Manuscript accepted on :18-11-2021
Published online on: 30-11-2021
Plagiarism Check: Yes
Reviewed by: Dr. Jonathan Nyce

Second Review by: Dr. Swastika Maity

Final Approval by: Dr. Fai Poon
Vanshika Shrivastava1* , Naveen Sharma2
, Naveen Sharma2 , Vikas Shrivastava1
, Vikas Shrivastava1  and Ajay Sharma3
and Ajay Sharma3
1Amity Institute of Biotechnology, Amity University Madhya Pradesh Gwalior, India-474005
2Amity Institute of Pharmacy, Amity University Madhya Pradesh Gwalior, India-474005
3Department. of Pharmacognocy, Delhi Pharmaceutical sciences and Research University, New Delhi-110017
Corresponding Author E-mail: naveenpcol@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2279
Abstract
Nothapodytes nimmoniana is an endangered medicinal plant widely distributed throughout the Western Ghats of India. The plant contains camptothecin (CPT) which is renowned anticancer drugs. Though, CPT found in many plant species but maximum amount of CPT has been reported from N. nimmoniana. Due to very good source of CPT, this plant has been explored for its Phytochemical, Biotechnological and Pharmacological aspects. Looking to the huge global demand for CPT, overexploitation of N. nimmoniana, unplanned deforestation, and lowest production of CPT from intact plant, reduction of seed germination, high market cost and not have economically feasible process of production has optimistic us to investigate this plant in a systematic manner. The proposed article can be utilized for the establishment of extraction methods and analytical protocol for CPT. Also, Bioreactors production of CPT using high yielding cell line of N. nimmoniana. The pharmacological data will be applicable for discovery of new Drug and development lead to novel compounds which are safe and effective.
Keywords
Anticancer; Camptothecin; Biotechnological; Nothapodytes Nimmoniana; Medicinal plant; Pharmacological
Download this article as:| Copy the following to cite this article: Shrivastava V, Sharma N, Shrivastava V, Sharma A. Review on Camptothecin Producing Medicinal Plant: Nothapodytes nimmoniana. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Shrivastava V, Sharma N, Shrivastava V, Sharma A. Review on Camptothecin Producing Medicinal Plant: Nothapodytes nimmoniana. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3ruI1WP |
Introduction
Nothapodytes nimmoniana (Syn. Mappia foetida, N. foetida) is related to the Icacinaceae family. N. nimmoniana commonly known as Amruta and vernacular names are Narkya, Ghanera, Kalgur and Kalagaura. It is an endangered forest tree and disseminated in Sri Lanka, China, South East Asia, Taiwan, North Sumatra, Luzon Philippines and India 1. In India, this plant is prevalent to Western Ghats of Maharashtra, Jammu and Kashmir, Goa, Tamilnadu, Assam and Kerala given in figure 1.
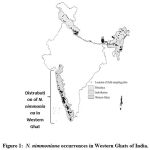 |
Figure 1: N. nimmoniana occurrences in Western Ghats of India. |
This medicinal plant has great property that is main source of alkaloid camptothecin. Camptothecin is alkaloid and cytotoxic compound that has monoterpene pentacyclic structure. The Camptotheca cuminata was first plant native to China for isolating of Camptothecin 2. Till now, camptothecin occurs in another plant species, including Nothapodytes nimmoniana, Ophiorrhiza species, Merrilliodendron megacarpum and Ervatamia heyneana. In 21th century, camptothecin is the third potential anticancer drug by interest of pharmaceutical industry around the world 3, 4. Firstly, camptothecin has been obtained from in vivo grown barks and leaves, further in-vitro grown tissue culture of N. nimmoniana 5, 6. Then, it has been proceed from different parts of plant in various quantity with greatest yield occurs in roots then stem, leaves and fruits 7, 8, 9. Camptothecin is useful for treatment of cancers.
Topotecan and Irinotecan are derivative of Camptothecin alkaloid that alternatives to cure of various kind of cancers. The marketing sales of these derivatives were estimated $1000 million annually and 1000- 1500 tons of N. nimmoniana wood chips used to synthesis for about 1 ton of raw material of camptothecin [10, 11]. The greater exporter of N. nimmoniana is wood chips for CPT to other countries for industrialization along with more than 1000 tons reported trade volume and twice unreported trade volume were reported. The Exporters were reported that twings of species sold at US$0.16 – $0.24/ kg and further sell out by pharmaceutical industries at US rate $15000/kg in the market of universe [10, 12]. Due to increasing pharmaceutical marketplace demands of camptothecin and rapid use in cancer disease around the world, population of N. nimmoniana have been under exploitation pressure therefore required conserve this plant by following strategies of mass cultivatation [6, 7]. Interest in plant secondary metabolites and its anticancer properties has need to prevalent study of other anticancer compounds from N. nimmoniana getting increase attention such as 9-methoxy camptothecin, Scopoletin, 10-hydroxycampthothecin, acetylcamptothecin, β-sitosterol, scopoletin, trigonelline and pumiloside [13, 14]. Individual plant species showed naturally camptothecin compound variability crosswise a broad ecological range 7
Plant tissue culture approaches create alternative prospect for CPT production lacking harvesting of in their natural habitat populations. This approach of N. nimmoniana maintains populations of harvested plant and rapid propagation for conservation purpose 15, 16. The localization of N. nimmoniana species , chemically characterization and identification of individuals which produced high concentration of CPT used for material produced by clonal propagation and cell lines growth with high amount of CPT 9, 17,, 18, 19. Further experimentally efforts were reported on culture media selection, culture media composition optimization or enrichment for enhancement in vitro response as well as CPT production [10, 16, 20]. This medicinal plant has widespread variety of pharmacologic activities against like cancer, HIV, bacterial infection, inflammatory, malaria, fungal infection, free radicals and anemia 21.
Mechanism of Action of Camptothecin 22
It has essential mechanism of action as a result of inhibition of DNA topoisomerase – I enzyme by following manner in figure 268.
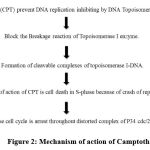 |
Figure 2: Mechanism of action of Camptothecin |
Biosynthetic pathway of Camptothecin 23
Camptothecin (indole alkaloid) synthesized by shikmate and MVA/DXP pathway. In Shikate pathway, firstly made chorismate which is synthesize anthranilate and trypthophan by synthase enzyme than tryptophan synthesize trpamine by decarboxylase enzyme. In MVA/DXP pathway firstly made Gerniol which is synthesize 10-Hydroxygeranial by geranial 10- hydroxylase and loganin synthesize by loganic acid o-methyl transferees than secologanin synthesize by secolganin synthase. Both of intermediate typtamine and secologanin alternatively synthesize strictosidine by synthase enzyme and camptothecin is synthesized following by 3-s pumiloside and 10- deoxypumiloside. The biosynthesis pathway of Camptothecin given in figure 3.
