Vedavati Goudar1 , Kanthesh B M2
, Kanthesh B M2  and Nagalambika Prasad1*
and Nagalambika Prasad1*
1Department of Microbiology, Faculty of Life Sciences, JSS Academy of Higher Education and Research, Mysuru.
2Division of Molecular Biology, Faculty of Life Sciences, JSS Academy of Higher Education and Research, Mysuru.
Corresponding E-mail: ambikap@jssuni.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2327
Abstract
The current research emphasis on the isolation and differentiation of Listeria monocytogenes from different food samples most frequently infected with Listeriosis outbreaks. Crude chicken meat, raw milk, pasteurized cheese, ice cream and raw fish are samples from the city of Bangalore. The selective medium mainly used for the isolation of Listeria is oxford agar. Using isolated L. monocytogenes from food samples, morphologic and biochemical identification was carried out. 2 samples (fresh milk and Ice cream) were positive out of 5 samples; 3 samples (raw chicken meat, raw fish, and pasteurized cheese) were negative. The results conferred during this study indicate the contamination of Ice- cream and Raw Milk samples with L. monocytogenes.
Keywords
Contamination; Food samples; Food borne pathogen; Listeria Monocytogenes; Listeriosis
Download this article as:| Copy the following to cite this article: Goudar V, Kanthesh B. M, Prasad N. Isolation and Identification of Listeria Monocytogenes in Bangalore City from Various Food Samples. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Goudar V, Kanthesh B. M, Prasad N. Isolation and Identification of Listeria Monocytogenes in Bangalore City from Various Food Samples. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3sq5sRG |
Introduction
Listeriosis is one among the least food borne disease compared to all other food borne illness. Listeriosis caused by the bacterium Listeria monocytogenes. It is one among the food borne pathogen and Listeriosis is one among the least food borne disease compared to all other food borne illness. Listeriosis caused by the bacteria, L. monocytogenes. It is one among the food borne pathogen and ubiquitous in nature. L. monocytogenes is Gram positive, rod shaped, Catalase positive, non-spore forming, facultative anaerobic, Oxidase negative, motile bacterium. It is widely distributed in water, soil and also present in various animal foods and food products 1. L. monocytogenes withstand at various temperatures for survival and forms flagella at above 27 °C. It can grow in a pH range from 4.6 to 9.5 and grow in low water activity environments2. Foods include unpasteurized dairy products , ready to eat foods , sea foods. It is an opportunistic pathogen for human beings and animals and although mortality rate that reaches 20-40% and incidence of critical sickness is very high 3. Food is an important vehicle of transmission in 99% of Listeriosis cases. The most affected peoples are immune compromised persons, pregnant women, neonates and children. Listeria is an emerging pathogen during the early 1980s, it’s difficult to detect, number of efforts to develop both cultural and rapid methods for its detection. The best choice for Listeriosis detection is Antibiotic therapy. Some of the antibiotics to treat Listeriosis disease are Ampicillin, Gentamicin etc 4. As a food borne pathogen, is a serious threat for human health and food safety. The consumption of raw food and food products that carry Listeria monocytogenes is a cause of Listeriosis. To control the Listeriosis consumer should follow WHO (World Health organization) rules and regulation for the food safety and healthy life. Consumption of proper cooked food (meat and meat products), proper handling of food, adequate heating and cooling of the food is very important aspects of controlling the food borne pathogen 5. The aim of this study is to isolate and identifies the L. monocytogenes from food samples & biochemical characterization for further identification.
Materials and Methods
Sample collection
In the present study, samples were collected from Dairy shop, Fish and Chicken markets from different areas in Bangalore city. The samples including the Milk products (Pasteurized Amul cheese, Ice-cream, Raw milk), poultry product (Raw Chicken), sea food (Raw Fish). The samples were suspected to be contaminated with Listeria. The samples were collected in sterile container (Box) under aseptic precautions and also the samples collection box at 4°C and were transported to the laboratory directly 6.
Processing of sample to Enrichment media
25 g of samples were taken aseptically and homogenized using sterile mortar and pestle later the crushed sample was inoculated on to 225 ml of enrichment media Fraser Broth (Table 1), Whereas solid sample was inoculated as into Frazer broth and incubated at 37°C for 24 hours 6.
