Priyanka S1* , Manju J2
, Manju J2 and Girish K1
and Girish K1
1Department of Pharmacology, Kempegowda Institute of Medical Sciences, Bangalore, India, 560070
2Department of Orthopaedics, Kempegowda Institute of Medical Sciences, Bangalore, India, 560070
Corresponding Author E-mail: drpriyanka110@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2374
Abstract
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) and muscle relaxants are widely prescribed for the highly prevalent painful muscle spasms. But there appears to be lack of published clinical study comparing the efficacy and tolerability with the intended study drugs. Aim: To compare the efficacy and tolerability of the fixed-dose combination (FDC) of Etoricoxib-Thiocolchicoside versus Thiocolchicoside alone in patients with painful muscle spasms. Materials and Methods: A prospective, open-label, randomized comparative study of 100 eligible outpatients with painful muscle spasms of either gender aged between 18 to 65 years were randomly assigned to receive oral medications b.i.d for 7 days of either FDC of Etoricoxib(60mg)-Thiocolchicoside(4mg)(GROUP-1) or Thiocolchicoside(4mg)(GROUP-2) alone. Results: The primary efficacy endpoint assessed by the percentage reduction in the intensity of pain utilizing a 10 cm Visual Analogue Scale (VAS) on movement and at rest was significant in both groups (p<0.0001) but more pronounced with GROUP-1 than GROUP-2 (p<0.05) on day 7 compared to day 1. The secondary efficacy endpoint assessed by patient and clinician using Global Assessment of Response to Therapy (GART) on a 5 point scale showed significantly larger number of patients with excellent to a very good response for GROUP-1 compared to GROUP-2. Though both the groups were well tolerated, the GART score for tolerability was more excellent in GROUP-2 than GROUP-1.Any adverse events during the study period was also recorded. Conclusion: The Etoricoxib -Thiocolchicoside combination is more effective with faster pain relief and less tolerable than Thiocolchicoside monotherapy, in treating patients with painful muscle spasms.
Keywords
Etoricoxib-Thiocolchicoside; Muscle Spasms; Thiocolchicoside; Visual Analogue Scale
Download this article as:| Copy the following to cite this article: Priyanka S, Manju J, Girish K. A Comparative Study of Efficacy and Tolerability of Fixed-Dose Combination of Etoricoxib and Thiocolchicoside versus Thiocolchicoside alone in Patients with Painful Muscle Spasms. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Priyanka S, Manju J, Girish K. A Comparative Study of Efficacy and Tolerability of Fixed-Dose Combination of Etoricoxib and Thiocolchicoside versus Thiocolchicoside alone in Patients with Painful Muscle Spasms. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3LEdSMC |
Introduction
Muscle spasms occurring in the general population are of varying prevalence depending upon various etiologies.1 They accompany degenerative or inflammatory diseases affecting the musculoskeletal system.2 Muscular spasms may occur when there is involuntary and forcible contraction of the muscle and cannot relax, as seen in conditions when it is overused, strained, fatigued as a result of stress, over exercising or dehydration. Usually it is self-limiting but if it delays, the consequences of it include absenteeism from work, unwarranted hospital and treatment costs and sometimes disablement. Its impact is seen on the society economically and socially.3 Therefore this condition should be addressed and effectively managed.
By definition, a muscle spasm is a sustained involuntary contraction of a muscle that is usually painful and cannot be relieved completely by voluntary effort.2 Conditions involving backache, neuralgias, torticollis, lumbosacral strain are associated with painful muscle spasms, which are treated with short-term use of centrally acting muscle relaxants combined with analgesics; but efficacy and tolerability are not impressive.4
Skeletal muscle relaxants are a class of drugs belonging to the diverse group, whose utility is seen in conditions involving centrally to reduce the muscle tone by acting at the cerebrospinal axis or to reduce muscular pain or spasms by acting peripherally at the neuromuscular junction or muscle fibre.5 Having a different mechanism of action, most of them act via their sedative property.
