Manuscript accepted on :20-11-2021
Published online on: 29-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Guy-Armel Bounda
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Gul Ozcan
Sindhuja A* , Vimalavathini R
, Vimalavathini R and Kavimani S
and Kavimani S
Department of Pharmacology, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, Gorimedu, Puducherry, India - 605006
Corresponding Author E-mail: vimalavathini@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2331
Abstract
Advanced glycation end products (AGEs) are formed excessively in pathological conditions due to non - enzymatic glycation of proteins, lipids or nucleic acids, affecting their structure and function. Isorhamnetin is a naturally occurring flavonoid with anti-inflammatory, anti-oxidant, anti-obesity, anticancer, anti-diabetic and anti-atherosclerosis activity. Structure activity studies of isorhamnetin reveal the presence of hydroxyl group in the B-ring of isorhamnetin may contribute to antiglycation activity. Hence we hypothised that isorhamnetin may have antiglycation activity owing to its structure as well as antioxidant and free radical scavenging activities by modulating various AGE pathway proteins. The aim of our study was to determine the antiglycation activity of isorhamnetin by targeting various molecular proteins of AGE pathway using insilico docking. The structure of isorhamnetin was imported and drawn in Marvin sketch (version 6. 3. 0). Nearly 17 molecular proteins of AGE pathway were docked with isorhamnetin using autodock tools 4.2 (version 1. 5. 6) software. The present study showed that isorhamnetin exhibited good docking profiles with receptor for advanced glycation End product (RAGE), protein kinase B (PKB/Akt2), activating transcription factor4 (ATF4), cAMP response element-binding protein (CREB), extracellular signal regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3-K) and signal transducer and activator of transcription (STAT) indicating it may exert good antiglycation activity by modulating these proteins of AGE pathways. However further invitro and invivo studies are required to establish the antiglycation activity of isorhamnetin.
Keywords
AGE; Antiglycation; Isorhamnetin; In silico Docking; RAGE; STAT
Download this article as:| Copy the following to cite this article: Sindhuja A, Vimalavathini R, Kavimani S. In Silico Docking Studies of Antiglycation Activity of Isorhamnetin on Molecular Proteins of Advanced Glycation End Product (AGE) Pathway. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Sindhuja A, Vimalavathini R, Kavimani S. In Silico Docking Studies of Antiglycation Activity of Isorhamnetin on Molecular Proteins of Advanced Glycation End Product (AGE) Pathway. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3Ju62Ur |
Introduction
AGEs are modification of proteins, lipids or nucleic acids when they are non – enzymatically glycated and oxidised when exposed to reducing sugar moiety1. Reactive sugars react with free amino groups especially lysine or arginine residue forming a non stable schiff base by Maillard reaction and more stable keto-amine products. The Schiff base and amadori products can react irreversibly with amino acid residues of peptides or proteins to form protein crosslinks. Further they can undergo oxidation, dehydration, polymerisation to give rise to numerous in vivo AGEs such as carboxymethyl lysine, pentosidine, carboxyethyl lysine and non fluorescent protein crosslinks such as glyoxal lysine dimer and methylglyoxal lysine dimer2. The accumulation of AGEs occurs under the circumstances of pathological conditions such as hyperglycaemia, oxidative stress, chronic renal failure, osteoarthritis, chronic heart failure, diabetes mellitus, cardiovascular, neurodegenerative diseases, auto-immune diseases, atherosclerosis and cancer3. Hence it would be imperative to derive drugs from natural origin to ameliorate those chronic diseases.
Isorhamnetin belongs to a group of secondary metabolites known as flavonoid. It is naturally present in red turnip, goldenrod, mustard leaf and Ginkgo biloba. It has been reported for its wide range of pharmacological effects such as anti-inflammatory, anti-oxidant, anti-obesity, anticancer, anti- diabetic and anti-atherosclerosis activity4. Docking is the computational determination of binding affinity between protein and ligand. The autodock 4.2 is molecular modelling simulation software, effective for protein ligand docking5.
