Adiatmo Pratomo1 , Nina Mariana1*
, Nina Mariana1* , Surya Otto Wijaya1
, Surya Otto Wijaya1 , Betha Ariesanty1
, Betha Ariesanty1 , Titi Sundari1
, Titi Sundari1 , Dian Wahyu Tanjungsari1
, Dian Wahyu Tanjungsari1 , Herlina1
, Herlina1 , Siti Maemun1
, Siti Maemun1 ,2, Farida Murtiani1
,2, Farida Murtiani1 , Vivi Lisdawati1
, Vivi Lisdawati1  and Mohammad Syahril1
and Mohammad Syahril1
1Sulianti Saroso Infectious Disease Hospital, Jakarta, Indonesia
2Department of Public Health, University of Respati Indonesia, Jakarta, Indonesia
Corresponding Author E-mail:- mynayla09@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2334
Abstract
Background: Coronavirus disease 2019 (COVID-19) was declared as a world pandemic since early 2020. There was no specific antiviral agent that appeared to be active against the virus, and antiviral agent such as remdesivir, favipiravir were in limited supply. We evaluated the use of convalescent plasma (CP) administered as adjuctive treatment to standard of care in moderate to severe COVID-19 patients. Methods: We conducted a series of 9 moderate to severe patients of COVID-19 older than 18 years received CP transfusion from 9 recovered donors at a single institution (Sulianti Saroso Infectious Disease Hospital, Jakarta, Indonesia) from January 2021 to June 2021. Results: Out of 9 patients (age range 30-81 years, 6 males and 3 female), and all patients received at least 1 or 2 unit of 200 mL of CP from 9 recovered donors. There were 4 patients (age range 30-71 years, 4 male) that were not treated with antiviral therapy. Of the 9 patients, 2 severe cases were died, while all of moderate cases survived and they were discharged from the hospital (length of stay: 8-22 days). Conclusion: Our experience showed that CP transfusion in moderate COVID-19 patients might provide clinical benefit and it was well-tolerated. However, further development clinical trials with better designs and greater power is needed to evaluate the efficacy and safety of this treatment.
Keywords
Convalescent plasma transfusion; COVID-19; Clinical responses
Download this article as:| Copy the following to cite this article: Pratomo A, Mariana N, Wijaya S. O, Ariesanty B, Sundari T, Tanjungsari D. W, Maemun H. S, Murtiani F, Syahril. The Use of Convalescent Plasma (CP) Transfusion Therapy in Moderate to Severe COVID-19 Patients: a Single Center Experience in Indonesia. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Pratomo A, Mariana N, Wijaya S. O, Ariesanty B, Sundari T, Tanjungsari D. W, Maemun H. S, Murtiani F, Syahril. The Use of Convalescent Plasma (CP) Transfusion Therapy in Moderate to Severe COVID-19 Patients: a Single Center Experience in Indonesia. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3ErXj1n |
Introduction
Coronavirus disease 2019 (Covid-19) was declared as a world pandemic on March 11, 2020.1 The manifestation of illness are mild disease to severe respiratory failure that requiring intensive care unit admission.2 As of September 24, 2021, it had caused a total of 230,418,451 cases of infection and resulted in 4,724,876 deaths globally.3 Dyspnea, comorbidities, elevated C-reactive protein levels, and desaturation predict mortality in moderate to severely ill Covid-19 patients.4
There was no antiviral agent that appeared to be active against the virus, and antiviral agent such as remdesivir, favipiravir were in limited supply.5 So far, the incomplete knowledge of Covid-19 is still the biggest challange and resulting in a lack of effective drugs and adequate treatment strategies.6 Treatment guidelines are updated periodically.4
Previous studies showed that CP’s efficacy was better if used at the early stages of infection, and it was the most effective when administered before the patients produces a significant amount of Immunoglobulin G (IgG) antibodies.6 However the quality control of CP and variation of cases in patients in different studies make it rather difficult to evaluate the efficacy and risk of CP therapy, especially in our country.2 Thus, in this study, we evaluated the use of CP administered in moderate to severe patients with COVID-19, such as variation of clinical outcomes, changes of laboratory parameters, the disappearance of SARS-Cov-2, and adverse events.
