CO2 incubator to release the cells attached on the surface of the flask. The trypsinized cell suspension was used for the treatment of cells with doxorubicin. The treatment was carried out using a 6 wells plate.
The control used was a medium containing RBC, RBC and P. falciparum, as well as RBC and doxorubicin 0.4 M. Treatment of P. falciparum cells with doxorubicin was carried out with three concentration variants (0.1 µM, 0.2 µM, and 0.4 µM). Each treatment was repeated twice. After doxorubicin administration has been carried out, the culture is put in a CO2 incubator for 2 x 24 hours.13
The controls used were wells containing medium, wells containing medium added with menadione with a concentration of 8µM, and wells containing medium and P. falciparum. Treatment wells consisted of medium added with doxorubicin with concentrations of 0.1µM, 0.2µM, and 0.4µM respectively. Each well was made with two repetitions. The cells were incubated for 48 hours in a CO2 incubator for further analysis.
Staining with Giemsa
Giemsa dissolved in PBS was used in this study (10% g/v). Giemsa staining was started by placing 10 µL of suspension on a slide. Then it was fixed with methanol and after that, add Giemsa dye for 10 minutes. The results were observed at 100x magnification to detect the presence of P falciparum in RBC.
Analysis
Cell culture observations were carried out twice (0 and 48 hours). The number of RBCs was calculated using a counting chamber or hemocytometer.14 The density of RBC and P. falciparum cells outside the RBC was determined by observation under a microscope.
Results
This research consists of two stages, the addition of P. falciparum to RBC culture to ensure that P. falciparum infects RBC and the treatment stage with doxorubicin.
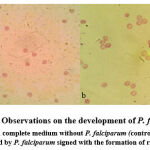 |
Figure 1: Observations on the development of P. falciparum (a) RBC in complete medium without P. falciparum (controll) (b) RBC infected by P. falciparum signed with the formation of ring (↑). |
The first results showed the presence of a ring form of P. falciparum in the RBC, which also proved that P. falciparum had successfully infected the RBC (Figure 1). Cell culture treatment with doxorubicin was initiated by ensuring that doxorubicin administration did not cause RBC lysis. It can be observed in control wells containing RBC in complete medium without the addition of doxorubicin and control wells containing RBC with the addition of 0.4 µM doxorubicin. The results revealed that the two wells had almost the same percentage reduction in RBC number (65% and 66%, respectively), prove that the highest dose of doxorubicin in this study (0.4 µM) did not cause RBC lysis (Table 1, Figure 2).
Table 1: Observation and Calculation of the Number of RBC and P. falciparum
| Observation time | |||||||
| Day 0 | Day 2 | Total number of P. falciparum observed outside red blood cell | |||||
| Total number of RBC | Density of RBC (per mm2) | Confluence of RBC (%) | Total number of RBC | Density of RBC (per mm2) | Confluence of RBC (%) | ||
| Control | |||||||
| (A) RBC | 160000 | 305485 | 80 | 55000 | 90810 | 50 | – |
| (B) RBC + Plasmodium falciparum | 160000 | 314767 | 80 | 6800 | 10810 | 30 | ++++ |
| (C)RBC + doxorubicin | 160000 | 318987 | 80 | 53000 | 89189 | 50 | – |
| Treatment | |||||||
| (D) RBC + Plasmodium falciparum + doxorubicin 0.1 μM | 160000 | 303797 | 80 | 8100 | 2702 | 10 | +++ |
| (E) RBC+ Plasmodium falciparum + doxorubicin 0.2 μM | 160000 | 302953 | 80 | 17500 | 31891 | 20 | ++ |
| (F) RBC + Plasmodium falciparum + doxorubicin 0.4 μM | 160000 | 305485 | 80 | 30000 | 58378 | 40 | + |
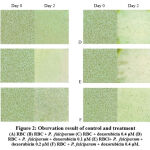 |
Figure 2: Obsrvation result of control and treatment (A) RBC (B) RBC + P. falciparum (C) RBC + doxorubicin 0.4 μM (D) RBC + P. falciparum + doxorubicin 0.1 μM. |
Furthermore, the observations and calculations of the number of RBC infected with P. falciparum after adding of doxorubicin showed a decrease in the number of lysed RBC on the second day. The percentage reduction in RBC at 0.1 µM, 0.2 µM, and 0.4 µM concentrations of doxorubicin were 94%, 89%, and 81%, respectively. Percentage reduction in RBC in the three variants of doxorubicin concentration was smaller than the control wells containing RBC and P. falciparum without doxorubicin which decreased the number of RBC by 95%. The wells containing RBC and P. falciparum added with doxorubicin 0.4 µM had the lowest red blood cell lysis (81%) compared to the other two variants of doxorubicin concentration, 0.1 µM and 0.2 µM with the decreasing number of RBC were 94% and 89%. These results suggested that doxorubicin can inhibit the development of P. falciparum, characterized by a reduced number of lysed RBC.