Based on the previous studies, it was clear that mechanism of action of isorhamnetin has not been elucidated clearly but being a flavonoid with presence of hydroxyl group in the B-ring6, anti-oxidant and free radical scavenging properties4, we proposed that isorhamnetin may have antigylcation activity. Hence our aim of the present study was to determine the insilico antiglycation activity of isorhamnetin by targeting various molecular proteins of AGE pathway such as receptor for advanced glycation end products (RAGE), signal transducer and activator of transcription (STAT), Janus kinase 2 (JAK2), cAMP response element-binding protein (CREB), activating transcription factor4 (ATF4), protein kinase RNA- like endoplasmic reticulum kinase (PERK), protein kinase 38 (p38), extracellular signal regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3-K), protein kinase B (PKB/Akt2), c- Jun N- terminal kinase (JNK), CCAAT/enhancer binding protein homologous protein (CHOP), nuclear factor-kB (NF-kB), p21, rapidly accelerated fibrosarcoma 1 (RAF1), rat sarcoma (RAS) and mechanistic target of rapamycin (mTOR) using autodock tools 4.2 (version 1. 5. 6) software. The objectives of our study were, 1. To determine the antiglycation activity of isorhamnetin on various AGE pathway proteins using insilico docking, 2. To dock AGE pathway proteins such as RAGE, STAT, JAK2, CREB, ATF4, PERK, p38, ERK, PI3-K, PKB/Akt2, JNK, CHOP, NF-kB, p21, RAF1, RAS and mTOR with isorhamnetin, 3. To dock AGE pathway molecular isoforms such as RAGE, CREB, ERK, JNK, JAK2, PI3-K and p38 with different resolutions that were available in Research collaborator for structural bioinformatics Protein data bank (RCSB PDB). 4. To determine binding energy, inhibition constant, number of hydrogen bonds and active residues interaction of isorhamnetin with the above mentioned AGE pathway proteins.
Materials and Methods
The structure of isorhamnetin (PubChem ID: 5281654) was downloaded from NCBI PubChem databases. Isorhamnetin was drawn in Marvin sketch which was converted to PDB format using OpenBabel-2.3.1.The molecular proteins of AGE pathway were imported from RCSB PDB with their respective PDB ID as listed below,
RAGE (3O3U, 3CJJ), STAT (3WWT), JAK2 (3EYG, 6VN8), CREB (4NYX, 5CGP), ATF4 (1CI6), PERK (4X7K), p38 (5OMG, 2FST, 2FSL), ERK (6SLG, 5LCJ), PI3-K (2WWE, 6BTY), Akt2 (2UZR), JNK (4H39, 4W4V), CHOP (3T92), NF-kB (1A3Q), p21 (821P), RAF1 (1C1Y), RAS (4L8G) and mTOR (5WBH). The heteroatoms of these proteins were removed and were saved in the PDB format. Isorhamnetin was docked with the above 17 molecular proteins of AGE pathways using Autodock 4.2 (Version 1.5.6)7.
Molecular Docking studies by Autodock 4.2 (Version 1.5.6)
The interaction between isorhamnetin and AGE mediating proteins was predicted using autodock 4.2 software. In this tool, the preparation of receptor was done by adding all hydrogen atoms (polar only) to the macromolecule to correct the calculation of partial atomic charges. Gasteiger charges were calculated for each of the atom in the protein receptor molecule in autodock 4.2. Grid parameter of size 60*60*60 Å was created with 0.375 Å spacing followed by picking an atom and centering the geometric centre of the targeted protein to run the autogrid file. The selected important docking parameters for the Lamarckian Genetic Algorithm were as follows: population size of 150 individuals, mutation rate of 0.02, crossing over of 0.8 and 25 docking runs. The ligand was docked with proteins using autodock 4.2 (Version 1. 5. 6) software with the above mentioned procedure. The binding energy, inhibition constant, number of hydrogen bonds, active residues interaction based on their ranking order were assessed8,9,10,11.
Results
Table 1: Docking score of isorhamnetin with various molecular proteins of AGE pathway using autodock 4.2.
