Rajathilagam T1* , Thuthi Mohan2
, Thuthi Mohan2 , Aruna B Patil3
, Aruna B Patil3 , Mohanavalli S4
, Mohanavalli S4 and Seethalakshmi S1
and Seethalakshmi S1
1Department of Pharmacology, ESIC Medical College and Post Graduate Institute of Medical Sciences and Research, KK Nagar, Chennai- 600078, Tamil Nadu, India.
2Department of Biochemistry, ESIC Medical College and Post Graduate Institute of Medical Sciences and Research, KK Nagar, Chennai- 600078, Tamil Nadu, India.
3Department of Community Medicine, ESIC Medical College and Post Graduate Institute of Medical Sciences and Research, KK Nagar, Chennai- 600078, Tamil Nadu, India.
4Department of Dentistry, ESIC Medical College and Post Graduate Institute of Medical Sciences and Research, KK Nagar, Chennai- 600078, Tamil Nadu, India.
Corresponding Author E-mail: rajathilagamtr@yahoo.co.in
DOI : https://dx.doi.org/10.13005/bpj/2332
Abstract
Periodontitis is a common multifactorial inflammatory disease with gradual loss of supportive tissues around the teeth which eventually leads to decrease in the quality of life. Blocking Interleukin-6 (IL-6), a multifunctional cytokine with pro-inflammatory properties has demonstrated therapeutic efficacy in inflammatory diseases like Rheumatoid arthritis, SLE and multiple sclerosis. Host immune response, the underlying cause for this progressive disease is targeted by Host modulatory therapy (HMT), an emerging treatment modality. Omega-3 polyunsaturated fatty acids (ώ 3 PUFAs), one of the relatively safe HMTs, reduces tissue destruction, stabilizes or even regenerates the periodontium through its anti-inflammatory & immunoregulatory properties. ώ 3 PUFAs are essential for the synthesis of eicosanoids which are involved in anti-inflammatory, antiplatelet aggregatory, vasodilation, vasoconstriction, immune response, cell growth and proliferation. The key factor examined and extrapolated in this study is the anti-inflammatory property of ώ 3 PUFA. The aim of the study was to evaluate the immunological and clinical response to ώ 3 PUFA supplementation therapy in chronic periodontitis by measuring the inflammatory cytokine, IL-6 levels in serum. In this open label exploratory study, 40 patients with a Female: Male ratio of 4:1were enrolled and assessed clinically by measuring Oral Hygiene Index-Simplified (OHI-S), Probing Pocket Depth (PPD), Clinical Attachment Level (CAL) and their serum for IL-6 levels. Subsequently 300 mg (concentration of EPA 180/DHA120) of ώ 3 PUFA was prescribed twice daily for 3 months and periodically reviewed to assess their IL-6 levels and periodontal status. IL-6 levels which were at a maximum mean of 10.2 pg/ml prior to treatment, showed a gradual and notable reduction to 2.3 pg/ml at the end of the study following ώ 3 PUFA supplementation therapy. The coefficient of variation R2 and ANOVA showed statistically significant periodic variation in biomarker IL-6 and in all clinical measurements at all time intervals. ώ 3 PUFA adjunctive therapy significantly reduces the inflammatory cytokine (IL-6) levels and causes noteworthy improvement of the most relevant clinical parameters (OHI-S, PPD, CAL). Hence ώ 3 PUFA can be recommended as a dietary supplementation and a safe host modulatory treatment in chronic periodontitis.
Keywords
Anti-inflammatory; Host Modulatory Therapy; Inflammatory cytokine; IL-6; Interleukin 6; Periodontitis; ώ 3 PUFA
Download this article as:| Copy the following to cite this article: Rajathilagam T, Mohan T, Patil A. B, Mohanavalli S, Seethalakshmi S. IL-6, a Therapeutic Target and Omega-3 PUFA, a Host Modulator in Chronic Periodontitis. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Rajathilagam T, Mohan T, Patil A. B, Mohanavalli S, Seethalakshmi S. IL-6, a Therapeutic Target and Omega-3 PUFA, a Host Modulator in Chronic Periodontitis. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3GVg8vH |
Introduction
Periodontitis is a chronic inflammatory condition, with progressive loss of both soft and hard tissues surrounding the teeth1,2,3,4. Since periodontitis is one of the commonest disease of the oral cavity, it is always a major public health concern 4,5. It is a disease individually accelerated or decelerated by multiple factors like hypertension, diabetes, genetic factors, unhealthy diet, high cholesterol, obesity and tobacco use. The interaction between dental plaque bacteria and the immune inflammatory response, induced by mediators such as arachidonic acid metabolites, cytokines and enzymes causes progressive tissue destruction in periodontitis. Eventual loss of tooth-supporting structures, including connective tissue attachment and alveolar bone is the end result of periodontal inflammation5,6,7. Interaction of environmental and genetic factors, host defense mechanisms, microbial agents are said to cause the expression of this common chronic inflammatory disease.1,3.