Reduction of lysed RBC was also confirmed by observing the RBC density per mm2 and red blood cell confluency in the control and treatment groups. In general, the density and confluency of RBC on the second day in the treatment group was greater than that of the control wells containing RBC and P. falciparum without the addition of doxorubicin. Density and confluency of RBC were found to be the highest at a concentration of 0.4 µM doxorubicin compared to other variants of doxorubicin concentration. These observations are also in line with the lowest concentration of P. falciparum cells outside the RBC observed at a doxorubicin concentration of 0.4 µM. Thus, the calculation of the number and density of RBC and P. falciparum revealed that doxorubicin could prevent infected RBC from lysis and inhibit the development of P. falciparum with the most significant effect found at the concentration of 0.4 µM doxorubicin.
Disscussion
Antimalarial drug discovery is crucial to overcome resistance problems. On the other hand, searching for potential antimalaria from existing drugs is preferable to simplify this endeavor. Some reports exhibited anticancer can also have antimalarial activity with different delivery ways: the combination of antimalaria with low-dose anticancer15 or repurposing anticancer as antimalarial drugs.16,17 Doxorubicin was established as a therapeutic agent.18,19,20 Hence this drug is one of the potential drug candidates to combat malaria disease. Several findings revealed that a compound can have multiple activities as anticancer and antilamaria. Anticancer targeting particular metabolism such as dihydrofolate reductase inhibitors (methotrexate, aminopterin, pemetrexate, edatrexate, pralatrexate or piritrexim), microtubulin assembly inhibitors (vinblastine, paclitaxel, tubulozole, docetaxel or dolastatin) and proteasome inhibitor (bortezimib) also active against Plasmodium.21 Anticancer drugs such as desatinib, oxaliplatin or irinotecan exhibit significant antiplasmodial activity.22 In addition, natural compounds such as vitamin C inhibit eukaryotic cell proliferation by causing oxidative stress in cancer cell23 and blood-stage Plasmodium.24
This investigation supports the antimalarial potential of doxorubicin in RBC infected with P. falciparum in vitro. Based on the data obtained, doxorubicin 0.4 µM had the greatest inhibitory effect on the development of P. falciparum, indicated by a decrease of lysed RBC. One of parameters that should be considered if doxorubicin will be tested in vivo is cytotoxicity of the drug against mammalial cell due to the compound can generate cardiomyopathy.25,26 The following are IC50 of doxorubicin on various cell lines when tested in vitro: 0.908 and 0.343 µM for prostate cancer cell line PC3 and DU145 respectively,27 1.1 and 0.72 µM for hepatocellular carcinoma cell line Huh7 and HepG2, respectively,28 2.5 µM for human lymphoma Ramos cell line,29 0.8 µM for VX2 cell line,30 27.96 and 9.93 µM for osteosarcoma cell lines D17 and U2OS, respectively,31 12.2009 µM for breast cancer MCF-7 and 10.339 µM for normal cell mouse fibroblast cell NIHT3T3.32 For those results we can concluded that the greatest concentration used in this study that cause greatest inhibitory of Plasmodium growth is still relatively low compared to most of IC50 of doxorubicin against various cell lines, hence increase of doxorubicin concentration is still possible. Moreover Lucas et al conducted a new doxorubicin delivery system to decrease cardiomyopathy: by loading the drug into RBC via electrophoretic method.33 The finding not only give knowledge that delivery doxorubicin inside RBC will reduce cytotoxic effect on heart of animal model, but also prove that doxorubicin didn’t destroy RBC if antimalarial effect of doxorubicin will be tested in vivo deliver via intravenous. Our research also fond that the greatest concentration of doxorubicin (0.4µM) did not generate RBC lysis in the absence of P. falcifarum.
Doxorubicin is an antibiotic derivative drug that can work in living cells by increasing oxidative stress levels.34 The same thing was also proven in a study conducted by Wang et.al. that doxorubicin can induce apoptosis in cells through various mechanisms.11 Doxorubicin inhibit particular enzymes involve in DNA metabolism such as DNA topoisomerase II10 and DNA methyltransferase I (DNMTI)35. Interestingly, doxorubicin also increases oxidative stress in cells by the oxidation of the molecule into less stable semiquinones.9 The release of Reactive Oxygen Species (ROS) in cells can induce cell membrane damage, lipid peroxidation, increased oxidative stress, and DNA damage that can lead to cell apoptosis. This mechanism is different from the increased oxidative stress by artemisinin which requires interaction with heme first to form free radicals. Therefore doxorubicin is promising potential drug as substitute of artemisinin.