| S. No | Protein | PDB ID | Binding energy (kcal/mol) | Inhibition constant (μM) | Number of H bonds | Protein residues |
| 1 | RAGE | 3O3U | -9.02 | 0.243 | 2 | 3o3u:N:TRP62:HE1
3o3u:N:ARG66:HE |
| 3CJJ | -6.05 | 36.66
|
4 | 3cjj:A:TYR150:HH
3cjj:A:ALA152:HN 3cjj:A:ARG179:HE 3cjj:A:ARG179:HH22 |
||
| 2 | STAT | 3WWT | -5.91 | 46.41 | 4 | 3wwt:A:LYS40:HZ2 1
3wwt:A:GLN41:HE21 1 3wtt:A:SER62:HG 1 3wtt:B:LYS183:HZ1 1 |
| 3 | JAK2 | 6VN8 | -7.93 | 1.53 | 3 | 6vn8:B:LYS1030:HZ2
6vn8:B:VAL1033:HN 6vn8:A:GLN854:HN |
| 3EYG | -7.29 | 4.54 | 1 | 3eyg:A:GLY887:HN | ||
| 4 | CREB | 4NYX | -8.74 | 0.393 | 3 | 4nyx:A:MET1133:HN
4nyx:A:ASN1163:HD22 4nyx:A:ASN1168:HD21 |
| 5CGP | -5.92 | 46.0 | 2 | 5cgp:A:ASN1162:HD21
5cgp:A:ASN1162:HD22 |
||
| 5 | ATF4 | 1CI6 | -5.49 | 94.16 | 2 | 1ci6:A:LYS: 316:HZ3
1ci6:ASN261:HD21 |
| 6 | PERK | 4X7K | -9.87 | 0.058 | 2 | 4x7k:A:CYS891:HN
4x7k:A:ASP955:HN |
| 7 | p38 | 5OMG | -8.22 | 0.948 | 1 | 5omg:A:ARG149:HH12 |
| 2FST | -8.11 | 1.14 | 1 | 2fst:X:ARG149:HH22 1 | ||
| 2FSL | -7.56 | 2.88 | 1 | 2fsl:X:ARG70:HH21 | ||
| 8 | ERK | 6SLG | -6.48 | 17.85 | 4 | 6slg:A:ALA35:HN 1
6slg:A:TYR36:HH 1 6slg:A:ARG67:HH11 1 6slg:A:ASP167:HN 1 |
| 5LCJ | -6.74 | 11.38 | 4 | 5lcj:A:LYS54:HZ2
5lcj:A:LYS54:HZ3 5lcj:A:LYS151:HZ1 5lcj:A:SER153:HG |
||
| 9 | PI3-K | 6BTY | -10.16 | 35.84 | 3 | 6bty:A:LYS1611:HN
6bty:A:VAL1628:HN 6bty:B:VAL1628:HN |
| 2WWE | -7.06 | 6.68 | 2 | 2wwe:A:ALA1207:HN
2wwe:A:ASN1280:HD21 |
||
| 10 | PKB/
Akt2 |
2UZR | -6.51 | 17.04 | 2 | 2uzr:A:GLN61:HE21
2uzr:A:LEU62:HN |
| 11 | JNK | 4H39 | -8.11 | 1.13 | 5 | 4h39:A:LYS93:HZ1
4h39:A:ARG110:HE 4h39:A:ARG110:HH22 4h39:A:ASP207:HN 4h39:A:PHE208:HN |
| 4W4V | -6.91 | 8.57 | 3 | 4w4v:A:ARG107:HE
4w4v:A:LYS191:HZ3 4w4v:A:THR226:HN |
||
| 12 | CHOP | 3T92 | -7.82 | 1.85 | 2 | 3t92:A:GLN14:HE21
3t92:A:LYS72:HZ1 |
| 13 | NF-kB | 1A3Q | -7.94 | 1.52 | 5 | 1a3q:C:DA509:H61
1a3q:C:DA510:H62 1a3q:D:DA609:H7 1a3q:D:DA609:H62 1a3q:B:LYS221:HZ3 |
| 14 | p21 | 821P | -7.83 | 1.83 | 3 | 821p:A:SER17:HN
821p:A:LYS117:HZ2 821p:A:LYS147:HN |
| 15 | RAF1 | 1C1Y | -10.21 | 0.032 | 5 | 1c1y:A:GLY15:HN
1c1y:A:LYS16:HZ1 1c1y:A:LYS16:HZ21 1c1y:A:SER17:HG 1c1y:A:ASP33:HN |
| 16 | RAS | 4L8G | -5.28 | 2.58 | 1 | 4l8g:A:LYS16:HZ1 |
| 17 | mTOR | 5WBH | -6.77 | 10.85 | 3 | 5wbh:C:ARG2110:HH11
5wbh:D:ARG2076:HE 5wbh:D:ARG2076:HH21 |
In our insilico study, isorhamnetin exhibited good binding energy on docking with AGE pathway proteins of RAGE, PKB/Akt2, ATF4, CREB, CHOP, ERK, JAK2, JNK, mTOR, NF-kB, p38, p21, PI-3K, PERK, RAS, RAF1 and STAT. Among the 17 AGE pathway proteins, RAGE with both isoforms 3O3U and 3CJJ on docking with isorhamnetin showed binding energy of -9.02 kcal/mol and -6.05 kcal/mol respectively. However 3CJJ isoform of RAGE (Figure 1) exhibited inhibition constant of 36.66 µM with 4 hydrogen bonds. The binding energies of 5CGP and 4NYX isoforms of