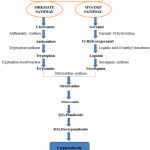 |
Figure 3: Biosynthetic pathway of Camptothecin. |
Plant Description
The plant Nothapodytes nimmoniana is a small tree and 3-8 meter tall. Branches are a little slanting, corky and scar leaves. Flowering season is during the month of July to August and ripened fruits are available during the month of November to December 16, 24, 25. Organoleptic evaluation of N. nimmoniana given in table 1. 26
Table 1: Organolyptic Evaluation of N. Nimmoniana.
| Characters | Leaf | Stem | Root | Flowers | Fruits | Bark |
| Color | Fresh leaves as Green, dry leaves as blade black. | Fresh ones Yellowish brown or mature ones dark brown | Fresh roots Light yellow or brownish color | Creamish yellow, Petals are yellow | blackish purple
|
grey color
|
| Odour and Taste | Unpleasant, Sweet | Unpleasant, Sweet | Unpleasant, Sweet | stinking smell | Unpleasant | |
| Size and shape | Leaf length 8-25cm, width-
3-15 cm in Ovate, leathery in texture with egg shaped to elliptical-oblong |
Stem length Up to 10 m and width 25 cm
Cylindrical, rough and fibrous |
length 80-110 cm and width
15- 30 cm Cylindrical, rough and fibrous |
Length 4-5 mm | about 2 x 1cm
quadrilateral to ellipsoid |
approx 5 mm wide and soft |
Taxonomical classification of Nothapodytes nimmonian
Kingdom – Plantae
Phylum – Tracheophyta
Class- Magnoliopsida
Family – Icacinaceae
Genus – Nathapodytes
Different parts of plant given Figure 4
 |
Figure 4: Different parts of N. nimminiana. |
Phytochemical Properties of N. Nimmoniana
Preliminary phytochemical tests show the occurrence of many compounds such as alkaloids, phenolics, carbohydrates, steroids, saponins, terpenoids, coumarins and fixed oil etc given in table 2 27
Table 2: Phytochemicals Present in N. Nimmoniana Plant Parts 28.
| Plant Constituents Occurrence | Leaves | Stem | Root |
| Alkaloids tests | Yes | Yes | Yes |
| Glycosides | No | No | Yes |
| Tannins | Yes | Yes | Yes |
| Phenols | Yes | Yes | Yes |
| Proteins | Yes | Yes | Yes |
| Carbohydrates | Yes | Yes | Yes |
| Resins | No | No | No |
| Flavonoids | No | Yes | No |
| Saponins | Yes | Yes | Yes |
| Coumarins | Yes | No | No |
| Phytosterols | Yes | Yes | Yes |
| Terpenoids | Yes | Yes | No |
Nothapodyte nimmoniana is one of potent sources of alkaloid compounds Camptothecin (CPT), 9-methoxy camptothecin and minor alkaloids like mappicine. Camptothecin (CPT) has proved notable compound which used as medicinal purpose with define results. The topotecan and irinotecan (Camptothecin analogues) are demonstrated specially treatment of cancer disease. Other bioactive compounds are isolate and characterize from this plant like 9-Aminocamptothecin, 10-hydroxycampthothecin, acetylcamptothecin, β-sitosterol, scopoletin, trigonelline, pumiloside and linoleic acid isolated from N. nimmoniana stem part [13].. the different phytochemicals identified from Nothapodytes nimmoniana given in table 3[29] and structure of some major phyto-constituents given in figure 5.
Table 3: Different Phytochemicals Identified from Nothapodytes Nimmoniana.
| S. No. | Compound | Plant Tissue |
| 1. | Camptothecin | All |
| 2. | 9-Methoxy- camptothecin | stem bark |
| 3. | 10-Hydroxy-camptothecin | stem bark |
| 4. | 5-hydroxy-9-methoxy-O-acetyl-camptothecin | Seeds, stem bark |
| 5. | 5-Hydroxy-9-methoxy-camptothecin | Leaves, stem bark, stem |
| 6. | 18,19-Dehydrocamptothecin | Seeds, leaves |
| 7. | 9-Methoxy-mappicine-20-O-glucopyranoside | Leaves, seeds,stem |
| 8. | 5-hydroxy-mappicine-20-O-glucopyranoside | leaves |
| 9. | Apigenin-7-O-glucopyranoside | stem |
| 10. | Apigenin | stem bark, seeds |
| 11. | Cinnamyl O-β-glucopyranoside | Leaves, stem |
| 12. | Omega-Hydroxypropioguaiacone | stem bark |
| 13. | Scopoletin | leaves |
| 14. | Sitosterol-β–D-glucopyranoside | Leaves, stem bark |
| 15. | 3-Hydroxystigmast-5-en-7-one | stem |
| 16. | Stigmastenone | leaves, fruits, seeds, stem, stem bark |
| 17. | Trigonelline | leaves |
| 18. | Pumiloside | fruits , stem bark |
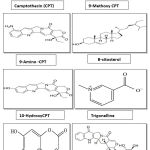 |
Figure 5: Structure of some major phyto-constituents. |
Camptothecin (CPT) Extraction and Isolation
Soxhlet extraction was done for the CPT from stems of N. nimoniana by using various solvents such as methanol, chloroform, DMSO, n-haxane, toluene, petroleum ether, ethyl acetate and acetone. The results showed that the camptothecin was occurs in maximum concentration in In results, the camptothecin was found in maximum concentration in chloroform(0.98%) and methanol extracts (1.12%) whereas camptothecin was found in minimum concentration in DMSO extract (0.18%). Camptothecin was not found in petroleum ether, water and n-hexane extracts [30 Various extraction method such as soxhlet apparatus extraction method, microwave-assisted extraction (MAE), ultrasonic sound extraction method and stirring extraction method were performed for Camptothecin (CPT) and 9-methoxycamptothecin (9-MCPT) extraction from N. nimmonimana using ethanol (90%, v/v) and methanol (90%, v/v). The extraction yield high frequency of CPT and 9-MCPT using methanol comparatively ethanol. The result showed CPT was obtained in maximum percentage (2.67%, w/w) through MAE extraction method. Microwave extraction method involve for 3-5 min, while stirring extraction and ultrasonic extraction method involve for 30 min. and Soxhlet extraction method involve for 30, 120 and 30 min, correspondingly to maximum proportion of CPT and 9-MCPT [31]. The direct injection capillary electrophoresis technique employed for the marker compound camptothecin prepared by DMSO solvent from N. nimmoniana. In results, camptothecin were successfully established and was obtained camptothecin in linear range 5 to 400 µg/mL. The monitoring of camptothecin concentration successfully proved for the duration of the development of the therapeutic plant 32.