Methods
A case series was conducted at sulianti saroso infectious disease hospital, Jakarta, from January 2021 to July 2021. This study was a part of a multicenter clinical trial of The National Covid-19 consortium that approved by the Ethics Committee of the Research and Development Agency, Ministry of Health, Indonesia (No.02.01/2/KE.351/2020) and each patient gave written informed consent.
Patients
This study evaluated 9 patients who were diagnosed with COVID-19 by RT-PCR; aged ≥ 18 years, had moderate to severe of Covid-19. Severe COVID-19 described by signs of severe respiratory such as any of oxygen saturation < 93 % on room air and respiratory rate > 30 breaths/min. Moderate Covid-19 described by signs of pneumonia and as absence of any criteria for severe COVID-19. The 9 patient had signed informed consent form. The patients’s serum was cross-checked for potential compatibility with the CP donor before the transfusion, and each patient received at least 1 or 2 unit of 200 ml CP (200 ml – 400 ml) according to the ABO blood types.
Donors
This study recruited 9 the CP donors based on the following criteria: aged 18-60 years that had a history of hospitalization with a diagnosis of COVID-19 and asymptomatic for more than 14 days. The 9 donors were recruited to donate their convalescent plasma after they signed the informed consent form. All donors tested negative of SARS-CoV-2, hepatitis B virus, hepatitis C virus, HIV, and syphilis at the time of blood donation. We used the Abbott Sars-CoV-2 IgG test (Architect) for detection of specific IgG levels against SARS-CoV-2 by the index (S/C) ≥ 4.5 of serum SARS-CoV-2 IgG.8 This immunoassay detected IgG antibodies in plasma and serum samples, which bind to SARS-CoV-2 recombinant antigen coated micro particles.7 The signal/cut off index (S/C) for this study was ≥ 4.5 as acceptable to be used for the purpose of qualifying high titer Covid-19 convalescent plasma.7,8 Following donation, 2 unit of CP (each unit being approximately 200 mL in volume) was obtained from each donor by aphaeresis.
Clinical Information
The 9 subjects were assessed on day 0 (before CP transfusion), day 1, day 3 and day 7 after CP transfusion such as clinical data, including body temperature, PAO2/FIO2, and Sequential Organ Failure Assessment (SOFA) score (range 0-11); laboratory data, including white blood cell count, blood gas analysis, cycle threshold value (Ct value), inflammatory factors C-reactive protein (CRP), procalcitonin, and interleukin-6; the various treatments; the data of chest X-ray; the clinical information of acute respiratory distress syndrome (ARDS). The other of clinical information such as demographic data, days of admission from symptom onset, any clinical symptom and also any comorbidity obtained from the medical record. We collected the all of data in tabular fashion.
Treatment outcome
We evaluated the use of CP administered in moderate to severe patients with COVID-19, such as variation of clinical outcomes, changes of laboratory parameters, the disappearance of SARS-Cov-2, and adverse events.
Result
A total of 9 Covid-19 patients (age range 30-81 years, 6 males and 3 females) and none were smokers. Figure 1 showed the flowchart resuming of 9 patients. The most common comorbidity were Hypertension. The characteristics of patients who receiving CP transfusion as showed Table 1.
 |
Figure 1: Flowchart resuming of 9 patients |
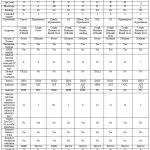 |
Table 1: Characteristics of Patients Who Receiving CP Transfusion |
DM: diabetes mellitus; TRALI: Transfusion-related acute lung injury; DXM: Dexamethasone; MPM: Meropenem; AZT: azithromycin; LFX: levofloxacin; RMS: Remdesivir; FPV: Favipiravir
The majority patients were cough and shortness of breath. Radiological results showed the majority of subjects have infiltration. Range of Interval between symptom onset and admission or positive Covid-19 was 1 until 11 days, and interval between symptom onset and plasma transfusion were 7 until 16 days (table 1).