CREB when docked with isorhamnetin were -5.92 kcal/mol and -8.74 kcal/mol respectively. But 5CGP of CREB (Figure 2) resulted with good inhibition constant of 46.0 µM with 2 hydrogen bonds when compared to that of 4NYX. On comparing docking score of isorhamnetin with two isoforms of ERK, 6SLG (Figure 3) showed higher inhibition constant of 17.85 µM with 4 hydrogen bonds than 5LCJ showing binding energy of 6.48 kcal/mol and -6.74 kcal/mol respectively. Among the two docked isoforms of PI-3K, 6BTY (Figure 4) showed binding energy and good inhibition constant of -10.16 kcal/mol and 35.84 µM respectively with 3 hydrogen bonds while 2WWE came out with the binding energy of -7.06 kcal/mol with isorhamnetin.
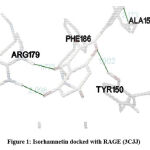 |
Figure 1: Isorhamnetin docked with RAGE (3CJJ) |
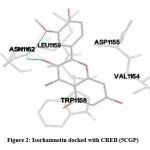 |
Figure 2: Isorhamnetin docked with CREB (5CGP). |
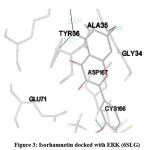 |
Figure 3: Isorhamnetin docked with ERK (6SLG). |
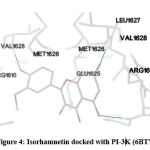 |
Figure 4: Isorhamnetin docked with PI-3K (6BTY) |
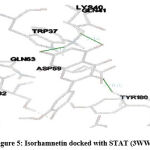 |
Figure 5: Isorhamnetin docked with STAT (3WWT) |
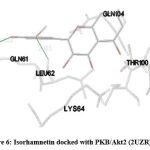 |
Figure 6: Isorhamnetin docked with PKB/Akt2 (2UZR) |
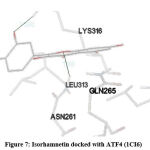 |
Figure 7: Isorhamnetin docked with ATF4 (1CI6) |
STAT (3WWT) (Figure 5), PKB/Akt2 (2UZR) (Figure 6) and ATF4 (1CI6) (Figure 7) were found to show higher inhibition constant and good binding energy of 46.41 µM, 17.04 µM and 94.16 µM and -5.91 kcal/mol, -6.51kcal/mol and -5.49 kcal/mol respectively other than RAGE (3CJJ), CREB (5CGP), ERK (6SLG), PI-3K (6BTY). The number of hydrogen bonds formed by isorhamnetin with STAT, PKB/Akt2 and ATF4 (Table 1) were 4, 2 and 2 respectively.
Discussion
Isorhamnetin (3, 5, 7-Trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one) is a flavonoid of quercetin derivative12 and consists of three carbon aromatic rings and many phenolic hydroxyl groups. Presence of ortho hydroxyl structure in B ring and a C2-C3 double bond in conjugation with a 4-oxo function6 in the C ring (benzopyran-4-one) accounts for free radical scavenging13. The hydroxyl groups at positions 3 and 5 in the A and C ring respectively, contributes to free radical scavenging activity by providing hydrogen bonding to the oxo-group14. The ortho-dihydroxy structure in the B ring provides stability for the flavonoid. Phenoxyl radicals contribute hydrogen atom release15, electron delocalization and hydrogen bonding ability. The C2-C3 structure in conjugation with a 4-oxo function in the C ring provides co-planarity of the hetero ring and increases radical stability by electron delocalization over all three-ring system. Presence of hydroxyl group on B-ring of isorhamnetin may be one of the key factors for its antiglycation activity6.