The methanolic extracts of various kind of camptothecin producing plant such as Camptotheca acuminate, N. nimmoniana and Ophiorrhiza pumila employed using reverse-phase HPLC technique coupled with photodiode array detection and electrospray-ionization ion-trap mass spectrometry. In results, the detection of camptothecin 0.146 % in C. acuminata and 0.048 % in N. nimmoniana [33]. The desorption electrospray ionization technique performed for the detection of 9-methoxycamptothecin (9-MCPT) and camptothecin (CPT) from of N. nimmoniana. The different parts were taken like leaves, stems and bark. In results, CPT precursor ion obtained fragmentation at m/z 349 showed a loss of 44 Da and producing the product ion at m/z 305 and the 9-MCPT precursor ion obtained fragmentation at m/z 379 showed a loss of 44 Da and producing the product ion at m/z 335. So, the desorption electrospray ionization is advanced technique for analysis of camptothecin and its derivative. This method suggested that the ion intensities and high amount of compound present in bark compared to the leaves and stems [34]. The hot extraction method proceeds for the CPT production from N. nimmoniana. The plant parts have been stem and twigs. The extraction followed by different solvents. In results, the extracts gave CPT with 0.15% yield [35]. The compound specific extraction of marker compounds by Accelerated Solvent Extractor (ASE). Two medicinal plants Nothapodytes nimmoniana and Piper nigrum was selected for the extraction of camptothecin (CPT) from stem and piperine from the fruits. The results showed the Effects of different temperatures improved yield high amount of CPT at 60-80⁰C compared to piperine yield at 37- 40⁰C from this extraction method. The total CPT content was 0.1875g 100 g/dw. The retention time were obtained for CPT at 5.9326 ± 0.051 min. and for piperine at 7.7694 ± 0.0900 min 36. The large scale isolation of camptothecin from Nothapodytes foetida: An improved process, through hot extraction process and precipitation process with some organic solvents. The camptothecin was yield upto 0.15% through the precipitation with a mixture of acetonitrile and dichloromethane 37.
Phytochemical Analysis
The CPT accumulation, in particular organ, via variable extraction method and in vitro propagation method both qualitatively and quantitatively by TLC and HPLC analysis confirm the existence of CPT in dry leaf, fresh barks, induced callus and somatic embryo. The results showed that bark extracts possessed a maximum amount of CPT, i.e. 377.77 μg/g. and soxhlet bark extract possesses the maximum amount of CPT [38]. The quantification of camptothecin was done by the liquid chromatography mass spectrometry (LCMS) from N. nimmoniana. In the results, the highest production of camptothecin was present in bark of stem (303.81μg/ml) while in leaves (31.75 μg/ml) [28]. The quantification of compounds 9-methoxycamptothecin and camptothecin (CPT) was obtained from N. nimmoniana by semi preparative HPLC. Camptothecin content was collected at 15 to 16 min and 9-methoxycamptothecin was collected at 20 to 25 min. The purification of the specific compounds was obtained by liquid chromatography-mass spectrometry (LC-MS) analysis technique. The purification was obtained 95% for CPT as compared to 9-MCPT [39]. The quantification technique HPLC analysis was performed for detection of the camptothecin content from the bark part of N. nimmoniana according to different seasonal variation. The bark drug collected in three season’s viz. monsoon, winter and summer in months of August, December and May. In results, monsoon (August) to accumulate higher levels of CPT (1.337 gram/100 gram dry bark powder) in barks of N. nimmoniana as compared to summer (May) [40]. The quantification of camptothecin content in N. nimmoniana using H-NMR method. In result, Camptothecin, 9-methoxycamptothecin, trigonelline and pumiloside were well separated and quantification analyzed by HPLC method. The determination of calibration curve of CPT and their derivatives were in the range 0.1-8.0 mg/ml and linearity were to be higher than 0.999. The higher concentration of CPT (4.85±0.13; 4.87±0.09), 9-MCPT (4.73±0.11; 4.75 ±0.11), Pumiloside (8.68±0.26; 8.71±0.20) and Trigonelline (6.68±0.13; 6.69±0.08) found in roots analyzed by H-NMR and HPLC compared to stems and leaves [41].The occurrence of camptothecin analyzed by HPLC technique from different kinds of parts of N. nimmoniana. The bark of N. nimmoniana was contributed CPT (0.27% dw) and 9-MCPT (0.11% dw) rather than stem, root and leaves. In another plant parts like young seeds yield high amount of CPT (0.32% dw) and 9-MCPT (0.16% dw) compared to old seeds. The young seeds was contributed CPT (0.11% dw) and 9-MCPT (0.04% dw). The higher amount of CPT (0.42% dw) and 9-methoxycamptothecin (0.18% dw) were contributed in the young seeds [42]. The variation of camptothecin of the distribution in N. nimmoniana by means of gender, age and seasonality. In this report they initiate considerable variation in CPT substance along with individuals and chemically screening was done for identification of rich sources of the camptothecin. In results, the fairy good concentration of CPT from two year old seeds obtained in root tips (0.4%) compared to mature leaves (0.21%), immature leaves (0.20%), wood (0.20%) and root bark (0.16%) [9]. The demonstration of N. nimmoniana plants species for the Camptothecin (CPT) was accumulated in high percentage 0.3% w/w. The root and stem bark were accumulated considerable difference in CPT compound. 148 individuals chemically profiled from 11 population of N. nimmoniana and 23 populations produced above 1% CPT. this estimation are 3-8 folds above in previously literature [7]. The quantification study of Camptothecin analyzed from the stem of N. nimmoniana using highly sophisticated technique high-performance thin layer chromatographic (HPTLC). The results showed the marker compound CPT was contributed 0.059% of content [43]. The LC-MS and HPLC techniques contributed the maximum concentration of CPT was obtained from N. nimmoniana. In the results, first time some minor compounds like 10-hydroxy camptothecin obtained in the extracts of roots and stem. The 17 trees studied for the detection of CPT observed low range 0.4 -1.86 % and 6 trees out of 17 trees estimated excess of 1 %(w/w) CPT obtained by the LC–MS analysis 17. The camptothecin content analysis by HPLC analysis in different kind of parts of N. nimmoniana collected on a variety of time periods from October to February month. In results, the roots (2.62%) had been contained high CPT compound in the February month rather than fruits contained 1.22% CPT in January month, then in stems obtained 0.81% CPT in January month and in leaves 0.70% CPT in February month. In results, the CPT was accumulated in the roots which were collected in February about 13 fold high following by October 8. CPT content influenced by geographical factors in N. nimmoniana and quantified by HPLC. The results showed that environmental and seasonal conditions extremely affect the camptothecin content in N. nimmoniana. In results, greatest proportion of camptothecin obtained 2.62% in roots parts collected from Mahabaleshwar area, 0.88% obtained from Sirsi area and 1.21% obtained from Patan area rather than 1.45% obtained stem collected from Patan area, 0.70% obtained from Sirsi region and 0.43% obtained from Mahabaleshwar areas. The minimum percentage of CPT obtained in leaves 0.29% from Sirsi area, 0.37% from Patan region and 0.70% from Mahabaleshwar area. Correspondingly, Fruits were accumulated highest concentration of camptothecin approx. 