Moderate and severe cases of Covis-19 were 6 and 3 cases, respectively. After the transfusion of convalesent plasma, two patient of severe cases required intensive ward and mechanical ventilation. Two patients were died, while all of moderate cases were survive and discharged from the hospital (length of stay: 8-22 days). All of 9 patients immediately received therapy standard at the hospital and also received CP transfusion. The range days of receiving CP transfusion on moderate cases and severe cases of Covid-19 were 7-16 days, 8-10 days, respectively. Meanwhile 2 patients of severe cases experienced Transfusion-related acute lung injury (TRALI). Majority patients received steroids therapy. Four patients received remdesivir, one patients received favipiravir, only 4 patients were not treated with antiviral therapy. They did not receive antiviral agents due to limited supply at the time. All of patients were treated with antibiotics, 2 patients got combination such as azithromycin and levofloxacinin. (Table 1)
Nine patients received at least 1 or 2 unit of 200 ml CP transfusion (200 ml – 400 ml) from 9 recovered donors. Table 2 showed Characteristic and antibody titer of convalesent plasma donors. The donors had recovered from SARS-Cov-2 infection and the majority of severities of Covid-19 were moderate to severe cases. Our study screened for the IgG test using an Abbott Sars-CoV-2 IgG test (Architect). Interval between moderate or severe symptom onset and IgG test were 39 – 180 days.
Table 2: Characteristic and IgG test of CP donors.
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Blood type | B | A | B | AB | B | O | B | A | A |
| Interval between symptom onset and IgG test, days | 39 | 37 | 72 | 60 | 60 | 60 | 63 | 150 | 180 |
| The history of severity of Covid-19 | moderate | severe | moderate | severe | moderate | moderate | moderate | severe | Severe |
| IgG titer* | index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
index (S/C)
≥ 4.5 |
| Donated plasma volume, ml | 400 | 400 | 400 | 400 | 400 | 200 | 400 | 400 | 400 |
*Sars-Cov-2 IgG (Architect ) assay
 |
Figure 2: Comparison of Clinical Characteristic and Laboratory of Patients during Before and After CP Transfusion |
Figure 2 showed that the serial evaluation of overall SOFA scores decreased, except that SOFA scores of 2 patients increased. The range of SOFA Score was 0 until 11 that higher scores indicating more severe illness. The majority of SARS CoV-2 RT PCR cycle threshold (Ct) value after CP transfusion was not defined as undetectable (cut off index CT value in speciment negatif was > 31,5).
Discussion
Our study explored the potential benefit of CP transfusion in 9 of Covid-19 patients consisting of 3 severe cases and 6 moderate cases of Covid-19. Six moderate cases of Covid-19 patients and 1 severe case with 8 – 22 days of length of stay received 1 or 2 unit of CP with a total of 200–400 ml. They were 7 of the survivor patients. The majority of survivor patients had one comorbid disease, meanwhile interval between symptom onset and CP therapy was 7-16 days. Serial evaluation of overall SOFA scores decreased that it related to clinical improvement, from the beginning to 7 days after CP transfusion. From Dr. Kariadi Hospital, Semarang, Indonesia, a study of total 73 patients received CP transfusion and 38 did not received it. The study showed that CP significantly improved SOFA scores (median, min-max;4, 4, 2-10 and 2.5, 0-9; < 0.001, before and after CP transfusion).9
The treatment timing is an important factor of effectiveness of CP transfusion.2 The passive immune therapy might present by the method of convalescent plasma. One possible mechanism for the efficacy of CP therapy is the high titer and high affinity IgG antibodies which may lead to the clearance of viremia.2 Patients develop a primary immune response by days 10 -14, which is followed by virus clearance.2 In fact, 4 of survivor patients were not treated with any antiviral agents such as remdesivir or favipiravir due to limited supply at the time. However, only 3 patients achieved Sars-cov-2 negative by PCR after therapy.