Table 1: Fraser broth composition (mg/l)
| S No | Name of the Chemicals | Concentration (g/l) |
| 1 | Meet peptone | 5.0 g |
| 2 | Sodium chloride | 20.0 g |
| 3 | Krypton | 5.0g |
| 4 | Yeast extract | 5.0 g |
| 5 | disodium hydrogen phosphate | 12.0g |
| 6 | Potassium dihydrogen phosphate | 1.35g |
| 7 | Aesculin | 0.5g |
| 8 | Distilled water | 1000ml |
| 9 | pH | ± 7.2 |
Isolation of the Listeria spp
The selective media used for isolation of L. monocytogenes is Oxford agar. The media were ready as per the composition (Table 2). The culture (1ml) is taken from enrichment medium and streaked on the Oxford agar plates and incubated at 37°C for 48 hours. Colonies formed after incubation, were likely to be L. monocytogenes and that them were sub cultured on to nutrient agar for more identification 6.
Table 2: Oxford agar composition (mg/l)
| S No | Name of the Chemicals | Concentration (g/l) |
| 1 | Aesculin | 0.5 g |
| 2 | Sodium chloride | 5.0 g |
| 3 | Peptone | 28.0g |
| 4 | Starch | 1.0 g |
| 5 | Ferric Ammonium citrate | 0.5g |
| 6 | Lithium chloride | 15.0g |
| 7 | Agar | 15.0g |
| 8 | Distilled water | 1000ml |
| 9 | pH | ± 7.2 |
Identification methods
In addition, on the basis of their morphological observation, staining and biochemical characterization, cultures of isolated bacteria (L. monocytogenes) have been classified 7.
Result
The isolation of L. monocytogenes is carried out by using Fraser Broth enrichment as selective enrichment media followed by Oxford agar. Totally few different samples were collected from different places such as super market, vegetable market and food shops from different areas in Bangalore and sample details were represented in Table 3. Oxford agar is a solid medium, mainly used for the selective medium for isolation and identification of L. monocytogenes in food samples. L. monocytogenes hydrolyze the esculin to esculetin. Esculetin reacts with ferric ammonium citrate resulting in a black precipitate and a positive reaction.
Table 3: Collected Sample details and occurrence of L. monocytogenes
| S. No | Name of the Food sample | Total Number of the sample | Total Number of positive samples | Total Number of Negative sample |
| 1 | Chicken | 01 | – | 01 |
| 2 | Fish | 01 | – | 01 |
| 3 | Milk | 01 | 01 | – |
| 4 | Ice –Cream | 01 | 01 | – |
| 5 | Cheese | 01 | – | 01 |
Out of five different samples were processed for isolation of sample and only Ice-cream and Milk samples showed positive results for L. monocytogenes. Several authors observed that Fraser broth is the effective enrichment broth for L. monocytogenes. The isolated colonies on Oxford agar plates were used for morphological, identification and staining, motility and biochemical characterization of typical L. monocytogenes colonies. The staining shows Gram positive rods and motile nature (Table 4).
In Biochemical confirmation miscellaneous test (Indole, Methyl red and Voges- Proskauer), Fermentation test (Sugar –Glucose and lactose), Substrate utilization test (Citrate Utilization and H2S), and Enzymes tests (Urease, Catalase and Oxidase).
For isolation of L. monocytogenes from different food samples from Bangalore city, using conventional methods: as per the growth on Oxford agar medium, Gram staining and Catalase test; totally 2 samples (40%) suspected L. monocytogenes were isolated from 5 samples.
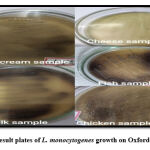 |
Figure 1: Result plates of L. monocytogenes growth on Oxford agar plates. |
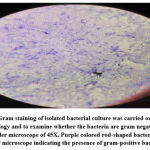 |
Figure 2: Gram staining of isolated bacterial culture was carried out to detect the morphology and to examine whether the bacteria are gram negative or gram positive under microscope of 45X. |
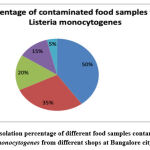 |
Figure 3: The isolation percentage of different food samples contaminated with L. monocytogenes from different shops at Bangalore city. |
Table 4: Showing Biochemical characterization of L. monocytogenes
|
BIOCHEMICAL TESTS |
SAMPLES
|
||||
| Chicken | Fish | Milk | Ice | Cheese | |
| Indole test | – | – | – | + | – |
| VP test | – | – | + | – | + |
| Catalase test | + | + | + | + | – |
| H2S test | – | – | – | – | – |
| Urease test | – | – | – | – | – |
| Methylred test | – | + | + | + | – |
| Vogesproskauer test | – | – | + | + | – |
| Citrate test | – | – | – | – | – |
| Oxidase test | – | + | – | + | – |
| Flagella | Flagellated | Flagellated | Flagellated | Flagellated | Flagellated |
| Nitrate Reduction test | – | – | – | – | – |
| Motility test | Motile | Motile | Motile | Motile | Motile |
Five samples were analyzed for the detection of a pathogenic micro-organism. L. monocytogenes isolated from 2 samples Milk and Ice-cream. The 2 isolates recovered from the samples were known supported the morphological, cultural and biochemical characteristics (table 4). It has been found that about 30 percent of milk products are infected with Listeria. According to our report, ice-cream (15%) samples tainted with L. monocytogenes are raw milk (26%).