Cytokines are chemically composed of water-soluble glycoproteins secreted in response to infection from hematopoietic and non-hematopoietic cells. The onset and/or progression of the disease is associated with the inflammatory cytokines induced during an inflammatory response. Hence, these inflammatory cytokines which predominantly contribute to the pathogenesis of periodontal disease, may be considered as diagnostic markers3,8,9. Therefore detection and estimation of inflammatory cytokine levels to evaluate periodontal disease or outcomes of periodontal treatment is gaining much importance1,8.
Interleukin-6 (IL-6), a multifunctional inflammatory cytokine plays a pivotal role in the regulation of host response to infection and tissue injury1,10,11. IL-6 has its effect on inflammation and bone resorption by stimulating osteoclastic activity 8,9. It may also promote the degeneration of inflamed periodontal tissues3,12. Since it is said that the expression of IL-6 is in proportion to the severity of periodontal disease and age, it has become a critical parameter in periodontal research 3,8.
The initiating event responsible for the ensuing inflammation and tissue destruction is the bacterial infection and hence traditional therapies for periodontitis focus on targeting the same. The therapeutic modalities should not only arrest and prevent periodontal tissue destruction, but should also aim at re-establishing and regenerating the periodontal tissues previously lost to disease13. The host response to the bacterial infection plays a more crucial role in the pathogenesis of periodontitis and its associated systemic effects and hence it is targeted by recent therapeutic strategies2,14. Host modulatory therapy (HMT), a new treatment approach, down-regulates the destructive aspects of the host response and hence reduces tissue destruction. It also up-regulates the protective or regenerative responses and thus stabilizes or even regenerates the periodontium 5,6,12,15.
Nonsteroidal anti-inflammatory drugs, tetracyclines, and bisphosphonates are a few of the spectrum of drug classes which have been evaluated as host modulating agents. These drugs have their own adverse effects and limitations when prescribed for HMT6,16. However ώ 3 PUFAs, which includes docosahexaenoic acid (DHA; C22:6, n-3) and eicosapentaenoic acid (EPA; C20:5, n-3), had proven protective and anti-inflammatory effects in atherosclerosis, cancer, cardiovascular disease, rheumatoid arthritis, cystic fibrosis, ulcerative colitis, asthma and periodontitis5,6,14. They also have immunoregulatory, antiarrhythmic, antiatherogenicity, platelet anti aggregatory, hypolipidemic and antioxidant enhancing properties with a negligible adverse reaction profile5. Initially, the beneficial effects of ώ 3 PUFAs were attributed to a reduction in the production of classic inflammatory mediators such as AA-derived eicosanoids like Prostaglandins PGE2 and inflammatory cytokines. Later studies reveal a novel series of anti-inflammatory lipid mediators, resolvins and protectins, which are enzymatically transformed by ώ 3 PUFAs and finally behave as substrates for the anti-inflammatory reaction. The molecular basis for this anti-inflammatory effect of ώ 3 PUFAs, lie in the enzymatic pathways of inflammation resolution by down regulating the production of nuclear transcription factors and cytokines 2,6,7,14,16.
Since the host modulating effect of ώ 3 PUFA was the key factor examined in our study, we estimated serum biomarker (IL-6) levels to further substantiate our findings.
Materials and Methods
Objectives:
The primary objective of the study was to evaluate the action of ώ 3 PUFA supplementation therapy on clinical outcomes in chronic periodontitis. The secondary objective was to quantify the effect of ώ 3 PUFA supplementation therapy on serum IL-6 levels in chronic periodontitis.
Methodology
This study was conducted in the Dental outpatient department (OPD) of ESIC Medical College Hospital. The study was done for 6 months from July 2019 to December 2019 to study the immune modulatory effects of dietary supplementation with ώ 3 PUFA on patients with chronic periodontitis.
Study design: An Open Label, exploratory study.
Study period: 6 months.
Sample size and sampling method: 40 patients were recruited based on the consecutive entry in dental OPD using eligibility criteria selection. The sample size was calculated using nMaster (v2.0).
Inclusion and exclusion criteria
Forty patients in the age group between 30 to 55 years, having chronic periodontitis, who were not on any antibiotics, immunotherapy with monoclonal antibodies, multivitamins or anti-inflammatory medications for the preceding three months were chosen to be included in the study. Vegetarian or vegan patients unwilling to consume fish and fish products or having allergy to fish, patients with chronic systemic diseases like Diabetes, TB, HIV, Hepatitis & epilepsy, patients with viral, fungal & bacterial infections, smokers, those who consume alcohol, pregnant and lactating females were not eligible for participating in this study. Patients with other chronic inflammatory diseases like Psoriasis, Rheumatoid arthritis, SLE, Multiple Sclerosis, patients with fever, sepsis and pancreatitis were not included in the study population. Patients with a history of close contact with pet animals, use of cosmetics with biotin were also screened and excluded from this study.
