Sadanand Mallurwar1* , Kratika Daniel2
, Kratika Daniel2 and Mahesh Bhat3
and Mahesh Bhat3
1faculty of pharmacy, Mandsaur University, Mandsaur, Madhya Pradesh, India 458001
2Faculty of pharmacy,(B. R. Nahata College of Pharmacy),Mandsaur University, Mandsaur, Madhya Pradesh, India 458001
3Nuper Therapeutics, A division of Jain Pharmaceuticals, Off. No. 106, Nyati Emporious, Near Balewadi Stadium, Baner, Pune-411045
Corresponding Author E-mail: mallurwarsd@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/2395
Abstract
Purpose: The primary motive of this study was to examine advantages of allometry scaling strategies for correct prediction of pharmacokinetics of Baricitinib in human from preclinical species. Baricitinib is basically Janus kinase (JAK) inhibitor used for the treatment of rheumatoid arthritis. Currently approved by FDA in combination with remdesivir for treatment of COVID-19 hospitalized patient. Methods: The literature published pharmacokinetic parameters (Cl and Vd) of preclinical species (Rat, Dog and monkey) were utilized for the allometry scaling of Baricitinib. The connection among the primary pharmacokinetic parameters [Volume of distribution (Vd) and clearance (Cl)] and body weight (BW) were studied across three preclinical species, we used the double logarithmic plots for prediction of the human pharmacokinetic parameters i.e. Cl and Vd with use of simple allometry and with additional correction factors for better prediction. The dose extrapolation of baricitinib was carried out by FDA guidelines. Results: By application of the allometric scaling methods and principles correlation was found to be satisfactory for the prediction of intravenous human Cl and Vd for baricitinib. The volume of distribution (Vd) predicted by simple allometry (65.3 L) was found to be in agreement with the reported value (75.5 L); clearance (Cl) prediction by simple allometry was found to be at least 1.06 -closer to the reported value (245 mL/min); CF were used to predict the clearance. Both brain weight (B.W) and maximum life span potential (MLP) predicted the Cl with 0.52- and 0.61 -fold difference. The application of monkey liver blood flow predicted Cl with 0.81 fold which was also in close agreement with reported value. The Cl prediction was also extrapolated using LBF (Liver blood flow) method and observed that the higher species (Dog and Monkey) have predicted Cl with better accuracy than rat. Conclusions: Overall, the simple allometry (SA), monkey liver blood flow (MLBF) and application of liver blood flow (LBF) methods showed excellent correlation with human. The time vs. plasma concentration simulated graph also showed the similar closeness with human profile. The inclusion of plasma protein binding factor didn’t improve the prediction accuracy. The FIH dose extrapolation were showed that PK guided approach and exponent for BSA based approach was found closer to actual human dose of 4.0 mg/Kg.
Keywords
Clinical Pharmacokinetics; FIH; Interspecies Scaling; Preclinical Pharmacokinetics; Simulations
Download this article as:| Copy the following to cite this article: Mallurwar S, Daniel K, Bhat M. Advantages of Allometric Scaling Methods for Predicting Human Pharmacokinetics of Novel JAK Inhibitor -Baricitinib and Dose Extrapolation. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Mallurwar S, Daniel K, Bhat M. Advantages of Allometric Scaling Methods for Predicting Human Pharmacokinetics of Novel JAK Inhibitor -Baricitinib and Dose Extrapolation. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3BDeNbe |
Introduction
The novel JAK inhibitors tap down cytokine mediated signal via the JAK-STAT pathway, these have crucial role in immune regulation and growth. These ‘small molecule’ drugs are highly specific for blocking targets identified within cells that cause chronic inflammation 1. Rheumatoid arthritis is categorized as autoimmune disease and occurred in females than males and more frequently in elder population. The occurrence rate mentioned in year 2002 ranged from 0.5% to 1% 2
Though allometric scaling methods needs supplementary refinements and has certain limits, it is still considered as potential tool and balanced option for the estimate of pharmacokinetic parameters in species for which there are no data reported or to get good interpret preclinical efficacy and safety trials 3. Rheumatoid arthritis is a devastating disease that affects the quality of life and efficiency of millions worldwide. This disease is characterized by inflammation of the joints that leads to damage to the cartilage 4.