Discussion
Listeriosis is one of the zoonotic diseases and is contracted principally from the consumption of contaminated food products (8). The existence of L. monocytogenes is the atmosphere is omnipresent. Increasing evidence suggests that food borne transmission of Listeria monocytogenes is attributable to a sustainable part of the human Listeriosis cause 9. There are several sporadic and outbreak breaks globally involving foods contaminated with Listeria that are reportable. The current study analyzed total 5 different food samples raw fish; raw milk, raw chicken meat, pasteurized cheese, and Ice cream were analyzed. Out of 5 samples only 2 samples (Raw milk, Ice cream) showed positive result. 3 samples (Raw chicken meat, raw fish, and pasteurized cheese) showed negative result. Two samples are raw milk (26%), Ice cream (15%) were positive for L. monocytogenes. In the present study L. monocytogenes were isolated from the different food samples by using selective Oxford agar media. Typical eubacteria colonies on the selective agar plates were sub cultured on the Nutrient agar plates. And additional identification had done by performing series of tests to substantiate Morphological, biochemical characteristics. L. monocytogenes is a gram positive, motile, encapsulated, glucose fermenting, Facultative anaerobic, Urease positive, Oxidase negative, Catalase positive organism belonging to the Eubacteria family. The isolated L. monocytogenes colony was identified by Gram’s staining, colony characteristics and biochemical tests such as Catalase test Urease test, Indole test, H2S test, Motility, Citrate test, Oxidase disc test, Aesculin hydrolysis test, VP test, MR test. In the present work, Raw milk and Ice –cream samples showed positive results for all biochemical tests, raw milk was found to be the main source of L. monocytogenes. About 25% raw milk was contaminated with Listeria. This is due to the lack of hygiene, environmental contamination and milk handlers. And other sample Ice creams (15%) were contaminated with Listeria. Ice cream is produced from Milk, Fruits, eggs these components may provide adequate nutritional support for Listeria growth and multiplication 10.
Some of important work carried out by Schlech, III W F. et al., 2000, studied that Food includes ready to eat foods, raw vegetables, unpasteurized dairy products, sea foods, meat products and 40% of traditional foods contaminated with Listeria monocytogenes about 40% 11. Gunasena et al., 1995, were studied the presence of L. monocytogenes in market samples different food items indicated that 38% of the samples contained L. monocytogenes of them 34% of chickens and 26% of dairy products were contaminated with L. monocytogenes 12. Rahimi 2012 et al., were studied that highest prevalence of Listeria was found in traditional Ice-cream (16.7%), followed by cheese (15.0%) samples 13. Enurah et al., 2013 were studied the prevalence of L. monocytogenes in fresh raw milk (15%) 14. Molla et al., was they reported a prevalence of 32.6% Listeria species in all food samples 15. These findings were comparable to the results of surveys undertaken in other countries 16, 17, 18, 19, 20. This suggests that the presence of a significant public health joined to the consumption of foods contaminated with L. monocytogenes. The overall findings were almost comparable with Gunasena et al., 1995. This study has incontestable the presence of Listeria in several types of raw and prepared to eat food products in Bangalore city. Also suggests the requirement for improved food safety is important aspect of controlling food borne pathogen.
Summary
Food from different sources in Bangalore city was collected to analyze for detection of Listeria monocytogenes. Samples were collected with adequate measures and taken to the laboratory for the isolation and identification, biochemical characterization. Totally 5 samples were collected for the identification of Listeria monocytogenes, samples are (raw chicken meat, raw milk, raw cheese, raw fish and Ice- cream samples). Among the 5 samples two samples Ice-cream (15%) and raw milk (25%) was showed positive results for the all biochemical tests. According to researches Listeria monocytogenes were highly contaminated with milk and milk products.
Conclusion
Introducing hygiene measures from production to consumption at the lowest level, the current study jointly sets out the criteria for increasing food safety. Listeria monocytogenes can survive extreme environmental conditions and pose a high risk to human health. A large proportion of the food samples were silently infected with L. monocytogenes recommend measures to improve the hygiene, proper handling and cooling storage conditions of the area unit when treating the infection.
Author Contribution
Vedavati and Nagalambika Prasad conceptualized the study. Vedavati and Nagalambika Prasad Collection and assembly. Vedavati and Nagalambika Prasad drafted the Manuscript. Nagalambika Prasad and B.M. Kanthesh helped Data analysis and interpretation with the Manuscript and Discussion.