Conclusion
The outcome of this research is that doxorubicin can reduce the number of lysed RBC due to P. falciparum and inhibit the development of P. falciparum with the greatest effect at a concentration of 0.4 µM. These results indicate that doxorubicin has potential as an alternative drug candidate for malaria.
Ackowledgement
Acknowledgments are addressed to Direktorat Riset dan pengabdian Kepada Masyarakat dan Inovasi – Universitas Padjadjaran (DRPMI-UNPAD) which has funded this research with Hibah Internal Universitas Padjadjaran-Riset Kompetitif Dosen Universitas Padjadjaran (HIU-RKDU) that made this research possible to be carried out.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding source.
References
- WHO. World Malaria Report 2017. World Health Organization. 2017.
- Epidemiologi Malaria di Indonesia. Kementerian Kesehatan RI. Jakarta; 2011;4.
- Atkinson J-AM, Fitzgerald L, Toaliu H, Taleo G, Tynan A, Whittaker M, et al. WHO | Malaria. Hum Resour Health. 2015;
- World Health Organisation. World Malaria Report. World Malaria Report. 2018.
- Buku Saku Penatalaksanaan Kasus Malaria. Jakarta: Kementerian Kesehatan RI; 2017.
- Rudrapal M, Chetia D. Endoperoxide antimalarials: development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold. Drug Des Devel Ther [Internet]. Dove Medical Press; 2016 Nov 1;10:3575–90. https://doi.org/10.2147/DDDT.S118116.
CrossRef - Ng CL, Fidock DA, Bogyo M. Protein Degradation Systems as Antimalarial Therapeutic Targets. Trends Parasitol [Internet]. 2017/07/05. 2017 Sep;33(9):731–43. https://doi.org/10.1016/j.pt.2017.05.009
CrossRef - WHO – Global Malaria Program. Status report on artemisinin resistance. 1. Subregion GM. Status report on artemisinin resistance. 2014;(January):1–7. 2014.
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011. https://doi.org/10.1097/FPC.0b013e32833ffb56
CrossRef - Taymaz-Nikerel H, Karabekmez ME, Eraslan S, Kırdar B. doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-31939-9.
CrossRef - Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms: Intermediacy of H2O2- and p53-dependent pathways. J Biol Chem. 2004. https://doi.org/10.1074/jbc.M400944200.
CrossRef - Sun Y, Xia P, Zhang H, Liu B, Shi Y. P53 is required for doxorubicin-induced apoptosis via the TGF-beta signaling pathway in osteosarcoma-derived cells. Am J Cancer Res [Internet]. e-Century Publishing Corporation; 2015 Dec 15;6(1):114–25. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27073729
- Walker J. Malaria: Methods and Protocols. Second Edi. Menard R, editor. Springer; 2013.
- Vembadi A, Menachery A, Qasaimeh MA. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front Bioeng Biotechnol [Internet]. Frontiers Media S.A.; 2019 Jun 18;7:147. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31275933
CrossRef - Aderibigbe BA and Ray SS. Preparation, characterization and in vitro release kinetics of polyaspartamide-based conjugates containing antimalarial and anticancer agents for combination therapy. Journal of Drug Delivery Science and Technology. Volume 36, December 2016, Pages 34-45. https://doi.org/10.1016/j.jddst.2016.09.006
CrossRef - Le Govic Y, Houzé S, Papon N. Repurposing Anticancer Drugs To Tackle Malaria. ChemMedChem. 2021 Jul 20;16(14):2192-2194. https://doi.org/ 10.1002/cmdc.202100176.
CrossRef - Yadav K, Shivahare R, Shaham SH, Joshi P, Sharma A, Tripathi R. Repurposing of existing therapeutics to combat drug-resistant malaria. Biomed Pharmacother. 2021 Apr;136:111275. https://doi.org/ 10.1016/j.biopha.2021.111275.
CrossRef - Yun UJ, Lee JH, Shim J. et al.Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab Invest . 2019. 99, 1157–1172. https://doi.org/10.1038/s41374-019-0193-1
CrossRef - Ouyang J, Yang M, Gong T, Ou J, Tan Y, Zhang Z, Li S. Doxorubicin-loading core-shell pectin nanocell: A novel nanovehicle for anticancer agent delivery with multidrug resistance reversal. PLoS One. 2020 Jun 22;15(6):e0235090. https://doi.org/10.1371/journal.pone.0235090.