Baricitinib have anti-inflammatory, immunomodulatory and antineoplastic activities. It binds to JAK1/2 and inhibitsits activation and leads to the inhibition of the JAK-STAT signaling pathways. This inhibition lowers the production of inflammatory cytokines and prevents an inflammatory response and reduces tumor cell growth. Baricitinib approved for the treatment of moderate- to-severely rheumatoid arthritis in adults and was hypothesized to be a better therapeutic option for COVID–19. It was measured amongst all molecules studied to have a four role by inhibiting relevant cytokine signaling and have activity against NAKs, AAK1, and GAK 2,3 , which stimulate AP–2-linked host viral propagation 5–7.
Approved by the FDA for RA in June 2018 However, currently in phase 2 trials for psoriasis and AD 8
 |
Figure 1: Structure of baricitinib |
BenevolentAI identified this compound as a prospective treatment for COVID-19 in its AI-based hypothesis. Data from Eli Lilly’s trial showed that baricitinib significantly reduces mortality in hospitalized COVID-19 patients by 38%. Review of the literature suggested that allometry scaling of novel JAK inhibitor Baricitinib hasn’t been published.
Methods
We used literature published database to find relevant articles of baricitinib for allometry scaling exercise. Baricitinib preclinical (Rat, Beagle dogs and Monkey) and clinical data published were considered for allometry predictions. The observed human pharmacokinetic data were also gathered in order to enable the comparison of the predicted vs. observed pharmacokinetic data. The published intravenous data of baricitinib in three preclinical species were used for the allometry scaling study of baricitinib. The correlation between the main primary PK parameters [volume of distribution (Vd) and clearance (Cl)] and body weight (BW) was studied across three pre-clinical species by using double logarithmic plots for prediction of the human pharmacokinetic parameters of Cl and Vd using simple allometry methods.
The collected data of volume of distribution (Vd) and clearance (Cl) values were transformed into L and mL/min, respectively. Table 1 shows the PK parameters collected from published literature from three animal species (Rat, beagle dogs and Monkeys) that were used in this allometric scaling exercise.
The published studies indicate that the total body clearance is ~17 L/h and the renal clearance contribution is ~13.4 L/h in human healthy subjects; it showed that parent fraction of baricitinib is predominantly excreted via urine. The literature showed the major transporters involvement of P-gp, OAT-3 and MATE2-K for renal excretion 9.
Table 1: Preclinical PK parameters of baricitinib from published data
| Species | Body Wt.(Kg) | AUC0-t (hr*ng /mL) | T½
(h) |
Cl
(mL/min/Kg) |
Vd (L/Kg.) |
| SD Rat | 0.204 | 134 | 1.16 | 21.6 | 2.10 |
| Beagle dogs | 10.0 | NA | 3.47 | 6.66 | 1.40 |
| Cynomolgus Monkeys |
8.00 | NA | NA | 5.99 | 1.10 |
PK Data from EMA assessment report [9]; NA: Not available.
Simple allometry: The scaling of Vd and Cl was carried out as per below equations.
Vd = aWx…………………….(1)
Cl = bWy……………………(2)
Where, W is the body weight in kg of all species.
a and b are the coefficients and x and y are exponents of allometry.
The pharmacokinetic parameter (Vd or Cl) and W (Body Weight) were converted logarithmically and fitted to the below equation:
log (Vd or Cl) = log a + b log W, by linear least-square regression analysis.
The correction factors applied in this study are with consideration of mathematically derived constants like maximum life span potential (MLP), brain weight (BW) as well as the physiological process like kidney blood flow (KBF), Monkey liver blood flow (MLBF) and excretory mechanisms as bile correction factor; Glomerular filtration rate (GFR).
Inclusion of correction factors (CF): These CF was summarized and was included into allometric equations are as below equation:
log (Cl × MLP/BrW/GFR/KBF/bile correction) = log b + y log W …………… (3)
Human Cl (Predicted) = Monkey Cl × (Human LBF/Monkey LBF) ………….. (4)
The LBF method for prediction of human clearance (Cl) from each of the pre-clinical species as a fraction of liver blood flow as follows:
Human Cl (Predicted) = Animal clearance * (Human LBF/animal LBF) …(5) [10]
 |
Figure 2: Utility of allometric scaling in drug development. |
The allometric scaling of the clearance (Cl); the physiological parameters were taken into consideration like as brain weight (BrW) or maximum lifespan potential (MLP) of each species were included as correction factor. The values for body weight, Brain weight and MLP used in for the exercise are presented in Table 2.