0.63% collected from Mahabaleshwar area comparatively obtained 0.36% by Patan region 25. The sophisticated technique high performance thin layer chromatography (HPTLC) for the quantification and validation of CPT. This validation was detection for precision, linearity, limit of detection (LOD), limit of quantitation (LOQ), accuracy and repeatability. The peak was shown linear with the selection of 80- 480 ng/spot and also shown correlation coefficient 0.998±0.020. The Repeatability was found 1.01 (% CV) of standard and 1.08(% CV) of sample. Limit of quantitation (LOQ) and limit of detection (LOD obtained 80 ng/spot and 40 ng/spot. The accuracy was determined through the typical proportion of recovery of 99.13% [44]. The quantification analysis technique for CPT in N. nimmoniana collected from various regions by high performance thin layer chromatographic technique. The following analysis was given the calibration curve for CPT that was linear in scale with the range of 0.3- 3 μg/ml with the correlation coefficient of 0.982 [45].The studies of many medicinal plants like N. nimmoniana, Ophiorrhiza sp. was given for production of CPT. In results, N. nimmoniana was contributed overall camptothecin taken from sum of stem, leaves and roots was obtained 0.170% in dry weight and 9-methoxy camptothecin was obtained 0.002% in dry weight. The results suggested that camptothecin (CPT) and 9-methoxy camptothecin (9-ACPT) was accumulated in maximum percentage in N. nimmoniana [46]. CPT variation in leaf and stem materials from ten individuals of N. nimmoniana collected from three sites of Western Ghats regions and quantified by reversed phase ultra-performance liquid chromatography photo diode array (RP-UFLC-PDA) technique. Then simple micro extraction method was performed with methanol. The highest accumulation of CPT content obtains into stems over leaf materials. The lowest CPT content was found 0.002 ± 0.000 g/100 g individually collected from Joida while highest CPT content was obtains 0.123 ± 0.006 g/100 g collected from Amgaon. Using this data, CPT yielding plants were categorized into five groups viz. I: Very low: <0.020, II: Low: 0.021-0.039, III: Moderate: 0.040-0.059, IV: High: 0.060-0.079 and V: Very high: >0.080. The CPT content was obtains in leaves sample in very low concentration under Ist category and CPT content was obtains in stem samples under ‘II’ category [47]. Studies of Nothpodytes nimmoniana was contributed detection of camptothecin from various parts by RP-HPLC. In analysis different parts of plant was used include seed coat, seeds, leaves and callus induced by leaves. HPLC analysis showed the presence of maximum camptothecin 0.040% recorded in seeds then dried leaf samples and minimum camptothecin 0.011% recorded in seed coat recorded least. These results suggested that approximately every parts of the plant contain camptothecin [48]. Nothapodytes nimmonina contribute CPT (% dry wt.) content in diverse parts of plant analysis from different sophisticated techniques given in table 4.
Table 4: Nothapodytes Nimmonina Contribute Cpt (% Dry Wt.) Content in Diverse Parts of Plant Analysis from Different Sophisticated Techniques.
| Plant Tissues | CPT (% dry wt.) | Chromatographic analysis
|
References
|
| Stem | 0.15-0.28 | LC-MS | [29] |
| 0.14 | HPLC | [9] | |
| Stem bark | 0.2 | LC-MS | [29] |
| 0.3 | UV, IR, NMR, and MS | [6] | |
| 0.236 | HPLC | [9] | |
| Seeds | 0.1 | LC-MS | [29] |
| Seed coat | 0.011 | HPLC | [48] |
| Roots | 1.0-2.0
0.18 |
LC-MS
HPLC |
[29] [9] |
| Bark (Roots) | 0.333–0.775 | HPLC | [9] |
| Fruits | 0.031 | LC-MS | [29] |
| Young leaves | 0.081 | LC-MS | [29] |
| 0.081 | HPLC | [9] | |
| Leaves (sample dried in hot air oven) | 0.020 | HPLC | [48] |
| Leaves (sample dried in liquid Nitrogen) | 0.016
|
HPLC | [48] |
N. Nimmoniana: Biotechnological Work
Plant tissue culture technique
N. nimmoniana lead to in-vitro development by plantlet explants culture of plant 49. The most highly developed medium is Murashige and Skoog medium for plant tissue culture method. The Murashige and Skoog medium was supplemented with different kinds of plant growth hormones for increase accumulation of camptothecin by cell suspension culture method from N. nimmoniana. In results, cell suspension culture was produced high cell biomass with the existence of NAA compared to 2, 4-D. during cultivation of suspension culture supplemented with NAA (10.74 mM) and BA (2.22 mM) containing media obtained 31.3 g/l DW in shaking condition of flask after 20 days. The camptothecin and 9-methoxycamptothecin shown maximum amount 0.035 mg/ml and 0.026 mg/ml in the medium supplemented with NAA 50. The accumulation of camptothecines and 9-methoxy camptothecine from callus cultures of from leaf explants of Nothapodytes foetida. The callus showed that the presence of CPT (2.893±2.39mg %) and 9-MCPT (0.4±0.4mg %). The result showed the quantity of camptothecine and 9-methoxy camptothecine produced in callus culture was low, than the intact plant 51. The first time organogenesis generated on media supplemented with Thidiazuron (TDZ) revived from the callus cultures of Nothapodytes foetida. The dual role of Thidiazuron (TDZ) promoting proliferation of shoots and shoots differentiation from the callus. The enhanced production of CTP in regenerated plant of N. nimmoniana is using different media culture like semisolid and liquid cultures. The primary shoot proliferation obtained on phillps and Collins (L2)+ TDZ(0.44µM)+BAP(2.22µM)+L- glutamine(0.03mM) and respond 85.0% of shoots. The results showed that liquid culture are best for enhanced multiplication [18]. The multiplication of cell suspension culture for increased production of CPT from N. nimmoniana, culture optimized through different nitrogen content and different sugar feeding such as sucrose, fructose, glucose and maltose. In this study, MS medium optimized which was modified with supplementary 2 % sucrose (mix after 12 day) with 3% sucrose, a nitrogen source 50/10 mM NH4/NO3, 0.5 mM phosphate, 0.93 lM kinetin and 10.74 lM naphthaleneacetic acid (NAA). This modified culture medium produced higher camptothecin content about 1.7- and 2.3-fold more than following by the control, respectively. The result showed that the media enriched with high nitrate produced the biomass, while media with ammonium content enhanced CPT content and in case of sugar, sucrose supports maximum accumulation of CPT [20]. The callus was obtain in a variety of Murashige and Skoog (MS) media with various plant growth hormones such as BA, NAA, kinetin, Biotin and 2, 4-D. In results, the media supplemented with IAA and IBA were not obtained callus development. The most selectable callus culture was obtained 0.61% from immature leaves as compared to mature leaves 52. N. nimmoniana also contributed in-vitro embryo culture and seed germination. The seed germination was achieved in highest percentage (69%) from uncoated seeds treat with concentration of 10mg/L gibberellic acid. The medium supplemented with Kn (1.0mg/L) and NAA (0.2mg/L) was found maximum frequency of seed germination 53.