From the results of our study, it was found that isorhamnetin exhibited good binding energy of -6.05 kcal/mol and inhibition constant of 36.66 µM with 4 hydrogen bonds when docked with RAGE (3CJJ). RAGE is a single transmembrane receptor of the immunoglobulin superfamily16 and its over expression leads to activation of NF-kB, STAT, hypoxia-inducible factor and tumor necrosis factor alpha which has been associated with inflammation, diabetic complications, stroke, atherosclerosis, rheumatoid arthritis and neurodegenerative disorder and cancer17. Isorhamnetin interaction with NF-kB (1A3Q) exhibited binding energy of -7.94kcal/mol and weak inhibition constant and 1.52µM by forming 5 hydrogen bonds. Isorhamnetin with STAT (3WWT) showed binding energy and inhibition constant of -5.91kcal/mol and 46.41µM respectively by forming 4 hydrogen bonds. Isorhamnetin principally activates the JAK/STAT pathway to induce GLUT4 translocation by inducing STAT3 and STAT5 phosphorylation. Isorhamnetin increases glucose uptake by activating JAK/STAT pathway revealing the molecular mechanisms that support the use of isorhamnetin as a novel therapeutic strategy for prevention and treatment of hyperglycaemia and associated disorders18. Thus our insilico studies shows that isorhamnetin may possess anti-glycation activity by moderating RAGE, STAT and NF-kB proteins involved in various functions such as cell cycle, apoptosis, proliferation19.The hydroxyl group of B ring of isorhamnetin could have contributed to this anti RAGE activity6.
Docking profile of isorhamnetin with PI-3K (6BTY) showed binding energy and inhibition constant of -10.16 kcal/mol and 35.84 µM respectively with the formation of 3 hydrogen bonds. PI-3K phosphorylates Akt2 which then phosphorylates and regulates multiple target proteins, implicated in the regulation of apoptosis, metabolism, protein synthesis and cell division20. When PKB/Akt2 (2UZR) was docked with isorhamnetin, it resulted with binding energy, inhibition constant and hydrogen bonds of -6.51kcal/mol, 17.04 µM and 2 respectively which were satisfactory. Akt2 signalling has been implicated in maintaining neuronal and glial signalling, angiogenesis, neurogenesis, peripheral and cerebral blood flow and modulates glucose uptake and brain ageing21. The altered Akt signalling causes cancer22, cardiovascular diseases23 and diabetes mellitus24. Isorhamnetin may modulate the PI3 kinase/Akt signalling pathway by moderating activity of PTEN (phosphatase and tensin homolog deleted on chromosome 10), a tumour suppressor implicated in cancers. Studies show that PTEN is an inverse regulator of PI3K/Akt signalling pathway and is modulated by flavonoids21. PI-3K pathway along with AKT/mTOR pathway regulates cell growth, survival, metabolism, motility, and angiogenesis. Studies showed that the loss of C2-C3 double bond structure of flavonoid gives potent PI-3K inhibitory activity2526. To add on isorhamnetin being a quercetin derivative can directly suppress Akt/mTOR pathway activation and induces autophagy20. This study is consistent with our docking report of mTOR (5WBH) showing possible feeble interaction with binding energy of -6.77 kcal/mol and inhibition constant of 10.85 µM with 3 hydrogen bonds with isorhamnetin.
Docking results of isorhamnetin with ATF4 (1CI6) showed good binding energy and inhibition constant of -5.49kcal/mol and 94.16 µM respectively with 2 hydrogen bonds. Autophagy, inflammation, oxidative and endoplasmic reticulum stress contributes to ATF4 activation27 leading to cancer28, diabetes29 and neurodegenerative disorders27. The binding energy and inhibition constant exhibited by isorhamnetin when it was docked with ERK (6SLG) were -6.48kcal/mol and 17.85 µM respectively with 4 hydrogen bonds imply good interaction between drug and ligand. ERK, a member of MAPK pathway controls proliferation, survival, differentiation and motility and its modification has been implicated in various diseases such as cancer30, cardiovascular disorder31, neurodegenerative disorder32 and diabetes mellitus33. ERK activates its transcription factors such as CREB resulting in modulation of phosphatase activity which are middle man in many signalling pathways25. Binding energy of -5.92 kcal/mol, inhibition constant of 46.0 µM and 2 hydrogen bonds were obtained on docking of isorhamnetin with CREB (5CGP) which is consistent with previous report that flavonoid family are capable of modulating the CREB-BDNF pathway34. CREB is involved in cell growth and survival and its over expression is implicated in various diseases such as cancer, autoimmune neurodegenerative and cardiovascular disease.