Table 2: Body weight, Brain wt. and MLP values considered for allometry
| Species | Weight (Kg) | Brain wt (Gm) | Maximum lifespan potential MLP (Years) |
| Rat | 0.240 | 1.80 | 4.68 |
| Dog | 10.0 | 80.0 | 19.7 |
| Monkey | 8.00 | 100 | 8.01 |
| Human | 70.0 | 1400 | 93.4 |
Digitalization of reported pharmacokinetic data
The reported Phase-I patients pharmacokinetic data of Baricitinib (Xia Zhaoet al., 2020);11 was collected from phoenix WinNonlin 8.1 using graph reader software as graph of time vs. plasma concentration was reported and no individual plasma concentrations was published. (Available at www.grpahreader.com ; accessed on 02 May. 2021) and Phoenix WinNonlin 8.1 software (Pharsight, Mountain View, CA, USA).
Prediction of intravenous plasma vs. time profiles of baricitinib in humans
Baricitinib plasma concentrations vs. time profile were derived by using simulation using Phoenix WinNonlin 8.1 software. The simulation of human intravenous profile were carried out at 2 mg/ kg dose employing one compartment model with bolus input (using WNL5 classic modeling, PK model #1).The pharmacokinetics of baricitinib in humans was characterized by rapid absorption with a long apparent elimination half-life; the decrease in plasma concentrations displayed a biphasic profile (Xia Zhaoet al., 2020).
Table 3: Comparative human digitalized values from graphreader with the reported values (Dose-2 mg/Kg)
| PK parameters | Predicted | Reported* |
| T max (h) | 1.00 | 1.00 (0.50-1.50) |
| Cmax (ng/mL) | 23.7 | 24.6 (4.11) |
| AUCinf,obs (ng× h/mL) | 138 | 140 (15.3) |
*Source: Xia Zhao et al., 2020
Dose extrapolation
The retrospective analyses of baricitinib dose were done with applications of different dose extrapolations methods as follows:
As per FDA draft guidelines (USFDA, 2005) the dose by factor approach was applied using translation of animal doses to human equivalent doses.
Table 4: Animal equivalent dose factors of different species BSA-CS 12
| Species | BSA-CS (Divide human dose by) |
| Mouse | 0.081 |
| Hamster | 0.135 |
| Rat | 0.162 |
| Guinea pig | 0.216 |
| Rabbit | 0.324 |
| Dog | 0.541 |
| Monkey (Cynomolgus, Rhesus) | 0.324 |
| Marmoset | 0.162 |
| Baboon | 0.541 |
| Micro-pig | 0.730 |
| Mini-pig | 0.946 |
Application of Rat NOAEL
As per published data (CDER, report 2018) reported NOAEL in rat was 8 mg/Kg; with application of BSA correction factor and safety factor the calculated HED the predicted dose was 47 mg in human.
HED =NOAEL of most sensitive species (mg=kg) x BSA -CF x 60 (kg)
Application of Mice NOAEL
Mice NOAEL reported value is 300 mg/Kg; with application of BSA correction factor and safety factor the calculated HED was 1458 mg in human.
Application of exponent for body surface area (0.67)
By use of this method which applies an exponent for BSA (0.67) and consider for difference in metabolic rate (MR), to translate doses between animals and humans. Thus, HED is derived by the equation considering dog NOAEL
HED (mg / kg) = Animal NOAEL (mg / kg) × (Weight animal[ kg]/Weight Human [ kg] (1-0.67)
The calculated dose by this method was 0.132 mg/Kg; for 60 Kg it was 8.0 mg/Kg; which was 2 fold higher than actual clinical dose.
Pharmacokinetically guided approach
Starting dose = AUC in index species X Estimated clearance in human
The AUC obtained at the NOAEL of dog (index species) reported AUC0-t (µM*hr) -1.21 and estimated clearance in human (L)-58.14
=1.21 X 58.14=70.18 mg
With application of safety factor of 10; the starting dose calculated was 7.0 mg for human 19
Overall the PK guided approach and exponent for BSA based approach was found closer to actual human dose of 4.0 mg/Kg.