The Endophtyic fungus for CPT production
The endophytic fungi strains were found about 52 kinds of strains isolated from mature and immature twigs of N. nimmonian collected from the regions of the Jammu and Mahabaleshwar The bioreactor studies was done for increased camptothecin content from Entrophospora infrequens, an the endophytic fungus and analyzed for accumulation of the CPT [54]. The endophytic fungus Neurospora sp. was studied isolated from seeds of N. nimmoniana cultivated in Sabouraud liquid media culture flask kept in rotatory shaker for shaking condition and estimated production of camptothecin. The confirmation of this compound from fungus culture was analyzed by chromatographic and spectroscopic analysis (LC-MS) and ion intensity of camptothecin showed m/z 349(M+H) [55]. The endophytic fungi strains isolated from different plant parts given in table 5.
Table 5: The Endophytic Fungi Strains Isolated from Different Plant Parts.
| Endophytes | Plant part isolated | References |
| Neurospora sp.
Aspergillus sp. |
Seeds, inner bark | [55, 56] |
| Entrophospora infrequens | mature and immature Twigs | [54] |
N. Nimmoniana: Pharmacological Studies
Antimicrobial activity and Antiglycation activity
The antibacterial activity of stems and leaves from N. nimmoniana with different extraction solvents i.e. methanol, chloroform and petroleum ether. The zone of inhibition of stems showed in range 9-15 mm in methanol compared to other solvents. In results suggested that the stem methanol extracts were most efficient against microbial activity 57. The Antimicrobial activity was shown in stem bark extract against Escherichia coli (MIC at 25mg/ml) and Pseudomonas aeruginosa (MIC at 25mg/ml) and in leaves against Pseudomonas aeruginosa (MIC at 50mg/ml) by using Tetrazolium micro plate microbial viability assay. The stem bark extract and leaves extract was also shown the antiglycation activity by producing glycation end product. This end product was decreased due to the stem bark extract (IC50 at 10.356μg/ml) and leaves extract (IC50 at10.995μg/ml) 28.
Antimalarial activit
The camptothecin has been effective and precise topoisomerase – I inhibitor drug against malaria parasites in vitro. In this study they were found principle action following by protein-DNA complexes trapped through camptothecin and repressed nucleic acid biosynthesis. Camptothecin was cytotoxic to P. falciparum, with an Ec50 value of 32 µM and 36 µM. 58.
Anti-inflammatory activity
The anti-inflammatory activity of the Nothapodytes nimmoniana by in vivo animal model following by carrageenan – was induced hind paw edema method in rats. The extracts were showed anti- inflammatory compared to control and standard ibuprofen induced group. These drugs were administered by oral rout. The result showed that ethanolic extract possess activity against inflammation which more efficient compared with petroleum ether extract and inflammation decreased significantly with the dose of ethanolic extract (200 mg/kg) compared to the standard ibuprofen 58.
Immunomodulatory activity
The endophytic fungus Entrophospora infrequens isolated from N. nimmoniana which possess immunomodulatory activity in vitro and in vivo (in Balb/c mice). This endophytes determined for production of camptothecin(CPT). In results, CPT-producing endophytic fungus was responsible for the immunomodulatory potential isolated from N. nimmoniana. 60, 69.
Antioxidant activity
The methanol extracts of leaf and stem of Nothapodytes nimmoniana showed Antioxidant activity were determined using various in vitro models like DPPH (1,1-diphenyl-2-picryl hydrazine) ABTS•+ (2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) Ferrous iron chelating ability, Reducing power. The result showed significantly antioxidant activity was found in stem 84.35 % to 250 μg/ml by DPPH method as compared to the standard and different parts of Nothapodytes nimmoniana 61. The methanolic extracts of different parts of N. nimmoniana showed significant antioxidant activity. Antioxidant activity of methanolic extracts were determined using various in vitro models like Diphenylpicrylhydrazyl (DPPH) radical, nitric oxide radical, superoxide radical and peroxide radical scavenging activity. The reducing power, anti-lipid peroxidation potential and total phenolic content of methanolic extracts were also determined. Among the various plant parts phenolic content ranged from 281.0 to 454.0 mg gallic acid equivalent (GAE) /100 g dry sample with maximum content found in methanolic extract of fruits (454.0 mg GAE/gm DW). Fruits shown maximum antioxidant activity with an IC50 value of 0.177 ± 0.2 mg/mL for DPPH radical, 0.177 mg/mL for H2O2 radical, 0.167 mg/mL for superoxide radical and 0.175 mg/mL for nitric oxide radical. Fruits showed maximum anti-lipid peroxidation effect (0.362 mg/mL) with higher reducing potential 3.65. The results were significant as compared to standard antioxidants such as l-ascorbic acid and α-tocopherol 62.