In general, majority of the centrally acting skeletal muscle relaxants used to treat muscle spasms have significant side effects such as sedation, drowsiness, mental confusion, weakness, ataxia, hallucinations, sudden withdrawal symptoms and anticholinergic side effects like dry mouth. Therefore, often the patient shows reduced compliance which leads to inefficacy in managing the painful spasms, resulting in reduced work capacity.4
Thiocolchicoside is derived semi-synthetically from colchicine which is extracted from the flower seeds of the species Gloriosa superb and Colchicum autumnale.6,7,8 It acts on the inhibitory neurotransmitters, glycine and gamma-aminobutyric acid (GABA)-A receptors, of the central nervous system. It is a muscle relaxant with anti-inflammatory and additive analgesic effects.9,10,11 It is clinically used from past four decades for the treatment of muscle pain and contractures in patients with orthopaedic, rheumatologic, or musculoskeletal disorders. It is used in various formulations-oral, parenteral, topical via transdermal and in the form of non-greasy topical foams or gels.11,12 Being well-tolerated, it has the advantage of reduced or no sedative effects, which is a drawback in the use of other muscle relaxants. It’s non-sedative property can be explained due to non-interference with nicotinic receptors. 8,13
Non-steroidal anti-inflammatory drugs are the medications which are routinely used for various musculoskeletal conditions worldwide. Currently, the selective Cyclooxygenase (COX)-2 inhibitors, a subclass of NSAIDs, have superseded the traditional NSAIDs which inhibit both consecutive COX-1 (responsible for gastrointestinal adverse effects) and inducible COX-2. Though the COX-2 NSAIDs and traditional NSAIDs show no significant difference statistically in the reduction of pain, the former has fewer gastrointestinal complications.14,15
Etoricoxib is a newer selective COX-2 inhibitor, having greater COX-2 selectivity compared to its congeners rofecoxib, valdecoxib, or celecoxib with gastroprotection being its advantage over other traditional NSAIDS.15 Fixed-dose combinations of NSAIDs and skeletal muscle relaxants may cause reduction in pain by acting through two different mechanisms and also the combined skeletal muscle relaxant can reduce the doses of NSAID making them much more favourable.3,16,17
Firstly, despite using skeletal muscle relaxants and analgesics for painful muscle spasms, the outcome is less promising and hence there is a need to reduce the social and economic disability by choosing an appropriate pharmacotherapy. Secondly, there are a lot of research done involving NSAIDs and muscle relaxants in FDC’s but no published data comparing the FDC’s of Etoricoxib and thiocolchicoside with thiocolchicoside monotherapy. Hence this comparative study to evaluate the objectives, efficacy and tolerability of the above two groups of drugs is conducted on the subjects with painful muscle spasms.
Materials and Methods
Study Design
This prospective comparative study which was randomized and open-label was conducted from December 2018 to May 2019 in the Orthopaedic outpatient department (OPD) of Kempegowda Institute of Medical Sciences, Bangalore. Approval of the study protocol was taken from the Institutional Ethics Committee and as well, obtaining written informed consent from all the study subjects. Registration of the trial was done with Clinical Trial Registry India (CTRI/ 2018/11/022223).
Study subjects
A total of 100 participants who fulfilled the specified inclusion and exclusion criteria were screened and then randomized to receive either of the two treatment protocols.
Inclusion criteria
All male and female subjects aged between 18-65yrs presenting to the Orthopaedic OPD with localized, uncomplicated, non-traumatic painful muscle spasm of recent onset (1-30 days) and who are willing to give written informed consent and available for regular follow up and examination were included in the study.
Exclusion criteria
Subjects with a history of kidney and liver damage, gastrointestinal bleeding or acid peptic diseases, cardiovascular disease, pregnant and lactating mothers, those allergic to NSAIDS and skeletal muscle relaxants or prior history of hypersensitivity reactions or asthma; those potentially requiring auxiliary treatment; those who were treated with NSAIDs or skeletal muscle relaxants 1 week before the study; those with severe concurrent systemic diseases including bleeding diathesis, on anticoagulant therapy and those with osteoporosis, malignancy, or previous surgical history of lumbar spine were excluded from the study.