Acknowledgement
The authors would like to acknowledge the Management of JSS Academy of Higher Education & Research, Mysuru, Karnataka, for supporting the basic research ideas and also for the resources provided.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding Sources
There is no funding source.
Reference
- Barbuddhe S. B, Malik S.V.S, Kumar J.A, Kalorey D.R and Chakraborty T. Epidemiology and risk management of Listeriosis in India. International Journal of Food Microbiology. 2012; 154:113-118.
CrossRef - Buchanan R, Lindquist R, Ross T, Smith M, Todd and Whiting R. Risk assessment of Listeria monocytogenes in ready to eat foods. 2004. Microbiological Risk Assessment Series,4. Food Agriculture Organization of the United Nations, Rome (Italy).
- Conner D. E, Scott V. N, and Bernard D. T. Growth inhibition and survival of Listeria monocytogenes as affected by acidic conditions. Journal of Food protection. 1990: 53:652-655.
CrossRef - EFSA (European Food safety Authority). Trends and sources of Zoonses, Zoonotic agents and antimicrobial resistance in the European Union in 2010. EFSA Journal. 2012: 2597
- Ray B., et al. Fundamentals Food Microbiology (3rd ed). 2004. Boca Raton, FL: CRC
- Alsheikh A. D. I, Mohammed G.E and Abdalla M.A. Isolation and identification of Listeria monocytogenes from Retail Broiler Chicken Ready to Eat Meat Products in Sudan. International Journal of Animal and Veterinary Advances. 2012; 5(1): 9-14, 2013.
CrossRef - Aneja, K. R. Experiments in Microbiology and plant pathology and Biotechnology. 2009. New Age International private limited Publishers.
- Farber J.M. et al. Present situation in Canada regarding Listeria monocytogenes and ready to eat sea food products. International Journal of Food Microbiology. 2000; 62:247-251.
CrossRef - Kozacinski L, Hadiosmanovic M, Meiotic B, et al. The meaning of Listeria monocytogenes in Veterinary sanitary inspection, Veterinary Achievement Journal. 2000:70-71.
- Doyle, M.P. Effect of Environmental and Processing conditions of Listeria monocytogenes. Food technology. 1988; 42: 169-171
- Schlech III W F. Food borne Listeriosis. Clinical Infectious Diseases. 2000; 31:770-775.
CrossRef - Gunasena Deepthi K, Chandra P. K, Kumudu G and Widanapathirana S. Occurrence of Listeria Monocytogenes in Food Sri Lanka. Journal of National Science Foundation of Sri Lanka. 1995; 23(3):107-114.
CrossRef - Rahimi E, Momtaz H, Sharifzadech A, Behzadnia A, Ashtari M. S, Zandi Esfahani S, Riahi M, Momeni M. Prevalence and antimicrobial resistance of Listeria species isolated from traditional dairy products in Charar Mahal and Bakhtiary, Iran. Bulgarian Journal of Veterinary Medicine. 2012; 15 (2):115-122.
- Enurah L. U, Aboaba O. O, Nwachukwu S. C. U, Nwosuh C. I. Antibiotic resistant profiles of food (fresh raw milk) and environmental (abattoir effluents) isolates of Listeria monocytogenes from the six zones of Nigeria. African Journal of Microbiology Research. 2013; 7(34):4373-4378.
- Molla B, Yilma R, Alemayehu D. Listeria monocytogenes and other Listeria species in retail meat and milk products in Addis Ababa, Ethiopia. Ethiopia Journal Health Dev. 2004; 18: 131-212.
CrossRef - Uyttendaele M, Troy P. D and Debevere J. Incidence of Listeria monocytogenes in poultry and poultry products obtained from Belgian and Freanch abattoris. Food Microbiology.1997; 14:339-345.
CrossRef - Cordano A.M and Rocourt J. Occurrence of Listeria monocytogenes in food in Chile. International Journal of Food Microbiology. 2001; 70: 175-178.
CrossRef - Soultos N, Koidis P and Madden R.H. Presence of Listeria and Salmonella in retail chicken in Northern Ireland. Letters in applied Microbiology. 2003; 37:421-423.
CrossRef - Bhilegonkar K. N, Killshrestha S. B, Kapoor K. N, Ashok Kumar, Agarwal R.K, Singh B.R and Kumar A. Isolation of Listeria monocytogenes from milk. International Journal food Science and technology Mysore. 1997; 34: 248-250.
- Jalali M and Abedi D. Prevalence of Listeria species in food products in Isfahan, Iran. International Journal of Food Microbiology. 2008; 122:336-340.
CrossRef