Zeng H et al showed that Convalesent Plasma with high (Neutralizing antibody titer 50) NAT50 (>1:640) was better than low NAT50 value (≤1:640) of CP, including shorter negative conservation time of viral RNA, and higher increment of IgG level after CP transfusion, reduce repeated stimulation of the immune system by killer T cell, prevent cytokine storm, and prevent the progression of infection from mild to critical.2,6 Neutralizing antibody titer 50 is neutralizing antibody titer which was calculated with the highest dilution of plasma that resulted in a 50% reduction of virus infection.2
There is evidence that mild patients frequently had a lower level of Sars-CoV-2 specific antibodies than severe patients.2 In fact, CP donors who usually recovered from mild infection may not generate adequate protective antibodies, and the minimum threshold of neutralizing antibody titers required to prevent Sars-CoV-2 re-infection are currently unestablished, more studies are needed.2,10,11
In this study, CP donors recovered from moderate to severe of Sars-CoV-2 infection that we assumed that had higher level of Sars-Cov-2 antibodies.12 Patients met the eligibility criteria of CP donor that the signal/cut off index (S/C) for this study was ≥ 4.5. We used the Abbott Sars-COv-2 qualitative test for detection of specific antibodies (IgG) levels against SARS-CoV-2.4 United State Food and Drug administration (FDA) reissued on August 23, 2020, letter of Authorization to add a test acceptable to be used in manufacture of Covid-19 convalescent plasma such as Abbott Sars-Cov-2 IgG test. Plasma donation must be tested by registered or licensed blood establishments for anti-Sars-Cov-2 antibodies as manufacturing step to determine suitability before release.7,12,13
In this study, one or two doses of CP with a total of 200–400 mL was well tolerated by 7 of survivor patients, while 1 out of 7 patients experienced adverse event such as TRALI, but the patient recovered and survived. Previous study has reported the safety of convalescent plasma transfusion, meanwhile they could not conclude that a better improvement in patients with moderate cases were solely due to CP treatment.1 A randomized control trial study mentioned that convalescent plasma seemed to improve resolution of shortness of breath and fatigue in patients with moderate covid-19 and led to higher negative conversion of SARS-CoV-2 RNA on day 7 post transfusion, but CP was not associated with a reduction in progression to severe covid-19 or all cause 28 days mortality.14
In this study, 2 severe cases of Covid-19 patients were died. One of severe case of Covid-19 patient had cancer as a comorbidity, while 1 elderly patient (81 years old) had hypertension. Interval between symptom onset and plasma transfusion administration of those patients was 8 and 14 days. One patient experienced TRALI at the day after CP transfusion, but she recovered from TRALI, although after 20 days she was died. We assessed that the death could be explained by worsening Covid-19 and other the underlying disease or secondary infection.15,16,17 Laboratory data of 2 severe cases showed an increase of CRP, IL-6, WBC, procalcitonin level and Sofa Score on day 3 after CP transfusion. It can be assumed that CP transfusion and other treatment has not been used successfully to treat late stages of infection.9,17 According to Eastern Virginia Medical School (EVMS) Critical Care COVID-19 Management Protocol, the course of COVID-19 consists of 4 phases: incubation, symptomatic, early pulmonary phase, and late pulmonary phase.19
However, we present 2 out of 9 patients who received standard of care and convalescent plasma and required intensive care and mechanical ventilation. More evidence of the efficacy and safety of CP transfusion are needed.11,21,22 Transfusion- Related Acute Lung Injury might be caused by the result of patient and blood component factors which lead to neutrophil activation and endothelial cell damage and capillary leak in the lung causing pulmonary edema and hypo perfusion.23 A cases of TRALI have been reported after administration of convalescent plasma in patients with Covid-19.23
There is evidence that dyspnea, the presence of any co morbid disease, elevated CRP levels, and low pulse O2 saturation levels predict mortality in moderate to severely ill Covid-19 patients.4.21 The patients who meet these criteria should be treated and monitored closely and also be aware of the possibility of TRALI.4,23
Our study has several limitations. We present 9 patients that might be difficult to draw scientifically conclusions about beneficial of CP transfusion. Meanwhile, variable of cases and antiviral therapy could have caused a difference in the outcomes. In this study that CP transfusion was administered 7 to 16 days after symptom onset that we should be confirmed about the different timing of CP administration which might be related to different outcomes. Furthermore, randomized control trial with large number of samples and better selection of severity cases is needed to better results.
Conclusion
Our experience showed that CP transfusion in moderate COVID-19 patients might provide clinical benefit and it was well-tolerated. However, further development clinical trials with better designs and greater power is needed to evaluate the efficacy and safety of this treatment.