CrossRef - Corti A, Sacchi A, Gasparri AM et al.Enhancement of doxorubicin anti-cancer activity by vascular targeting using IsoDGR/cytokine-coated nanogold. J Nanobiotechnol . 2021. 19, 128. https://doi.org/10.1186/s12951-021-00871-y
CrossRef - Nzila A, Okombo J, Becker RP, Chilengi R, Lang T, and Niehues T. Anticancer agents against malaria: time to revisit?. Trends in parasitology. 2010. 26(3), 125–129. https://doi.org/10.1016/j.pt.2009.12.002
CrossRef - Mogire RM, Akala HM, Macharia RW, Juma DW, Cheruiyot AC, Andagalu B, Brown ML, El-Shemy HA, Nyanjom SG. Target-similarity search using Plasmodium falciparum proteome identifies approved drugs with anti-malarial activity and their possible targets. PLoS One. 2017 Oct 31;12(10):e0186364. https://doi.org/10.1371/journal.pone.0186364.
CrossRef - Schoenfeld J D, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2(-) and H2O2-mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017. 31, 487.e8–500.e8. https://doi.org/10.1016/j.ccell.2017.02.018
CrossRef - Shi X, Wei M, Xu Z, Liu Y, Zhang M, Lv L, Wang Q. Vitamin C Inhibits Blood-Stage PlasmodiumParasites via Oxidative Stress. Front. Cell Dev. Biol., 11 May 2021 | https://doi.org/10.3389/fcell.2021.639944
CrossRef - Chatterjee, K., Zhang, J., Honbo, N., and Karliner, J. S. Doxorubicin cardiomyopathy. Cardiology.2010.115(2),155–162. https://doi.org/10.1159/000265166
- Abdullah, C.S., Alam, S., Aishwarya, R. et al.Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Sci Rep. 2019. 9, 2002 (2019). https://doi.org/10.1038/s41598-018-37862-3
CrossRef - Tsakalozou E, Eckman AM, Bae Y. Combination Effects of Docetaxel and Doxorubicin in Hormone-Refractory Prostate Cancer Cells. Biochemistry Research International. 2012. vol. 2012, Article ID 832059, 10 pages, 2012. https://doi.org/10.1155/2012/832059
CrossRef - Capone F, Guerriero E, Sorice A, Colonna G, Storti G, Pagliuca J, Castello G, Costantini S. Synergistic Antitumor Effect of Doxorubicin and Tacrolimus (FK506) on Hepatocellular Carcinoma Cell Lines. The Scientific World Journal.2014. vol. 2014, Article ID 450390, 9 pages, 2014. https://doi.org/10.1155/2014/450390
CrossRef - Dangkong D and Limpanasithikul W. Effect of citral on the cytotoxicity of doxorubicin in human B-lymphoma cells Pharmaceutical Biology. 2015. 53:2, 262-268, https://doi.org/10.3109/13880209.2014.914233
CrossRef - Pascale F, Bedouet L, Baylatry M, Namur J, Laurent A. Comparative Chemosensitivity of VX2 and HCC Cell Lines to Drugs Used in TACE. Anticancer Res. 2015 Dec;35(12):6497-503. PMID: 26637862.
- Małek A, Taciak B, Sobczak K, Grzelak A, Wójcik M, Mieczkowski J, Lechowski R, Zabielska-Koczywąs KA. Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma-In Vitro Studies. Molecules. 2021 Jun 8;26(12):3487. https://doi.org/10.3390/molecules26123487.
CrossRef - Tomankova K, Polakova K, Pizova K, Binder S, Havrdova M, Kolarova M, Kriegova E, Zapletalova J, Malina L, Horakova J, Malohlava J, Kolokithas-Ntoukas A, Bakandritsos A, Kolarova H, Zboril R. In vitro cytotoxicity analysis of doxorubicin-loaded/superparamagnetic iron oxide colloidal nanoassemblies on MCF7 and NIH3T3 cell lines. Int J Nanomedicine. 2015;10(1):949-961. https://doi.org/10.2147/IJN.S72590
CrossRef - Lucas A, Lam D, Cabrales P. Doxorubicin-loaded red blood cells reduced cardiac toxicity and preserved anticancer activity. Drug Deliv. 2019 Dec;26(1):433-442. https://doi.org/10.1080/10717544.2019.1591544.
CrossRef - Faraji A, Dehghan HR, Mobaraki M, Zare M, Houshmand M. Association of ABCB1 and SLC22A16 Gene Polymorphisms with Incidence of doxorubicin-Induced Febrile Neutropenia: A Survey of Iranian Breast Cancer Patients. PLoS One. 2016 Dec 30;11. https://doi.org/10.1371/journal.pone.0168519.
CrossRef - 35. Yokochi T, Robertson KD. Doxorubicin inhibits DNMT1, resulting in conditional apoptosis. Mol Pharmacol. 2004 Dec;66(6):1415-20. https://doi.org/10.1124/mol.104.002634.
CrossRef