Treatment procedure
After obtaining the demographic data and relevant medical history from the eligible study subjects, they underwent randomization through simple randomization procedure in the ratio 1:1 divided equally into two treatment groups, GROUP-1 and GROUP-2, each receiving the b.i.d dose of the treatments for 7 days. GROUP-1 (50 subjects) received FDC of Etoricoxib (60mg) with Thiocolchicoside (4mg) and GROUP-2 (50 subjects) received Thiocolchicoside (4mg) alone. Follow up of the study subjects was done for another week to ascertain complete remission of pain and none of them required any rescue medications.
Efficacy assessments
The primary efficacy outcome measure was evaluated for the intensity of pain at rest and on movement on day 1 (visit 0, baseline), day 3 (visit 1), and day 7 (visit 2) using a 10 cm Visual analogue scale (VAS) as reported by a patient where 0 implies no pain and 10 implies unbearable pain.
The secondary efficacy outcome measure included Global Assessment of Response to Therapy (GART). The clinician and the patient independently assessed the efficacy and tolerability at the end of the study (day 7) on a 5 point scale (0-Poor, 1-Fair, 2-Good, 3-Very Good, 4- Excellent).
At each visit the adverse events if any, were recorded.
Statistical analysis
A sample size of 100 participants was calculated (50 in each group) based on 90% prevalence of the disease as stated in previous studies, with a 95% confidence interval (CI), 5% type I error, and precision of 0.05.
Results for continuous data were expressed as mean ± standard; the discrete data were represented as numbers and percentages. Variables, within the group, were compared with paired t-test. Variables, between the groups, were compared with an independent t-test. Non-parametric data which included Global Assessment of Response to Therapy (GART) was assessed using Mann-Whitney U-test. 95% confidence level (p<0.05) was taken as statistical significance.
Results
In our study, 100 subjects were totally enrolled, with 50 in both the groups and all of them finished the study. Figure 1 depicts the flow of study participants. The demographics and the baseline characteristics are compared as in Table 1.
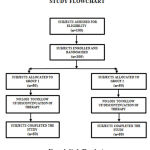 |
Figure 1: Study Flowchart. |
Table 1: Demographic and baseline characteristics.
| PARAMETERS | GROUP- 1
(ETORICOXIB + THIOCOLCHOCOSIDE) n=50 |
GROUP- 2
(THIOCOLCHICOSIDE ALONE) n=50 |
p Valueδ | |
|
AGE (yrs)
|
38.44 ± 11.32* | 38.36 ± 10.53* | 0.9709 | |
|
WEIGHT (kgs)
|
66.8 ± 8.02* | 68.12 ±7.11* | 0.3860 | |
|
HEIGHT (cm)
|
159.74 ± 4.81* | 161.43 ± 6.57* | 0.1454 | |
| GENDER (%)
|
Male | 18 (9%) | 22 (11%) |
0.7401 |
|
Female |
32 (16%) | 28 (14%) | 0.7805 | |
| *Results are expressed as mean ± standard deviation.
δSignificance (p value) obtained using an unpaired t-test |
||||
Of the 100 participants, 60% (n=60) were female participants and 40% (n=40) were male participants (p=0.13). There was no statistically significant difference with the gender distribution (p> 0.05). The incidence of muscle spasms was found to be more among the age group 35-45years (n=28, 28%) as shown in the Table 2 and Figure 2. Mean body weight was 65.15 ± 4.55 in GROUP-1 and 63.11 ± 5.43 in GROUP-2. Mean height was 160.23 ± 2.45 in GROUP-1 and 162.83 ± 5.67 in GROUP-2.