Parameters Assessed
Those patients who were eligible, were included in the study after obtaining informed consent. A structured patient profile form was used to enter the demographic details, brief medical history and assessment of clinical parameters [Oral Hygiene Index (OHI-S), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL)]. The enrolled patients underwent scaling as an oral prophylactic measure to remove plaque and bacteria below the gum lines.
Blood samples were collected for baseline investigations and to assess IL-6 levels. Then the patients were prescribed ώ 3 PUFA capsules 300 mg (concentration of EPA 180/DHA120) twice daily for 3 months. They were reviewed at the end of first week, second week, 1st month and 3rd month of treatment to assess the IL-6 levels & periodontal status.
Assessment of Clinical Parameters 2,8,9,17,18
Simplified Oral Hygiene Index (OHI-S)
OHI-S was used to assess the Oral hygiene status. It has two components, the Debris Index-Simplified (DI-S) and the Calculus Index-Simplified (CI-S). They are calculated separately and are summed up to get the OHI-S score. Mouth mirror and explorer were used to examine the oral hygiene status. The interpretation of index is as follows:
Good: 0 to 1.2
Fair: 1.3 to 3.0
Poor: 3.1 to 6.0.
Probing Pocket Depth (PPD)
William’s periodontal probe was used to record the distance in millimeters from the gingival margin to the base of gingival sulcus or periodontal pocket. The probe was inserted without any pressure into the gingival sulcus or pocket to the long axis of the tooth at four surfaces of each tooth (labial/ buccal, lingual /palatal, mesial and distal). The interpretation of PPD is as follows:
Mild: 3-5 mm
Moderate: 5-7mm
Severe: >7mm
Clinical Attachment Level (CAL)
William’s graduated periodontal probe was used to measure the distance between the cemento-enamel junction [CEJ] and the base of gingival sulcus or pocket in an apical direction. The probe was inserted into the buccal (labial), lingual (palatal), mesial and distal surfaces for each tooth. The interpretation of CAL is as follows:
Slight: 1-2 mm
Moderate: 3-4 mm
Severe: >5 mm
Methodology for estimating IL-6 in serum
Principle and Method
Quantitative determination of IL-6 in human serum was done by a simultaneous one step immunoenzymatic sandwich chemiluminescent assay on the Access 2 Beckman hormone analyzer. To the reaction vessels coated with paramagnetic particles, containing mouse monoclonal anti-human IL-6, the alkaline phosphatase conjugate and blocking agent, the serum sample is added. Materials bound to the solid phase hold on to a magnetic field created, while unbound materials are washed away after incubation. The substrate Lumi-Phos 530 which is chemiluminescent, is then added to the vessel. Measurement of the chemiluminescent light, generated by the reaction is done using a luminometer. The concentration of IL-6 in the sample is directly proportional to the light or luminescence produced. The quantity of IL-6, the analyte found in the sample was measured against a six point calibration curve using 0, 2.5, 25, 250, 750 and 1500 pg/ml of IL-6 calibrators, which were run in duplicate. The calibrators used are traceable to the NIBSC/WHO standard as per EN ISO 17511 requirements.
This assay is linear for concentration up to 1500 pg/ml. The analytical sensitivity of this assay is 0.5 pg/ml. 3 ml of venous blood was collected from a peripheral vein in a barcoded yellow topped, gel vacutainer tube with no additives or preservatives and transported to the lab, in closed temperature controlled containers. The tube was allowed to stand at room temperature for 20 minutes, till complete clot formation and serum separated by an Eppendorf 5810 centrifuge at 2500- 3000 rpm for 10 minutes. Serum was separated within 45 minutes of collection and sample processed within 4 hours of collection. Sample rejection criteria included delay in transport of samples, hemolysed, turbid, and contaminated samples.
Environmental and safety controls
Samples and reagents disposed as per Tamil Nadu Pollution control board guidelines
Calibration and Quality Control
A multi-point calibration using six calibrators ranging from 0.5 to 1500 pg/ml was done every 28 days during the study period and Beckman quality controls run for every batch and validated every 24 hours before patient samples were run.
| Sample Volume | 110 µl |
| Dead volume | 80 µl |
| QC Volume | 200 µl |
| QC frequency | 2 levels every 24 hours |
Reference Interval
Biological reference intervals followed in the study for serum Interleukin-6/IL-6 was 5.3 to 7.3 pg/ml in adults between the ages 22 to 73 yrs. Alert/Critical values considered were <2.5 pg/ml, >500 pg/ml.
Statistical analysis
The categorical data was represented by numbers and percentages and continuous data as mean and standard deviation (SD) along with all descriptive statistics.
ANOVA ‘F’ test was used to analyze the clinical treatment outcomes OHI-S, PPD, CAL and the biomarker IL-6. Post hoc analysis was performed using Dunnett T3 multiple comparison test; which assumes the variances are not equal.
Total observations (n) in this study were forty. Patients were selected on consecutive basis but not at random. The clinical treatment measurements did not follow normal distribution. Though the test of normality showed significant difference and the Null hypothesis was rejected that the data distribution was normal. Then the non-parametric Fried man test was used to compare the independent variables. After observing, both Parametric test (ANOVA) and nonparametric test (Fried man) results rejected the same Null hypothesis of study. However, the robust ANOVA test was used to compare the treatment variables over the non-parametric test Fried man. The linear regression method was used to predict the effect on IL- 6 when there was change in clinical measurements.