Anticancer/cytotoxic activity
Nothapodytes nimmoniana produced the alkaloid compound camptothecin(CPT). This study suggested that CPT inhibited specifically DNA topoisomerase enzyme and caused DNA damage. This drug effectively demoralized various types of cancer disease 63. The principle action of camptothecin(CPT) produced by Nothapodytes nimmoniana which activated S or G2-M arrest and the homologous recombination repair pathway in tumor cells 63. Nothapodytes nimmoniana produced new compound, 10-methoxy-9-nitrocamptothecin which showed anticancer activity. In results, this compound showed great anticancer activity along with enhancing the cellular accumulation of DNA damage 63. The 18 known compounds in which acetylcamptothecin was a new naturally occurring alkaloid compound from Nothapodytes foetida (Wight) Sleumer. Among these camptothecin, scopoletin, O-acetylcamptothecin and 9-Omethoxy camptothecin showed significant anticancer activity 42. The endophytic fungus accumulated such compounds which showed anticancer activity isolated from Nothapodytes nimmoniana. The results showed effectively influenced against all cancer cell lines 26, 66. The anticancer effect of its hydro alcoholic extract on HeLa cell lines, the results showed that the cytotoxic effect on HeLa cell lines was determined using MTT assay, where IC50 for leaves, stem bark and standard CPT was 178μg/μl, 1540μg/μl, and 29μg/μl respectively 28.
Nephro Protective and Hepatoprotective activity
The evaluation of new activities was determined nephro-protective and hepatoprotective activities of Nothapodytes nimmoniana. The phytoconstituents like alkaloid (camptothecin) and flavonoids shown nephroprotective and hepatoprotective activity from N. nimmoniana 67.
Conclusion
Nothapodytes nimmoniana is a natural source of Camptothecin(CPT). Looking to its high demands, this plant needs exploration of the phytoconstituents aspects and therapeutic potential. The exploration of biotechnological properties of N. nimmoniana through cell suspension culture and callus culture are used as alternative for camptothecin production which is one of the medically important alkaloid and is of great demand in the pharmaceutical world as it is used for the formation of anti-cancer drugs. Therefore, this plant species needs improving their conservation using various techniques like maintaining genetic impact of species and micro propagation technique. The present review study revealed that Nothapodytes nimmoniana is alternative sources of natural drug for treatment of cancers.
Future Prospects
Vaious phytochemical extaction methods need to optimize comparatively for production of Camptothecin. Plant biotechnological techniques like cell suspension and callus culture are occupying better results for the production of Camptothecin and another phytoconstituents like 9-methoxy Camptothecin, 9-amino Camptothecin,10- hydroxyl Camptothecin, β-sitosterol, trigonelline, scopoletin and pumiloside. Advance interest of development of callus cultures by using up scaling bioreactor and hairy root cultures produce high amount of alkaloid compounds. Endophytes also have industrial applications for the production of high yield of CPT. Optimization of cultures through modified elicitors and precursor feeding enhance production of CPT a CPT and other alkaloid compounds that will be great interest of exploration. Micro-propagation of N. nimmoniana is need for conservation of plant species that reduce overexploitation.
Acknowledgment
The authors would like to thank Madhya Pradesh Council of Science & Technology (MPCST) sponsored research project letter No. A/ R&D/ RP-2/ Phy & Engg./ 2018-10/273 for funding research work.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There are no funding source.
References
- Karehed J .Trees of tropical Asia old icacinaceae. Am. J. Bot., 2001; 88: 2259–2274.
CrossRef - Wall M, Wani M, Cook C, Palmer K, Phail A, Sim G et al. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumour inhibitor from Camptotheca acuminata. J. Am. Chem. Soc., 1966; 88(1): 3888-90.
CrossRef - Lorence A and Nessler L. Camptothecin, over four decades of surprising findings. Phytochem., 2004; 65(4): 2731-2841.
CrossRef - Panneerselvam K, Bhavanisankar K, Jayapragasam K et al. Effect of growth regulators and planting media on rooting of cuttings of nimmoniana Mabberly. Ind. J. Plant. Physiol., 2004; 9: 308–312.
- Roja G and Heble M. R. The quinoline alkaloids camptothecin and 9-methoxycamptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochem., 1994; 36: 65-66.
CrossRef - Govindachari T and Viswanathan N. Alkaloids of Mappia foetida. Phytochem., 1972; 11(2):3529–31.
CrossRef - Suhas S, Ramesha B, Ravikanth G, Rajesh P, Vasudeva R, Ganeshaiah K et al. Chemical profiling of Nothapodytes nimmoniana populations in the Western Ghats, India for anti-cancer compound, camptothecin. Curr. Sci., 2007; 929(4): 8-12.
- Namdeo G and Sharma A. HPLC analysis of camptothecin content in various parts of Nothapodytes foetida collected on different periods. Asian Pac. J. Trop. Biomed., 2012; 2(5): 389-393.
CrossRef - Padmanabha B, Chandrashekar M, Ramesha B, Vasudeva R, Ganeshaiah K and Shaanker R. Patterns of accumulation of camptothecin, an anti-cancer alkaloid in Nothapodytes nimmoniana Graham in the Western Ghats, India: Implications for identifying high-yielding sources of the alkaloid. Curr. Sci., 2006; 90(2):95–100.
- Patwardhan B, Vaidya A. D. B and Chorghade M. Ayurveda and natural products drug discovery. Curr. Sci., 2004; 86: 789-9
- Watase I, Sudo H, Yamazaki M and Saito K. Regeneration of transformed Ophiorrhiza pumila plants producing camptothecin. Plant Biotech., 2004; 21: 337-342.
CrossRef - Dubey N, Kumar R and Tripathi P. Global promotion of herbal medicine: India’s opportunity. Curr. Sci., 2004; 86(1): 37-41.
- Jedage H. D, Velhal A. B, Khan Z. K, avate G. D, Raskar S. M, Salunke P. B and Patil P. S. A Review on: Nothapodytes nimmoniana Graham. Pharmacogn. & Phytochem., 2018; 10(3): 251-256.
CrossRef - Wu T, Leu Y, Hsu H, Ou L, Chen C, Chen C et al. Constituents and cytotoxic principles of Nothapodytes foetida. , 1995; 39(2): 383-85.
CrossRef - Smetanska I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Engin/Biotechnol., 2008; 111: 187–228.
CrossRef - Rajasekharan P. E, Abdul Kareem V. K and Vasantha K. T. Optimization of protocols for in vitro multiplication and conservation of Nothapodytes nimmoniana, an endangered medicinal plant. Acta. Hrt., 2010; 865: 53–58.