Docking of isorhamnetin with JAK2, PERK, p38, JNK, CHOP, mTOR, NF-kB, p21, RAF1 and RAS yielded binding energies of above -5 and were in the range of -7.29 kcal/mol, -9.87 kcal/mol, -8.22 kcal/mol, -6.91 kcal/mol, -7.85/2 kcal/mol ,-6.77 kcal/mol, -7.94 kcal/mol , -7.83 kcal/mol , -10.21 kcal/mol , -5.28 kcal/mol respectively. But their inhibition constants were very less. This implies that isorhamnetin can bind to these proteins and may or may not mimic its action. But this insilico study revealed that isorhamnetin exhibited good binding energy and inhibition constant when it was docked with remaining AGE pathway proteins of RAGE (3CJJ), PKB/Akt2 (2UZR), ATF4 (1CI6), CREB (5CGP), ERK (6SLG), PI3-K (6BTY) and STAT (3WTT) and the number of hydrogen bonds exhibited by the above 7 molecular proteins were 4, 2, 2, 2, 4, 3 and 4 respectively. Thus our study showed that isorhamnetin exhibited antiglycation activity by binding and inhibiting RAGE protein and modulating other AGE pathway proteins such as PKB/Akt2, ATF4, CREB, ERK, PI-3K and STAT. Hence therapeutic targeting of these proteins by isorhamnetin will go a long way in alleviating diseases caused by dysregulation of AGE pathways such as cancer, diabetes mellitus, cardiovascular, neurodegenerative and autoimmune disorders. However further in vitro and in vivo studies are required to establish better knowledge on antiglycation activity of isorhamnetin.
The insilico docking studies of isorhamnetin with 17 AGE molecular proteins were performed and the results were analysed. The inference of this study reveals that isorhamnetin showed profound inhibitory activity against RAGE, PKB/Akt2, ATF4, CREB, ERK, PI-3K and STAT. Therefore this study recommends that isorhamnetin can be profiled for the futuristic in vivo and in vitro studies to establish its antiglycation activity for the diseases caused by AGE pathway proteins.
Acknowledgement
There is no acknowledgement.
Conflict of interest
There is no conflict of interest.
Funding source
We worked on this project without any funding.
References
- Goldin A, Beckman J. A, Schmidt A. M, and Creager M. A. Advanced glycation end products, sparking the development of diabetic vascular injury. Circulation, 2006; 114:597-605.
CrossRef - Gkogkolou P and Bohm M. Advanced glycation end products. Key players in skin aging?. Dermatoendocrinol., 2012; 4(3): 259-70.
CrossRef - Yamagishi S and Matsui T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorder and therapeutic intervention. Nutrition, 2016; 32(2): 157-65.
CrossRef - Gong G, Guan Y, Zhang Z, Raman K, Wang S, Zhou S, Luan X and Zhang H. Isorhamnetin: A review of Pharmacological effects. Biopha., 2020; 110301.
CrossRef - Meng X, Zhang H, Mezei M and Cui M. Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des., 2011 June 1; 7(2): 146-157.
CrossRef - Ronsisvalle S, Panarello F, Longhitano G, Siciliano E. A, Montenegro L and Panico A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics, 2020; 7(3); 71.
CrossRef - Vimalavathini R, Thamizharasi S, Vishvaja S, Srinithi S and Yuvaraj G. Insilico docking of aminopyrimidines targeting receptor for advanced glycation end products. ejpmr, 2020; 7(9) : 231-236.
CrossRef - Norgan A. P, Coffman P. K, Kocher J. P. A, Katzman D. J and Sosa C. P. Multilevel Parallelization of Autodock 4.2. Journal of Cheminformatics, 2011; 3: 12.
CrossRef - Morris G.M, Huey R, Lindstrom W, Sanner M. F, Belew R. K, Goodsell D. S and Olson A. J. Autodock4 and Autodock Tools4: Automated docking with selective receptor Flexibility. J Comput Chem., 2009; 30(16): 2785-2791.
CrossRef - Seeliger D and Groot B. L. Ligand docking and binding site analysis with pyMOL and Autodock/Vina. J comput Aided Mol Des., 2010; 24: 417-422.