The Statistical significance was considered at p <0.05. SPSS statistical software (version 21.0) was used for statistical analysis. The graphics were provided by Excel Microsoft 10.0.
Results
80% of the patients shortlisted for the study were females and 60% of them were housewives. A higher proportion of the patients (30%) were between 41-45 years of age. They had 1 to 5 acute exacerbations during the chronic course of the disease ranging from 3-14 months.
Table 1: Demographic profile.
|
Descriptive Statistics (categorical data) |
|||
| Characteristics | Category | N | Percentage |
| Gender | Male | 8 | 20% |
| Female | 32 | 80% | |
| Age group (years) | <= 30 | 2 | 5% |
| 31–35 | 8 | 20% | |
| 36–40 | 8 | 20% | |
| 41–45 | 12 | 30% | |
| 46–50 | 3 | 8% | |
| 51–55 | 7 | 18% | |
| Occupation | Housewife | 24 | 60% |
| Factory workers | 10 | 25% | |
| Housemaid | 3 | 8% | |
| Office workers | 3 | 8% | |
| Chief
Complaints |
Pain, sensitivity | 14 | 35% |
| Pain, sensitivity, tooth mobility | 14 | 35% | |
| Pain, sensitivity, tooth mobility, associated symptoms | 7 | 18% | |
| Pain, sensitivity, associated symptoms | 1 | 3% | |
| Pain, tooth mobility | 4 | 10% | |
| Duration of disease (months) | 3 | 2 | 5% |
| 4 | 6 | 15% | |
| 5 | 7 | 18% | |
| 6 | 15 | 38% | |
| 7 | 3 | 8% | |
| 8 | 2 | 5% | |
| 12 | 4 | 10% | |
| 14 | 1 | 3% | |
| Number of episodes | 1 | 4 | 10% |
| 2 | 17 | 43% | |
| 3 | 10 | 25% | |
| 4 | 5 | 13% | |
| 5 | 4 | 10% | |
The participants were clinically examined periodically to measure OHI-S, PPD and CAL. The patients’ serum was assessed for any significant change in the IL-6 levels along with clinical measurements. The observed variations are noted in Table-2 and depicted by Mean plot in Figure -1. These patients who received our treatment schedule exhibited both clinical and statistically significant reduction in the pathology of the chronic periodontitis and also corresponding variations in the biomarker (IL-6) levels. The Boxplot in Figure 2 clearly illustrates this therapeutic response.
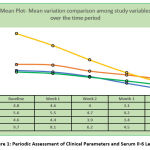 |
Figure 1: Periodic Assessment of Clinical Parameters and Serum Il-6 Levels. |
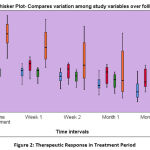 |
Figure 2: Therapeutic Response in Treatment Period. |
Table 2: Periodic assessment of clinical parameters and serum IL-6 levels
| Descriptive Statistics (Continuous data) | ||||||||
| Assessed
parameters |
Time Interval(N=40) | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
| OHI-S | Baseline | 4.8 | 0.7 | 0.1 | 4.6 | 5.0 | 3.2 | 5.8 |
| 1st week | 4.6 | 0.6 | 0.1 | 4.4 | 4.8 | 3 | 5.7 | |
| 2nd week | 4.0 | 0.7 | 0.1 | 3.7 | 4.2 | 2.4 | 5.1 | |
| 1st Month | 3.1 | 0.9 | 0.1 | 2.8 | 3.4 | 1.6 | 4.8 | |
| 3rd Month | 2.3 | 1.2 | 0.2 | 1.9 | 2.7 | 0.7 | 4.7 | |
| PPD | Baseline | 5.6 | 0.8 | 0.1 | 5.4 | 5.9 | 3.7 | 6.8 |
| 1st week | 5.5 | 0.8 | 0.1 | 5.2 | 5.7 | 3.7 | 6.6 | |
| 2nd week | 4.7 | 0.9 | 0.1 | 4.4 | 5.0 | 3 | 5.8 | |
| 1st Month | 4.2 | 1.0 | 0.2 | 3.9 | 4.5 | 2.6 | 5.6 | |
| 3rd Month | 3.6 | 1.2 | 0.2 | 3.2 | 4.0 | 2 | 5.5 | |
| CAL | Baseline | 4.6 | 0.6 | 0.1 | 4.4 | 4.8 | 3.4 | 5.2 |
| 1st week | 4.4 | 0.6 | 0.1 | 4.2 | 4.6 | 3.1 | 5.2 | |
| 2nd week | 3.9 | 0.8 | 0.1 | 3.7 | 4.2 | 2.6 | 4.9 | |
| 1st Month | 3.4 | 1.0 | 0.2 | 3.0 | 3.7 | 1.8 | 4.6 | |
| 3rd Month | 2.8 | 1.1 | 0.2 | 2.5 | 3.2 | 1.2 | 4.5 | |
| IL- 6 | Baseline | 9.7 | 1.7 | 0.3 | 9.1 | 10.2 | 6.4 | 12 |
| 1st week | 8.1 | 1.9 | 0.3 | 7.5 | 8.8 | 5.2 | 11.5 | |
| 2nd week | 6.2 | 2.6 | 0.4 | 5.3 | 7.0 | 2.3 | 10.6 | |
| 1st Month | 4.5 | 2.7 | 0.4 | 3.6 | 5.3 | 1.2 | 9.7 | |
| 3rd Month | 3.1 | 2.3 | 0.4 | 2.3 | 3.8 | 0.7 | 7.6 | |
Inferential statistics presented in Table 3 enables us to realize that the treatment option adopted in this study required 2 weeks to 3 months to significantly influence the disease process for effective outcome.