Table 2: Age and gender distribution
|
Age and gender distribution |
|||
| AGE (YRS) | MALE | FEMALE |
Percentage of Participants |
| 18-25 | 2 | 3 |
5% |
| 26-35 | 7 | 17 |
24% |
| 36-45 | 11 | 17 |
28% |
| 46-55 | 14 | 13 | 27% |
| 56-65 | 6 | 10 | 16% |
| TOTAL | 40% | 60% | p value = 0.13 |
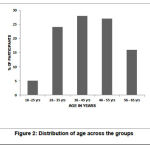 |
Figure 2: Distribution of age across the groups |
Primary Efficacy endpoint
Pain scores as assessed by VAS showed a statistically significant reduction (p<0.0001) with movement and at rest from day 1 to day 7 within the group. Also between GROUP -1 and GROUP-2 there was a statistically significant difference in the reduction of pain scores (p<0.05, 95% CI). The above findings are depicted in Table 3. The corresponding percentage improvement in pain relief from day 1 to day 3 to day 7 with movement and at rest in both the groups is shown in Figure 3a and Figure 3b respectively.
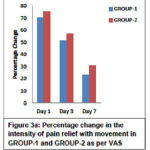 |
Figure 3a: Percentage change in the intensity of pain relief with movement in GROUP-1 and GROUP-2 as per VAS |
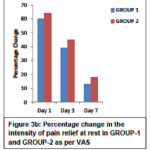 |
Figure 3b: Percentage change in the intensity of pain relief at rest in GROUP-1 and GROUP-2 as per VAS |
Table 3: Evaluation of primary efficacy endpoint (intensity of pain relief) as per VAS
| Evaluation of primary efficacy endpoint (intensity of pain relief) as per VAS | |||||
| INTENSITY OF PAIN | GROUP- 1
ETORICOXIB+THIOCOLCHICOSIDEα (n=50) |
GROUP- 2
THIOCOLCHICOSIDEα (n=50) |
P-value* | 95% CI | |
| Baseline
Day 1 Mean± SD |
With movement | 7.02 ± 1.22 | 7.54 ± 0.84 | 0.0148 | 0.1043-0.9357 |
| At Rest | 6.04 ± 1.59 | 6.44 ± 0.96 | 0.1310 | -0.1213-0.9213 | |
| Day 3
Mean ± SD |
With Movement | 5.08 ± 1.47 | 5.66 ± 1.12 | 0.0288 | 0.0614-1.0986 |
| At Rest | 3.94 ± 1.57 | 4.48 ± 1.16 | 0.0533 | -0.0078 -1.0878 | |
| Day 7
Mean ± SD |
With Movement | 2.28 ± 1.60 | 3.1 ± 1.29 | 0.0058 | 0.2432 – 1.3968 |
| At Rest | 1.26 ± 1.32 | 1.82 ±1.22 | 0.0299 | 0.0556 -1.0644 | |
| P-valueδ
(Between day 1 to day 7) |
With movement and at rest | < 0.0001 | <0.0001 | ||
| αResults are expressed as mean ± standard deviation(SD)
*Significance (P value) obtained using an unpaired t-test δSignificance (P value) obtained using a paired t-test |
|||||
The mean (± SD) VAS score with movement reduced from 7.02 ± 1.22 at baseline to 2.28 ± 1.60 on day 7 in GROUP-1 and 7.54 ± 0.84 at baseline to 3.1 ± 1.29 on day 7 in GROUP-2. Similarly the mean (± SD) VAS score at rest reduced from 6.04 ± 1.59 at baseline to 1.26 ± 1.32 on day 7 in GROUP-1 and 6.44 ± 0.96 at baseline to 1.82 ± 1.22 at rest on day 7 in GROUP-2. However, the percentage reduction in the pain severity was greater within GROUP-1 than GROUP-2 (p<0.05, 95% CI).
Secondary efficacy endpoint
The Global efficacy assessment by the patient was rated as excellent to a very good response for GROUP-1 (52%-44%) compared to GROUP-2 (46%-40%) as shown in Figure 4a. The Global efficacy assessment by the clinician was from excellent to very good in GROUP-1 (64%-30%) which was better compared to GROUP-2 (60%-32%) as shown in Figure 4b. The Global tolerability scores as assessed by the patient ranges from excellent to very good in both the groups but were found to be better in GROUP-2 ranging between (84% to 14%) compared to (78% to 10%) in GROUP-1 as shown in Figure 5a. Similarly, the Global tolerability assessment done by the clinician showed a better response ranging between excellent to very good in GROUP-2 (94% to 4%) compared to GROUP-1 (82% to 8%) as shown in Figure 5b.