Acknowledgement
We would like to acknowledge the valuable input of the following researchers at Sulianti Saroso Infectious Disease Hospital. The study was conducted by the hospital study team in 2020.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Support
This project was supported with grant by National Research and Innovation Agency Republic of Indonesia (No.91/F1/PKS-KCOVID-19E/VI/2020)
References
- Rejeki MS, Sarnadi N, Wihastuti R, Fazharyasti V, Samin WY, Yudhaputri FA, et al. Convalescent plasma therapy in patients with moderate-to-severe COVID-19: A study from Indonesia for clinical research in low- and middle-income countries. E Clinical Medicine. 2021 Jun;36:100931.
CrossRef - Zeng H, Wang D, Nie J, Liang H, Gu J, Zhao A, et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: a multi-center case series. Signal Transduct Target Ther. 2020 Oct 6;5:219.
CrossRef - World Health Organization. Novel Coronavirus . Available from: https://www.who.int/indonesia/news/novel-coronavirus. Accessed on 27 Sept 2021
- Islam M, Algin A, Eroglu S, Yaşar G, Ademoğlu E, Dölek Ü. Early predictors of mortality for moderate to severely ill patients with Covid-19. Am J Emerg Med. 2020 Aug 1;45.
CrossRef - Luther B, David M, Ralph B, Aravinda D. CONVALESCENT PLASMA TRIAL Available at https://collaboratory.unc.edu/wp-content/uploads/sites/476/2020/07/convalescent-plasma-trial.pdf. Accessed on 20 Sept 2021
- Xi Y. Convalescent plasma therapy for COVID-19: a tried-and-true old strategy? Signal Transduct Target Ther. 2020 Sep 15;5:203.
CrossRef - Commissioner O of the. FDA In Brief: FDA Updates Emergency Use Authorization for COVID-19 Convalescent Plasma to Reflect New Data; Available from: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-updates-emergency-use-authorization-covid-19-convalescent-plasma-reflect-new-data. Accessed on 27 Sept 2021
CrossRef - Castro MDM, Caicedo I, Ortiz-Rojas HJ, Castillo CM, Medina AG, Alexander N, et al. Performance verification of the Abbott SARS-CoV-2 test for qualitative detection of IgG in Cali, Colombia. PloS One. 2021;16:e0256566.
CrossRef - Damai S, Muchlis A, Retnaningsih, Eko A, Budi S, Nur F, et al. Convalesent plasma as an adjuctive treatment for severe and critically ill Covid-19. Bali Med J, 2021: 3: 851-859
- Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020 Jul 1;19:102554.
CrossRef - Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with Convalescent Plasma for Critically Ill Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020 Jul;158:e9–13.
CrossRef - Michael K, Maria R, Reinhard I, Jessica S, James L, Nicholas P. Longitudinal analysis of SARS-Cov-2 antibodies in 800 U.S. first time convalesent plasma donations. Transfusion;2021: 1-7
- Huo X, Sun X, Bragazzi N, Wu J. Effectiveness and feasibility of convalescent blood transfusion to reduce COVID-19 fatality ratio. R Soc Open Sci. 8:202248.
CrossRef - Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020 Oct 22;371:m3939.
CrossRef - Piechotta V, Chai KL, Valk SJ, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600.
CrossRef - Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its Impact on Patients with COVID-19. Sn Compr Clin Med. 2020 Jun 25;1–8.
CrossRef - Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using Open SAFELY. Nature. 2020 Aug;584(7821):430–6.
CrossRef - Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial | Critical Care Medicine, JAMA. Available from: https://jamanetwork.com/journals/ jama/fullarticle/2766943. Accessed on 28 Sept 2021
- EVMS Medical Group. EVMS Critical Care Covid-19 Management Protocol. 2020 Aug 1;1–25.
- Frontiers | Current Understanding of COVID-19 Clinical Course and Investigational Treatments | Medicine. Available from: https://www.frontiersin.org/articles/10.3389/fmed.2020.555301/full. Accessed on 28 Sept 2021
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020 Apr 28;117:9490–6.
CrossRef - Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci; 35 (14). Available from: https://synapse.koreamed.org/articles/1146001. Accessed on 27 Sept 2021
CrossRef - Kevin Y Pratik S, Matthew P. Convalescent plasma for COVID-19 complicated by ARDS due to TRALI. BMJ Case Rep 2021;14: e239762. doi:10.1136/bcr-2020-239762
CrossRef