Table 3: Treatment outcomes on assessed study variables
|
Multiple Comparisons (post hoc test-Dunnett T3†) |
|||||||
| Dependent Variablesa | (I) Time interval | (J) Time interval | (I-J) Mean Difference | Std. Error | p value | 95% Confidence Interval | |
| OHI-S | Baseline | 1st week | 0.14 | 0.15 | 0.986 (NS) | -0.28 | 0.55 |
| Baseline | 2nd week | .793* | 0.15 | 0.0001 | 0.35 | 1.24 | |
| Baseline | 1st Month | 1.64* | 0.17 | 0.0001 | 1.14 | 2.14 | |
| Baseline | 3rd Month | 2.448* | 0.21 | 0.0001 | 1.84 | 3.06 | |
| PPD | Baseline | 1st week | 0.14 | 0.18 | 0.996 (NS) | -0.38 | 0.66 |
| Baseline | 2nd week | .913* | 0.20 | 0.0001 | 0.34 | 1.48 | |
| Baseline | 1st Month | 1.428* | 0.21 | 0.0001 | 0.83 | 2.03 | |
| Baseline | 3rd Month | 2.01* | 0.23 | 0.0001 | 1.36 | 2.66 | |
| CAL | Baseline | 1st week | 0.16 | 0.14 | 0.935 (NS) | -0.24 | 0.56 |
| Baseline | 2nd week | .67* | 0.15 | 0.0001 | 0.24 | 1.11 | |
| Baseline | 1st Month | 1.25* | 0.18 | 0.0001 | 0.73 | 1.76 | |
| Baseline | 3rd Month | 1.77* | 0.20 | 0.0001 | 1.18 | 2.36 | |
| IL-6 levels | Baseline | 1st week | 1.51* | 0.40 | 0.003 | 0.36 | 2.66 |
| Baseline | 2nd week | 3.49* | 0.48 | 0.0001 | 2.09 | 4.89 | |
| Baseline | 1st Month | 5.203* | 0.50 | 0.0001 | 3.77 | 6.64 | |
| Baseline | 3rd Month | 6.575* | 0.45 | 0.0001 | 5.28 | 7.87 | |
| a. All dependent variables were shown ANOVA F test statistically significant at p<0.05 | |||||||
| † Equal variances are not assumed. (Test of Homogeneity of Variances were found statistically significant) * p<0.05, NS- Not Significant |
|||||||
The statistics in Table – 4a presents the good fit of Model to estimate the coefficients, in which R values showed the periodic correlation with the biomarker IL-6. The coefficient of variation R2 showed the periodic variation in biomarker IL-6 (predictive variable) and was explained by the variation in clinical measurements (predictors). The increasing trend of Adjusted R2 showed that each time interval was important to note the change in dependent variable biomarker IL-6. ANOVA results showed that the variation in all clinical measurements (predictors) was found statistically significant in comparison of all time intervals in this study.
Table 4a: A Model Fit summary and ANOVA results
| Dependent Variable: IL-6 | Model summary | ANOVAa test results | |||
| R | R Square | Adjusted R Square | F test | p value | |
| baseline | .459a | 0.211 | 0.145 | 3.205 | .035b |
| 1st week | .644a | 0.415 | 0.366 | 8.521 | .000b |
| 2nd week | .754a | 0.568 | 0.532 | 15.787 | .000b |
| 1st Month | .859a | 0.738 | 0.716 | 33.724 | .000b |
| 3rd Month | .929a | 0.862 | 0.851 | 75.080 | .000b |
| a.Dependent Variable:IL6, b. Predictors: (Constant), OHIS, PPD,CAL | |||||
Table 4b explains the influence of the Predictors OHI-S, PPD and CAL on serum IL-6 levels over the time period from baseline to 3rd month. At baseline, no statistically significant impact was seen. In 1st week and 2nd week, only PPD significantly influenced IL-6 levels. In the 1st month and 2nd month of treatment period, both OHI-S and PPD significantly influenced the reduction in IL-6 levels. The predictor CAL didn’t show significant effect on IL-6 values over all the time periods.