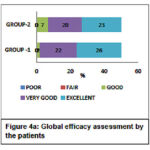 |
Figure 4a: Global efficacy assessment by the patients |
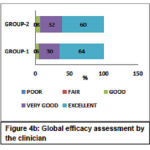 |
Figure 4b: Global efficacy assessment by the clinician |
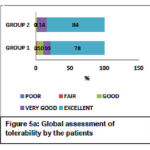 |
Figure 5a: Global assessment of tolerability by the patients |
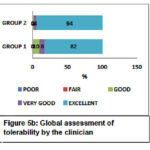 |
Figure 5b: Global assessment of tolerability by the clinician |
Safety
About 24% (n=12) of the subjects had adverse events in GROUP-1 compared to 14% (n=7) in GROUP-2 as depicted in Table 4. A total of 19 adverse events were reported. The commonest adverse event was gastritis complained by 7 subjects in GROUP-1 and 2 subjects in GROUP-2. Other adverse events complained in GROUP-1 were nausea (n=3), itching (n=1), drowsiness (n=1). In GROUP-2 the adverse events reported were nausea (n=1), dizziness (n=1), drowsiness (n=2) and dyspepsia (n=1). These reported adverse events were possibly associated to the study medications and were of mild to moderate intensity requiring not much intervention or discontinuation of the therapy.
Table 4: Adverse events reported in both the study groups
| ADVERSE EVENTS | GROUP 1 (n=50) | GROUP 2 (n=50) |
| GASTRITIS | 7 | 2 |
| NAUSEA | 3 | 1 |
| ITCHING | 1 | 0 |
| DIZZINESS | 0 | 1 |
| DROWSINESS | 1 | 2 |
| DYSPEPSIA | 0 | 1 |
| TOTAL | (n=12) 24% | (n=7) 14% |
Discussion
Painful muscle spasms are the most frequently encountered acute problem occurring in all age groups and are also seen in any occupation related to stress. Although it is mild and subsides in due course of time, quick relief and easy healing with minimal adverse effects is desired to comfortably get back to the daily routine in our life.18 Many therapeutic interventions have been tried for this purpose but remain unsatisfactory. Among the commonly prescribed medications worldwide, the traditional NSAIDs, newer selective COX-2 inhibiting NSAIDs, and muscle relaxants form the basis for treating muscle spasms due to their analgesic and anti-inflammatory properties.14,15
In this study, the results suggested that though both FDCs of the Etoricoxib with Thiocolchicoside and Thiocolchicoside monotherapy showed similar efficacy; Thiocolchicoside monotherapy had fewer adverse events compared to the FDCs.
In the present study, there was a higher percentage of female participants compared to male participants; though non-significant it is found in accordance with the previous study conducted by Ajya Sah and Rudip Thapa, where female participants were 57% and male participants were 43%.3 However, in the study conducted by Raut Asawari et al. the male participants (82.5%) were predominant to female participants (17.5%).19
The percentage of individuals presenting with painful muscle spasms are more in the age group of 36-45yrs (28%), 46-55yrs (27%) which is similar to the study Ajya Sah and Rudip Thapa3 and Anil Pareek et al16 suggesting that it is more common with increasing age (middle and elderly individuals ) due to the stress-related, work-related injuries or due to lack of adequate physical activity.20 Also, this age group represents the working-age group and, long-term absenteeism from work due to the ailment is not desired.1,14 Hence an appropriate and effective medication that offers quick and immediate relief has to be proposed.
Though the pain reduction has been seen significantly (p<0.0001) in both the groups, the primary efficacy endpoint which included pain scores has been reduced more in the Etoricoxib and Thiocolchicoside group than the Thiocolchicoside monotherapy group (p<0.05). There has been no study with this combination of drugs, but studies have been made with different combinations of NSAIDs with muscle relaxants. In the Ajya Sah and Anil Pareek studies, the efficacy of aceclofenac plus tizanidine has been better compared to aceclofenac alone.3,16 In Raut Asawari et al diclofenac and thiocolchicoside reduced more pain than diclofenac alone19 and in Sachdeva et al Aceclofenac and Thiocolchicoside reduced more pain scores compared to Aceclofenac.20 This shows the superiority of the FDC’s over Thiocolchicoside alone due to their additive effect.