- Rizvi S. M. D, Shakil S and Haneef M. A simple click by click protocol to perform docking: Autodock 4.2 made easy for non-Bioinformaticians. EXCLI J., 2013; 12: 831-57.
- Kandakumar S and Manju V. Pharmacological applications of isorhamnetin: A short review. IJTSRD., 2017; 1(4); 2456-6470.
CrossRef - Nayak Y, Jayashree B. S and Unnikrishnan M. K. Benzopyran-4-one is an Ideal Pharmacophore for Lead Optimisation in Antidiabetic Drug Discovery. RJPT., 2012; 5(8); 1025-1033.
- Tapas A, Sakarkar D and Kakde R. The chemistry and Biology of Bioflavonoids. , 2008; 1(3): 132-143.
- Marref S. E, Benkiki N and Melakhessou M. K. In vitro Antioxidant Activity, Total Phenolics and Flavonoids Contents of Gladiolus segetum Extracts. RJPT., 2018; 11(11): 5017-5023.
CrossRef - Riehl A, Nemeth J, Angel P and Hess J. The receptor RAGE; Bridging inflammation and cancer. Cell Commun Signal., 2009; 8: 7-12.
CrossRef - Chuah Y. K, Basir R, Talib H, Tie T. H and Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory disease. Hindawi, 2013; 15: 403460.
CrossRef - Jiang H,Yamashita Y, Nakamura A, Croft K and Ashida Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci Rep., 2019; 9: 2690.
CrossRef - Kishore R and Verma S. K. Roles of STATs signalling in cardiovascular diseases. JAKSTAT., 2012; 1(2): 118-124.
CrossRef - Syed N, Adhami V. M, Khan M. I and Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem., 2013; 13(7): 995-1001.
CrossRef - Spencer P. E. Beyond antioxidants: the cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc Nutr Soc. 2010; 69(2): 244-60.
CrossRef - Song M, Bode A. M, Dong Z and Lee M. AKT as a therapeutic target for cancer. Cancer Res., 2019; 79(6): 1019-1031.
CrossRef - Abeyrathna P and Su Y. The critical role of Akt in cardiovascular function. Vascul Pharmacol., 2015; 74: 38-48.
CrossRef - Zdychova J and Komers R. Emerging Role of Akt kinase/ Protein Kinase B signalling in pathophysiology of Diabetes and its complications. RAS., 2005; 54(1): 1-16.
- Yang J, Nie J, Ma X, Wei Y, Peng Y and Wei Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019; 18(1): 26
CrossRef - Zhang W, Hu J. J, Fu R. Q, Liu X, Zhang Y. H, Li J, Liu L, Li Y. N, Deng Q, Luo Q. S, Ouyang Q and Gao N. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Scientific Reports. 2018; 11255.
CrossRef - Pitale P. M, Gorbatyuk O and Gorbatyuk M. Neurodegeneration: Keeping ATF4 on tight leash. Front Cell Neurosci., 2017; 11: 410.
CrossRef - Singleton D. C and Harris A. L. Targeting the ATF4 pathway in cancer therapy. Expert Opin Ther Targets., 2012; 16(12): 1189-202
CrossRef - Chen Y, Wang J. J, Hosoya K. I, Ratan R, Townes T, Zhang S. X. Activating transcription factor 4 mediates hyperglycaemia – induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia, 2012; 55(9): 2535-45.
CrossRef - Kohno M and Poduyssegur J. Targeting the ERK signalling pathway in cancer therapy. Ann Med., 2006; 38(3): 200-11.
CrossRef - Gallo S, Vitacolonna A, Bonzano A, Comoglio P and Crepaldi T. ERK: A key player in the pathophysiology of cardiac Hypertrophy. Int J Mol Sci., 2019; 20(9): 2164.
CrossRef - Cruz C. D and Cruz F. The ERK 1 and 2 Pathway in the nervous system: From basic aspects to possible clinical applications in pain and visceral dysfunction. Curr Neuropharmacol., 2007; 5(4): 244-252.
CrossRef - Ozaki K. I, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, Tanimura S and Kohno M. Targeting the ERK signalling pathway: a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab., 2016; 310(8); E643-E651.
CrossRef - Sharma P, Kumar A and Singh D. Dietary Flavonoids Interaction with CREB-BDNF Pathway: An Unconventional Approach for Comprehensive Management of Epilepsy. Curr Neuropharmacol. 2019; 17(12): 1158-1175.
CrossRef