Table 4b: Influence of clinical outcomes on serum biomarker levels.
| Model | Unstandardized Coefficients | Standardized Coefficients | t test value | p value | 95% Confidence Interval for B | |||
| Dependent Variable: IL-6 | Predictors | B | Std. Error | Beta | Lower Bound | Upper Bound | ||
| Baseline | (Constant) | 3.59 | 2.03 | 1.77 | 0.09 | -0.53 | 7.71 | |
| OHI-S | 0.15 | 0.64 | 0.06 | 0.23 | 0.82 | -1.15 | 1.45 | |
| PPD | 0.56 | 0.44 | 0.28 | 1.26 | 0.21 | -0.34 | 1.46 | |
| CAL | 0.48 | 0.66 | 0.17 | 0.73 | 0.47 | -0.86 | 1.82 | |
| 1st week | (Constant) | -1.36 | 1.93 | -0.71 | 0.49 | -5.28 | 2.56 | |
| OHI-S | 0.51 | 0.57 | 0.17 | 0.90 | 0.37 | -0.64 | 1.66 | |
| PPD | 0.96 | 0.44 | 0.40 | 2.16 | 0.04 | 0.06 | 1.86 | |
| CAL | 0.42 | 0.56 | 0.14 | 0.76 | 0.45 | -0.72 | 1.56 | |
| 2nd week | (Constant) | -3.88 | 1.62 | -2.40 | 0.02 | -7.15 | -0.60 | |
| OHI-S | -0.21 | 0.69 | -0.06 | -0.31 | 0.76 | -1.61 | 1.18 | |
| PPD | 1.38 | 0.61 | 0.51 | 2.28 | 0.03 | 0.15 | 2.61 | |
| CAL | 1.11 | 0.80 | 0.33 | 1.40 | 0.17 | -0.50 | 2.73 | |
| 1st month | (Constant) | -4.72 | 0.97 | -4.89 | 0.0001 | -6.68 | -2.76 | |
| OHI-S | 1.40 | 0.51 | 0.46 | 2.76 | 0.01 | 0.37 | 2.42 | |
| PPD | 1.23 | 0.54 | 0.48 | 2.28 | 0.03 | 0.13 | 2.33 | |
| CAL | -0.11 | 0.62 | -0.04 | -0.17 | 0.87 | -1.36 | 1.15 | |
| 3rd month | (Constant) | -2.69 | 0.61 | -4.45 | 0.0001 | -3.92 | -1.47 | |
| OHI-S | 0.97 | 0.38 | 0.49 | 2.56 | 0.01 | 0.20 | 1.74 | |
| PPD | 1.42 | 0.44 | 0.72 | 3.27 | 0.002 | 0.54 | 2.31 | |
| CAL | -0.58 | 0.40 | -0.28 | -1.43 | 0.16 | -1.40 | 0.24 | |
Discussion
Chronic periodontitis is a progressive inflammatory disease with a multifactorial etiology, higher recurrence rates and ineffective treatment protocols 8,13. IL-6, a pleiotropic cytokine has diverse roles in acute phase response and chronic inflammation involved in this disease 10,11.
ώ 3 PUFAs reduce the rate of synthesis of pro‑inflammatory enzymes and cytokines, thereby reducing periodontal inflammation and loss of bone14. Hence recently, an increased interest in using ώ 3 PUFA as an additional therapy for longstanding periodontitis has been observed5.
Our study focused on the regulatory effects of ώ 3 PUFA on the intermediary molecules and cytokine induced inflammation (IL-6) and improved healing process in chronic periodontitis.
In this study we observed that the age and gender distribution of our group of patients was similar to that mentioned in the previous trials.
Estimation of serum IL-6 levels
In our study, this measurement was essential to quantify the effect of ώ 3 PUFA supplementation therapy on the inflammatory cytokines released which are in turn responsible for causing chronic periodontal inflammation.
In a study by Goutoudi et al the inflammatory cytokine levels were estimated in gingival crevicular fluid (GCF). IL-6 and IL-8 were significantly higher, in non-diseased versus diseased sites, while following periodontal treatment, they increased significantly. This could be due to reduction of GCF volume following therapy. It has been suggested that in GCF, compared to the concentration, the total cytokine amount might be more representative of the disease status. According to Chapple et al, GCF volumes do not correlate to the inflammatory status8.
Hence, we decided that estimating IL-6 levels in serum would be the most appropriate method
to substantiate the host modulating effect of ώ 3 PUFA.
We identified that IL-6 levels which were at a maximum mean of 10.2 pg/ml prior to treatment, showed a gradual & notable reduction to 2.3 pg/ml after ώ 3 PUFA supplementation therapy and they were statistically significant.
This was similar to that observed in studies conducted by Kujur et al and Stando et al to assess the effect of ώ 3 PUFA therapy on inflammatory cytokine levels.
These results indicated that the balance between the activities of pro-inflammatory and anti-inflammatory cytokines significantly altered the duration and intensity of periodontal inflammation.