In the secondary efficacy endpoint, the patient and the physician efficacy parameter was better in GROUP 1 compared to GROUP 2 showing the superiority of the combined combination over the single muscle relaxant.18,21
But in some cases and some studies, the skeletal muscle relaxants like Tolperisone and Thiocolchicoside used alone have shown better efficacy compared to placebo, and also for tolerability they have an upper hand compared to the NSAIDs.1,19
Though both groups are safe with very few adverse effects, in this study, adverse events were more in the combination group and more with Etoricoxib than thiocolchicoside. Etoricoxib has more adverse effects with gastritis hence less tolerable than thiocolchicoside. Unlike other muscle relaxants, Thiocolchicoside has been reported to have less gastrointestinal side effects and was well tolerated.23 The extension of its pharmacological actions has been shown as the side effect of a centrally acting skeletal muscle relaxant.
The limitations of the study are that the sample size is small and it is an open-label study. This can be addressed by conducting multicenter randomized control study to establish the beneficial effect of the FDC of Etoricoxib and Thiocolchicoside in various other acute pains with muscle spasms with a larger study population.
Conclusion
In the present study for the treatment of painful muscle spasms, the fixed-dose combination of Etoricoxib and Thiocolchicoside has shown better efficacy with faster pain relief compared to Thiocolchicoside alone though it is more tolerable than the combination therapy.
Acknowledgment
The author sincerely thanks the Orthopaedic Department for allowing to conduct the study and provide the clinical material. All authors have read and approved the final manuscript.
Conflicts of Interest
There are no conflicts of interest.
Funding Sources
There is no funding source for this study
References
- Prabhoo R, Keny S, Prabhoo T, et al. A Phase IV Observational Multi-centre, Open-Label Study on Efficacy and Safety of Tolperisone 150 mg in Patients with Painful Muscle Spasm Associated with Degenerative or Inflammatory Diseases of the Musculoskeletal System. The Journal of the Association of Physicians of India; 59: 33-37 (2011).
- Pratzel HG, Alken RG, Ramm S. Efficacy and tolerance of repeated oral doses of tolperisone hydrochloride in the treatment of painful reflex muscle spasm: results of a prospective placebo-controlled double-blind trial. Pain; 67(2-3): 417-425 (1996).
CrossRef - Sah Ajya, Thapa Rudip. Comparative Study On Efficacy Of Aceclofenac And Aceclofenac Plus Tizanidine In Patients With Low Back Pain At Nepal Medical College And Teaching Hospital. Indian Journal Medical Research and Pharmaceutical Sciences; 4(12): 39-44 (2017).
- Witenko Corey, Moorman-Li Robin, Motycka Carol, et al. Considerations for the Appropriate Use of Skeletal Muscle Relaxants for the Management Of Acute Low Back Pain. Pharmacy and Therapeutics; 39(6): 427-435 (2014).
- Chou R, Peterson K, Helfand M. Comparative Efficacy and Safety of Skeletal Muscle Relaxants for Spasticity and Musculoskeletal Conditions: A Systematic Review. Journal of Pain and Symptom Management; 28(2): 140-175 (2004).
CrossRef - Rajput JK, Patil PH, Surana SJ, et al. Analytical Methods for Determination of Muscle Relaxant Thiocolchicoside in Pharmaceutical Preparations- A Review. Open Pharmaceutical Sciences Journal; 2: 43-55 (2015).
CrossRef - Umarkar AR, Bavaskar SR, Yewale PN. Thiocolchicoside as Muscle Relaxant: A Review. International Journal of Pharmacy and Biological Sciences; 1(3): 364-371 (2011).
- Soonawalla DF, Joshi N. Efficacy of thiocolchicoside in Indian patients suffering from low back pain associated with muscle spasm. Journalof the Indian Medical Association; 106(5): 331-335 (2008).