CrossRef - Ramesha B, Amna T, Ravikanth G, Gunaga R. P, Vasudeva R, Ganeshaiah K and Qazi G. Prospecting for Camptothecines from Nothapodytes nimmoniana in the Western Ghats, South India: identification of high yielding sources of camptothecin and new families of camptothecines. J. Chromatogr. Sci., 2008; 46(4): 362-68.
CrossRef - Tejavathi D. H, Raveesha H. R and Shobha K. Organogenesis from the cultures of Nothapodytes foetida (Wight) Sleumer raised on TDZ supplemented media. Indian J. Biotech., 2012; 11: 205-9.
- Dandin V. S and Murthy H. N. Enhanced in vitro multiplication of Nothapodytes nimmoniana Graham using semisolid and liquid cultures and estimation of camptothecin in regenerated plants. Acta. Physiol. Plant, 2012; 34: 1381-1386.
CrossRef - Karwasara V. S and Dixit V. K. Culture medium optimization for camptothecin production in cell suspension cultures of Nothapodytes nimmoniana (J. Grah.) Mabberley. Plant Biotechnol. Rep., 2013; 7: 357-369.
CrossRef - Nataraj H. R, Rao P and Apoorva J. M. Anticancerous potentials of Nothapodytes nimmoniana (grah.) Mabb – A review. Int. J. Medi. Res. Pharmaceut. Sci., 2017; 4(5): 24-18.
- Svejstrup J. Q, Chris M. I, Ansen K et al. New technique for uncoupling the cleavage and religation reactions of eukaryotic topoisomerase I: The mode of action of camptothecin at a specific recognition site. J.Mol. Biol., 1991; 222: 669–678.
CrossRef - Isah T and Mujib A. Camptothcin from Nothapodytes nimmoniana: review on biotechnology application. Acta Physiol. Plant, 2015; 37; 106.
CrossRef - Cooke T. Flora of the presidency of Bombay. London; Taylor and Francis, 1901; 1: 225.
- Namdeo A. G, Sharma A, Fulzele D and Mahadik K. R. Influence of geographical and climatic conditions on camptothecin content of Nothapodytes nimmoniana. Natl. Prod., 2010; 1: 64-71.
- Khan N, Tamboli E.T, Sharma V. K and Kumar S. Phytochemical and pharmacological aspects of Nothapodytes nimmoniana. An overview. Herba. Polonica., 2013; 59(1): 54-65.
CrossRef - Sharma S, Kumar A and Namdeo A. Pharmcognostical and phytochemical analysis of Nothapodytes nimmoniana Int. J. Pharm. & Pharm. Sci. 2012; 4(4): 455-459.
- Dixit A, Gayakwad S, Shirolkar A, Warkad S, Devlae A, Murthy S. N and Pawar S. Phytochemical characterization and cells Based components of Nothapodytes nimmoniana (J. Graham). J. Curr. Microbiol. App. Sci., 2015; 2: 18-37.
- Sharma E and Arora B. S. Phytochemical study of Nothapoytes foetida. J. chem.. Environ. Sci. & Appli., 2015; 2 (1): 9-18.
CrossRef - Sharma S, Kumar A and Namdeo A. G. Pharmacognostical and phytochemical analysis of Nothapodytes nimmoniana Int. J. Pharm. Pharm. Sci., 2012; 4(4): 455-459.
- Fulzele D and Satdive R. Comparison of techniques for the extraction of the anti-cancer drug camptothecin from Nothapodytes foetida. Chromatogr., 2005; 1063(2): 9-13.
CrossRef - Hsiao H, Cheng T, Yang G, Huang I, Chen R. Determination of camptothecins in DMSO extracts of Nothapodytes foetida by direct injection capillary electrophoresis. Phytochem. Anal., 2008; 19(2): 136-40.
CrossRef - Yamazaki Y, Urano A, Sudo H, Kitajima M, Takayama H, Yamazaki M, Aimi N, Saito K. Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants. Phytochem., 2003; 62(3): 461-70.
CrossRef - Amitava S, Demian R. I, Hemanta R. N, Vasudeva B, Graham R. C and Pradeep T. Direct analysis of camptothecin from Nothapodytes nimmoniana by desorption electrospray ionization mass spectrometry (DESI-MS). Analyst, 2011; 136: 3066-68.
CrossRef - Srivastava S, Khan M and Khanuja S. Process for isolation of anticancer agent camptothecin from Nothapodytes nimmoniana. United States patent, 2005; 6: 893.
- Upadhya V, Pai S. R, Sharma A. K, Hegde H. V, Kholkute S. D and Joshi R. K. Compound Specific Extraction of Camptothecin from Nothapodytes nimmoniana and Piperine from Piper nigrum using Accelerated Solvent Extractor. J. Methods Chem., 2014; 1-6.
CrossRef - Neeraj V. Large Scale Isolation of camptothecin from Nothapodytes foetida: An improved process. Res. J. Chem. Env. Sci., 2014; 2(1): 42-3.
- Patil A. S, Kale A. S, Patil S. R and Paikrao H. M. Validation of Accumulation of Camptothecin Content, an Anti-Cancer Alkaloid in Nothapodytes Nimmoniana in Phenotypic Variants: Method for Identifying High-Yielding Sources of Alkaloid. Int. J. Pharm. Sci., 2016; 8(9): 19-23.
CrossRef - Puri S, Verma V, Amna T, Qazi G and Spiteller M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod., 2005; 68(12): 1717-19.
CrossRef - Pai SP, Pawar V. P, Nimbalkar M. S, Kshirsagar P. R, Kolar F. K and Dixit G. B. Seasonal variation in content of camptothecin from the bark of Nothapodyte nimmoniana (Grah.) Mabb. using HPLC analysis. Res., 2013; 5(3): 219–23.
CrossRef - Li C, Lin C and Wu T. Quantitative analysis of camptothecin derivatives in Nothapodytes foetida using 1H-NMR method. Chem. Pharm. Bull (Tokyo), 2005; 53(3): 347-49.
CrossRef - Fulzele D and Satdive R. Distribution of anticancer drug camptothecin in Nothapodytes foetida. Fitoterapia, 2005; 76(7): 643-48.
CrossRef - Dighe V, Ramesh T, Parekh G, Gokarn V and Dhotre O. HPTLC quantitation of camptothecin in Nothapodytes foetida (Wight) Sleumer stem powder. J. Planar Chromatogr. Modern TLC, 2007; 20(4): 131-33.