While evaluating the clinical outcome in our study, we observed that all the 3 periodontal parameters (OHI-S, PPD, CAL) improved significantly (p= 0.0001) after 2 weeks of ώ 3 PUFA supplementation therapy.
Our results were similar to findings in the study by Deore et al. who demonstrated that 300 mg ώ 3 PUFA dietary supplementation daily for 3 months along with periodontal treatment had significant reversal of progression and recovery on clinical parameters. The findings of our study were on par with that of Keskiner et al who had supplemented low dose ώ 3 PUFA for 6 months. Similar results were also seen in randomized controlled trials by Salman, Umrania, Kujur & Stando et al wherein higher doses of ώ 3 PUFA was utilised.
This is in contrast to the study done by Martinez who treated 15 patients having chronic periodontitis with non-surgical dental therapy and providing them 900 mg per day ώ 3 PUFA in divided doses for twelve months. They observed a decrease in AA to EPA ratio but no significant improvement in clinical outcomes. Similarly, Rosenstein and Campan et al did not detect positive effects in clinical outcome.
Our study results correlated with that of Elsharkawy et al, Elkouhli et al & Elwakeel and Hazaa who had used a combination of low-dose aspirin along with ώ 3 PUFA. Synergistic interaction of both molecules was observed which resulted in the progression of inflammation and improvement in clinical parameters. Low-dose aspirin as an adjunct to non-surgical treatment is a controversial subject. Long-term intake of aspirin is usually recommended as a primary and secondary prevention measure of cardiovascular events, where the possible adverse effects are less dangerous than the progression of disease. Thus, adjunctive therapy with low‑dose aspirin for chronic periodontal disease is a topic of dispute5.
At the end of our study, we detected improved healing which was clinically quantified by us as improvement in CAL and higher rates of closed periodontal pockets. This enabled us to emphasize the adjunctive role of ώ 3 PUFA in the effective treatment of chronic periodontitis.
We observed in our study that ώ 3 PUFA adjunctive therapy not only causes noteworthy improvement of the most relevant clinical parameters (OHI-S, PPD, CAL) but also significantly reduces the inflammatory cytokine (IL-6) levels. OHI-S, PPD, CAL and IL-6 are sensitive indicators of resolution of inflammation and tissue healing and are biomarkers which reflect a healthy and stable dentition in patients with periodontitis.
Strengths of our study
Dose
Different doses of ώ 3 PUFA supplementation have been used in other clinical trials. Martinez et al have used 3 capsules of 300 mg/day for one year, each capsule of which contained EPA 180 mg and DHA 120 mg. Using the same composition, Deore et al. had used one capsule/day for 3 months. Elkhouli combined three capsules of 1 g of ώ 3 PUFAs containing 300 mg of DHA and 150 mg of EPA with 75 mg of aspirin for 6 months. Sharkawy et al. used 3 g fish oil and 81 mg aspirin daily (Each capsule contained 900 mg of fish oil (EPA/DHA 30%) and 100 mg wheat‑germ oil ) for 6 months. The American Heart Association has approved a dose of 0.5–1.8 g/day of EPA + DHA to be regarded as safe in healthy people16.
Considering all these factors, a dosage of 600 mg/day was used in this study which was well within the range of therapeutic safety.
Serum IL-6 levels
Many studies in the past have assessed only the clinical improvement to ώ 3 PUFA therapy. But in our study we had further validated the response to treatment by monitoring the serum levels of inflammatory biomarkers (IL-6).
No adverse events were recorded during the course of treatment with ώ 3 PUFA.
Limitations
This open label, exploratory study was done based on the follow up three months’ time period. Instead of using a control group, the four time intervals were used in comparison with the baseline measurements. Sampling method was also used based on consecutive entry of the patients in the dental OPD. However, the care of bias issues was taken using appropriate statistical methods in data analysis.
The daily dietary intake of fatty acids and their body mass index of our patients may also have affected our results. These parameters were not taken into consideration in this study. Future research to validate the usage of dietary supplementation with ώ 3 PUFA as an adjunctive therapy option in preventing the progression and treatment of chronic periodontitis requires a larger sample size and long-term observation.
Conclusion
The emerging awareness of inflammation and its predominant role in the management of periodontal disease underscores the importance of exploring inflammatory pathways and mediators. This aspect of research helps to establish a new era in drug discovery and therapeutics for periodontal disease treatment. Hence therapies targeting the host response are one of the new promising treatment modalities in the management of periodontitis.
The results obtained in this study revealed that ώ 3 PUFA has favorable effects on preventing the progression of inflammation and tissue regeneration in periodontitis. Thus we can recommend dietary supplement of ώ 3 PUFA as a safe adjunctive treatment modality for periodontitis. It is possible that treating periodontitis with ώ 3 PUFA could have the added benefit of preventing other chronic diseases associated with inflammation, including ischemic cerebrovascular disease, given the evidence indicating a role for ώ 3 PUFA in other chronic inflammatory conditions. To support these findings, further multicentric clinical trials addressing issues such as dietary intake of fatty acids, BMI, safety, risk‑benefit analysis, and dosage are required.