- Janbroers JM. Review of the toxicology, pharmacodynamics and pharmacokinetics of thiocolchicoside, a GABA-agonist muscle-relaxant with anti-inflammatory and analgesic actions. Acta Therapeutica; 13(3): 221-250 (1987).
- Bashir SI, Rashid S. Randomised Comparative Study of Aceclofenac Thiocolchiside Fixed Dose Combination Against Aceclofenac Alone In Treatment Of Acute Nuchal Pain: Safety And Efficacy Assessment. International Journal Of Advances In Case Reports; 2(1): 37-40 (2015).
- Aguzzi C, Rossi S, Bagnasco M, et al. Penetration and Distribution of Thiocolchicoside through Human Skin: Comparison Between a Commercial Foam (Miotens) and a Drug Solution. AAPS PharmSciTech; 9(4): 1185-1190 (2008).
CrossRef - Bonina F, Puglia C, Trombetta D, et al. Vehicle effects on in vitro skin permeation of thiocolchicoside. Pharmazie; 57(11): 750-752 (2002).
- Patat A, Klein MJ, Surjus A, et al. Effects of acute and repeated doses of two muscle relaxants chlormezanone and thiocolchicoside, on vigilance and psychomotor performance of healthy volunteers. Human Psychopharmacology; 6: 285-292 (1991).
CrossRef - Roelofs PDDM, Deyo RA, Koes BW, et al. Non-steroidal anti-inflammatory drugs for low back pain. Cochrane Database of Systematic Reviews; (1): CD000396 (2008). doi: 10.1002/14651858.CD000396.pub3.
CrossRef - Brooks P, Kubler P. Etoricoxib for arthritis and pain management. Therapeutics and Clinical Risk Management; 2(1): 45-57 (2006).
- Pareek A, Chandurkar N, Chandanwale AS, et al. Aceclofenac-tizanidine in the treatment of acute low back pain: a double-blind, double-dummy, randomized, multicentric, comparative study against aceclofenac alone. Eur Spine J; 18: 1836-1842 (2009).
CrossRef - Rao R, Panghate A, Chandanwale A, et al. Clinical Comparative Study: Efficacy and Tolerability of Tolperisone and Thiocolchicoside in Acute Low Back Pain and Spinal Muscle Spasticity. Asian Spine Journal; 6(2): 115-122 (2012).
CrossRef - Kumar S, Rani S, Siwach R, et al. To compare the efficacy and safety of fixed dose combination of thiocolchicoside and aceclofenac versus chlorzoxazone, aceclofenac and paracetamol in patients with acute lower backache associated with muscle spasm. International Journal of Applied and Basic Medical Research; 4(2): 101-105 (2014).
CrossRef - Raut A, Reddy G, Patil S, et al. Comparative Efficacy Of Combined Use of Diclofenac with Thiocolchicoside and Diclofenac alone in Orthopedic Patients. International Research Journal of Pharmacy; 4(2): 164-166 (2013).
- Nikose S, Khan S, Gudhe M, et al. Efficacy of Diclofenac versus combination of Ibuprofen and Paracetamol, in Acute Low Back Pain with Sciatica. European Journal of Pharmaceutical and Medical Research; 3(12): 487-491 (2016).
- Desai AA, Sachdeva PD, Bhoomi D. A Comparative Study of combined use of Aceclofenac along with Thiocolchicoside and Aceclofenac alone in patients diagnosed of low back pain. An International Journal of Pharmaceutical Sciences; 2(2): 141-150 (2011).
- Patel HD, Uppin RB, Naidu AR,et al. Efficacy and Safety of Combination of NSAIDs and Muscle Relaxants in the Management of Acute Low Back Pain. Pain Ther; 8: 121-132 (2019).
CrossRef - Reddy DMK, Jagarlamudi P. To compare the efficacy and safety of thiocolchicoside and chlorzoxazone in muscle spasm associated with low back pain. J. Evolution Med. Dent. Sci; 7(48): 5150-5153 (2018).
CrossRef