Table 5: Evaluations of scaling methods
| Parameter | Types of Scaling | Predicted values | Reported values | Fold differences |
| Vd (L) | Allometric scaling by simple method (for Vd) | 65.3 | 75.7 | 0.86 |
| Cl (mL/ min) | Simple allometry | 231 | 245 | 1.06 |
| MLP correction factor | 405 | 0.61 | ||
| Brain weight (B.W) correction factor | 467 | 0.52 | ||
| Glomerular filtration rate (GFR) correction factor | 145 | 1.69 | ||
| Kidney blood flow (KBF) correction factor | 180 | 0.74 | ||
| Bile correction factor | 627 | 0.39 | ||
| Monkey liver blood flow | 200 | 0.81 |
Table 6: Prediction of Cl based on LBF method (equation 5).
| Species | Predicted human Cl (mL/min) | Actual reported Cl of Human (mL/min) | Fold difference |
| Rat | 551 | 245 | 0.44 |
| Dog | 311 | 0.79 |
Table 7: Free fraction data values of preclinical species extracted from literature
| Species | Free fraction in serum (%) |
| Rat | 47 |
| Dog | 61 |
| Monkey | 49 |
| Human | 50 |
*Source: EMA assessment report 9
Result and discussion
In order to understand the PK (Pharmacokinetics) predictions in human and providing an opportunity to refine various allometric scaling predictions this exercise will guide the scientist from drug discovery to development.
A simple allometry correlation was found to be satisfactory for the prediction of intravenous human clearance (Cl)/volume of distribution (Vd) for baricitinib. The excellent correlation was observed between monkey and human as monkey liver blood flow method predicted the Cl value with 0.81- fold difference; the application of Cl based on LBF methods showed that higher species have better correlation than rat.
The predicted values of clearance observed were within 2.0 fold of values by simple allometry this showed success of allometry scaling methods for baricitinib.
Based on our study and reviewed literature it is well identified that volume of distribution can be predicted using simple allometric scaling; still, the clearance was not establishing to be agreeable for simple allometry and therefore, from long time correction factors has been proposed and in many cases the use of such correction factors (CF) has led to improved predictions of clearance and half-life. (Mahmood I.) 13 Studied and clarified the significance of correction factors that may further convey allometry as a proficient predictive tool. Therefore, in this study we explored advantages of these methods which were not applied for novel JAK inhibitors.
The application of simple allometry resulted in a predicted Cl value of 231 mL/min, which was close agreement (1.06) with reported human value of 245 mL/min. The use of correction factors (CF) was generally considered to provide a better estimate of the human Cl.
The application of correction factors (CF) as bile flow and glomerular filtration rate of each species can also be applicable for drug classes that show similarity in the drug disposition as compared to baricitinib. In agreement, the inclusion of GFR, KBF and bile correction factor resulted in prediction of 0.74 -fold, 1.69-fold and 0.39 fold respectively; lower Cl value by KBF and GFR methods and higher by biliary clearance method than the reported human value.
In the absence of human PK data the application of monkey liver blood flow (MLBF) has been proved as better tool for getting a good correlation to the human values as monkey have better correlation to human. The application of species specific liver blood flow led to the prediction of human Cl within 0.81-fold to the reported value.
The prediction of human Vd of baricitinib by simple allometry resulted in an exponent of 0.85, which is within the satisfactory range of 0.8-1.10 the similar results were observed in the literature for most of the compounds; this resulted in a predicted value of 65.3 L which was observed in close of the observed human value of 75.7 L.
The additional efforts were tried to find out impact of plasma protein binding factors (Table 7) and observed that the prediction was not improved for simple allometry and for rest of the methods; as protein binding are not different form rodent to human. The many literature studies showed that protein binding factor were successful for vertical allometry and drugs with high extraction rate. Dose extrapolation study showed that PK guided approach and exponent for BSA based approach was found to predict FIH dose within two fold of 4.0 mg/Kg.
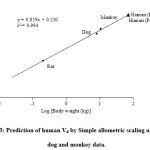 |
Figure 3: Prediction of human Vd by Simple allometric scaling using rat, dog and monkey data. |
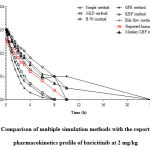 |
Figure 5: Comparison of multiple simulation methods with the reported human pharmacokinetics profile of baricitinib at 2 mg/kg |
Conclusion
Retrospective assessment of human pharmacokinetics prediction is necessary for improving prediction techniques and strategies for future candidate drugs.
Allometric scaling retrospectively predicted Vd and Cl parameters well within two fold of observed values. The overall PK of the baricitinib predicted with excellent accuracy with few exceptions of biliary corrections factor and could be used as a forthcoming tool for this class of drugs.