CrossRef - Namdeo A. G, Sharma A, Lohidasan S, Fulzele D. P and Mahadik K. R. HPTLC densitometric evaluation of tissue culture extracts of Nothapodytes foetida compared to conventional extracts for camptothecin content and antimicrobial Activity. Planta. Med., 2010; 76(5): 474-80.
CrossRef - Nazeerullah K, Tamboli E T, Madhukar G and Kumar S. Development and validation of HPTLC method for estimation of camptothecin. I J. Drug Formul. Res., 2011; 2(5): 333-37.
- Roja G. Comparative studies on the camptothecin content from Nothapodytes nimmoniana and Ophiorrhiza Nat. Prod. Res., 2006; 20(1): 85-88.
CrossRef - Ankad G, Upadhya V, Pai S. P, Nimbalkar M. S, Hegde H. V, Joshi R. J and Kolkute S. D. Evaluating Nothapodytes nimmoniana population from three localities of western ghats using camptothecin as phytochemical marker and selection of elites using a new-content range chart method. Mag., 2015; 11(41): 90–95.
CrossRef - Singh I, Kumaravadivel N, Gnanam R and Vellaikumar S. RPHPLC analysis for the camptothecin content in N nimmoniana, an endangered medicinal plant. J. Med. Plants Res., 2010; 4(3): 255–259.
CrossRef - Satheeshkumar K and Seeni S. In vitro multiplication of Nothapodites nimmoniana through seedling explants cultures. Indian J. Exp. Biol., 2000; 38(3): 273-77.
CrossRef - Fulzele D, Satdive R and Pol B. Growth and production of camptothecin by cell suspension cultures of Nothapodytes nimmoniana. Planta. Med., 2001; 67(3):150-52.
CrossRef - Sundravelan R, Desireddy B and Ciddi V. Production of camptothecines from callus cultures of Nothapodytes foetida (Wight) Sleumer. Indian J. Biotech., 2004; 3: 452-3.
- Thakre A, Kulkurni P, Datir K, Kulkarni S and Choudhari K. Study of different hormones on callus growth of Nothapodytes foetida and extraction of camptothecin from callus culture. Ameri. Int. J. Res. Formal Appli. & Nat. Sci., 2015; 10(1): 28-30.
- Kaveri S and Rao S. In vitro seed germination and Embryo Culture in Nothapodytes foetida (Wight) Sleumer. Letters Nat. Sci., 2015; 48: 23-31.
CrossRef - Amna T, Puri S, Verma V, Sharma J, Khajuria R, Musarrat J et al. Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Can. J. Microbiol., 2006; 52(3): 189-96.
CrossRef - Rehman S, Shawl A, Sultana S, Kour A, Riyaz-ul-Hassan S and Qazi G. N. An endophytic Neurospora from Nothapodytes foetida producing camptothecin. Prikl. Biokhim. Mikrobiol. 2008; 44(2): 225-31.
CrossRef - Sirikantaramas S, Asano T, Sudo H, Yamazaki M and Saito K. Camptothecin: therapeutic potential and biotechnology. Curr. Pharm. Biotechnol., 2007; (4): 196-202.
CrossRef - Kumar R, Vishwanathan H, Suresh T and Mohan P. Antibacterial activity of Mappia foetida leaves and stems. Fitoterapia., 2002; 73(2): 734-36.
CrossRef - Bodley A, Cumming J and Shapiro T. Effects of camptothecin, a topoisomerase-I inhibitor, on Plasmodium falciparum. Biochem. Pharmacol., 1998; 55(2): 709-15.
CrossRef - Sheeja E, Edwin E, Dhanbal S, Suresh B. Antiinflammatory activity of the leaves of Nothapodytes foetida Miers. Indian J. Pharm. Sci., 2005; 67(2): 251-53.
- Puri S, Amna T, Khajuria A, Gupta A, Arora R, Spiteller M and Qazi G. Immunomodulatory activity of an extract of the novel fungal endophyte Entrophospora infrequens isolated from Nothapodytes foetida (Wight) Sleumer. Acta Microbiol. Immunol. Hung., 2007; 4(3): 237-60.
CrossRef - Uma G, Jagathes K. S and Balasubramaniam V. In vitro antioxidant properties of Nothapodyte nimmoniana (Grah.) Mabb. (Icacinaceaae). Asian J. Pharm. Clin. Res., 2013; 6(1): 53-5.
- Namdeo A. G, Sharma A and Mahadik S. R. Antioxidant Activity of Methanolic Extracts of Nothapodytes Nimmoniana (J. Graham) Mabberly. Online., 2010; 1: 148-159.
- Cuong N, Hsieh M and Huang C. Recent development in nano-sized dosage forms of plant alkaloid camptothecin-derived drugs. Recent Pat. Anticancer Drug Discov., 2009; 7(2): 333-38.
- Huang M, Ze-Hong M, Hong Z, Yu-Jun C, Wei Lu and Jian Ding. Chk1 and Chk2 are differentially involved in homologous recombination repair and cell cycle arrest in response to DNA double-strand breaks induced by camptothecins. Mol. Cancer Ther., 2008; 7(6): 120-27.
CrossRef - Luo P, Qiaojun H, Xungui H, Yongzhou H, Wei Lu, Yiyu C et al. Potent antitumor activity of 10-methoxy9-nitrocamptothecin. Mol. Cancer Ther., 2006; 5(4): 302-10.
CrossRef - Rehman S, Shawl A, Sultana S, Kour A, Hassan S and Qazi G. N. In vitro cytotoxicity of an endophytic fungus isolated from Nothapodytes nimmoniana. Annals microbial., 2009; 59(1): 157-161.
CrossRef - Poudel S, Samaddar S, Pokharel A. D, Chaudhary S and Owoalade S. Review on Nothapodytes nimmoniana and its Nephro Protective, Hepatoprotective and Anti-Oxidant Activities. J. Technol. & Innovat. Res., 2018; 5(11): 436-443.
- Wang H, Ao M, Liu W, Bai Y, Zhu Y and Yu L. Topoisomerases inhibitory activities and DNA binding properties of 9-methoxycamptothecin from Nothapodytes nimmoniana (J. Graham) Mabberly. Nat. Prod. Res., 2019; 33(5): 727-731.
CrossRef - Durga B, Julius A and Raghavendra J. S. Review of Pharmacological Aspects of Nothapodytes nimmoniana Europ. J. Mol. & Clinic. Med., 2020; 07(03), 1727-32.