The correction factors evaluated in this study of baricitinib can be applicable for other or similar classes of drugs having similar metabolism and excretion pathways observed for baricitinib.
The reason for over prediction of biliary clearance may be due to lower recovery of baricitinib in feces as reported in published articles (Baricitinib recovery 15 % in feces by Sarah et al 2020) 14.
Allometry scaling will continue a method of choice for the prediction human PK parameters and the selection of first in human dose based solely on existing PK data of preclinical species.
Allometric scaling approaches will be decisive tool for scientists to optimize doses among species from preclinical to clinical stage of development.
Acknowledgement
None
Conflict of interest
The authors wish to declare that there are no conflicts of interests in the contents of the manuscript.
Funding Sources
There is no funding sources.
References
- Mallurwar SR, Nakra VK, Bhat MR. Clinical Prospectives of Janus Kinase Inhibitors: A review. Asian J Pharm Pharmacol. 2021;07 (2) : 85-91
CrossRef - Huang Q, Riviere JE, The application of allometric scaling principles to predict pharmacokinetic parameters across species Expert Opin. Drug Metab. Toxicol. 2014; 10 (9):1241-1253
CrossRef - Silman AJ, Pearson JE, Epidemiology and genetics of rheumatoid arthritis. Arthritis 2002; 4 (Suppl 3):S265-72
CrossRef - Scott DL, Wolfe F, Huizinga TW, Rheumatoid Lancet. 2010; 376: 1094–1108. http://dx.doi.org/10.1016/S0140-6736(10)60826-4
CrossRef - Inoue Y. et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007; 81(16): 8722-8729.
CrossRef - Bekerman E. et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest. 2017; 127(4): 1338-1352.
CrossRef - Owczarek K. et al. Early events during human coronavirus OC43 entry to the cell. Sci Rep.2018; 8(1): 7124.
- Papp KA. et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate‑to‑severe psoriasis. Br. J. Dermatol. 2016; 174 (6):1266‑76.
CrossRef - Committee for Medicinal Products for Human Use (CHMP)National Institute for Health and Care Excellence. Dec 2015 EMA/13493/2017
- Chiou WL, Robbie G, Chung SM, Wu TC, Ma C. Correlation of plasma clearance of 54 extensively metabolized drugs between humans and rats: Mean allometric coefficient of 66. Pharm Res. 1998; 15(9):1474–9.
CrossRef - Zhao X, Sheng XY, Christopher DP, Zhang X, Wang F et al. Pharmacokinetics, Safety, and Tolerability of Single- and Multiple-Dose Once- Daily Baricitinib in Healthy Chinese Subjects: A Randomized Placebo-Controlled Study Clin Pharm in Drug Development. 2020; 9(8) 952–960
- Nair AB, Jacob S A. simple practice guide for dose conversion between animals and human J Basic and Clin Pharm. 2016 ; 7(2): 27-31
CrossRef - Mahmood Pharmacokinetic allometric scaling of antibodies: Application to the First-in-human dose estimation. J Pharm Sci. 2009; 98 (10): 3850–3861
CrossRef - Sarah et. al. Baricitinib: A review of pharmacology safety, and emerging clinical experience in COVID-19. Pharmacotherapy 2020; 40(8):843–856
CrossRef - Mahmood I, Misconceptions and issues regarding allometric scaling during the drug development process Expert Opin Drug Metab Toxicol. 2018 ; 14(8):843-854
CrossRef - Sinha VK. et al. Towards a better prediction of peak concentration, volume of distribution and half-life after oral drug administration in man, using Clin Pharmacokinet. 2011; 50(2): 307-318.
CrossRef - Paine SW, Ménochet K, Denton R, McGinnity DF, Riley RJ. Prediction of human renal clearance from preclinical species for a diverse set of drugs that exhibit both active secretion and net Drug MetabDispos. 2011; 39 (6): 1008-1013.
CrossRef - Matthew W, CDER review Pharmacology/Toxicology review of OLUMIANT (baricitinib) CDER 2018
- www.grpahreader.com (Accessed on 15 may 2021)
- https://www.benevolent.com/news/baricitinib
- Sharma V, John HM. To scale or not to scale: the principles of dose extrapolations Br. J. Pharmacol. 2009; 157 (6):907-921.
CrossRef