Acknowledgement
The authors thankfully acknowledge the Department of Dentistry, Department of Biochemistry and ESIC Hospital & PGIMSR, Tamilnadu, India for their unconditional support.
Conflict of interest
The authors declare that they do not have any conflict of interest in the preparation and publication of this manuscript.
Financial support
The authors did not receive any fund for the preparation and publication of this manuscript.
References
- Paschalina G, Evdoxia D and Malamatenia A. Effect of periodontal therapy on crevicular fluid Interleukin-6 and Interleukin-8 Levels in Chronic Periodontitis. J. Dentistry., 2012; Article ID 362905:1-8.
CrossRef - Suzan A.S, Hadeel M. A and Omar H.A. Omega-3 as an adjunctive to non surgical treatment of chronic periodontitis patients. IOSR J. Dental and Medical Sciences., 2014;13,6: 8-11.
CrossRef - Abdul kareem H. A, Mustafa G. T, Hisham A. G and Afnan A. H. Estimation of the level of salivary Interleukin 6 (IL-6) and its’ correlation with the clinical parameters in patients with periodontal diseases. IOSR J. Dental and Medical Sci., 2015; 14, 9: 82-88.
- Syed W. P, Marei Hamad E.M, Omer Bashir A.T, Abdulgader G, Fatma M. A, Naveen Kumar P. G. Therapeutic role of dietary Omega‑3 fatty acids in Periodontal disease. Res. J. Dentistry., 2014; 4 (2): 82-86.
CrossRef - Mirella S, Pawel P, Magdalena N, Przemysław L and Natalia L. Omega-3 polyunsaturated fatty acids EPA and DHA as an adjunct to nonsurgical treatment of Periodontitis: A Randomized Clinical Trial. , 2020; 12:1-14.
CrossRef - Deore G.D, Gurav A.N, Patil R. Shete A.R, NaikTari R.S, Inamdar S.P. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double-blind, placebo-controlled, clinical trial. J. Periodontal Implant Sci., 2014; 44: 25–32.
CrossRef - Anne B. K, Carolyn D. K, Kirstin V, Petra R. K and Johan P. W. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids in Health and Dis., 2020;19:100.
CrossRef - Abdul kareem H. A. Determination of Interleukin-1β (IL-1 β) and Interleukin-6 (IL-6) in gingival crevicular fluid in patients with Chronic Periodontitis. IOSR J. Dental and Medical Sci., 2015; 14 (11): 81-90.
- Noor Mohammed H. L, Yamama A. A. R, Ali H. F, Dina A. A. Salivary IL-6 and TNF- α in patients with periodontitis. Annals of Tropical Medicine & Public Health., 2021; 317-325.
- Cem Gabay. Interleukin-6 and chronic inflammation. Arthritis Res. & Therapy., 2006; 8(Suppl 2):
CrossRef - Toshio T, Masashi N and Tadamitsu K. IL-6 in inflammation, immunity and disease. Cold Spring Harbor Perspect Biol., 2014; 6: a016295.
CrossRef - Muhammad S. S, Farzeen T, Pakiza R. H and Muhammad H. B. S. Host modulation therapeutics in Periodontics: Role as an adjunctive Periodontal therapy. College of Physicians and Surgeons Pakistan., 2014; Vol. 24 (9): 676-684.
- Shirin Z. F, Shahram A, Atefe M, Mehrdad B, Morvarid M. Adjunctive Low-Dose Aspirin plus Omega-3 fatty acid versus Low-Dose Doxycycline on Chronic Periodontitis. Journal of Islamic Dental Association of IRAN (JIDAI) Autumn., 2014; 26 (4): 230-236.
- Shirish K. K, Varsha G, Anand M. N, Gangesh S, Shweta B, Himanta G. Efficacy of Omega 3 fatty acid as an adjunct in the management of Chronic Periodontitis: A Randomized Controlled Trial. Indian J Dent Res., 2020; 31: 229-35.
CrossRef - Raju A, Dr. Ameet M, Dr. Marawar P.P. Host Modulatory Therapy: A novel approach in Periodontal therapy. IOSR Journal of Pharmacy., 2013; 3(4): 9-13.
CrossRef - Umrania VV, Deepika PC, Kulkarni M. Evaluation of dietary supplementation of omega-3 polyunsaturated fatty acids as an adjunct to scaling and root planning on salivary interleukin-1β levels in patients with chronic periodontitis: A clinico-immunological study. J Indian Soc Periodontol., 2017;21: 386-90.
- Nilima S. K, Rahul P, Abhijit N. G, Yojana P, Abhijeet S, Ritam N. T et al. Oral hygiene status, Periodontal status, and Periodontal treatment needs among institutionalized intellectually disabled subjects in Kolhapur district, Maharashtra, India. Oral Dis., 2014, Article ID 535316, 11 pages.
CrossRef - Cátia R, Maria C M, José Júlio P, Filomena S, Elsa M. C. Full-mouth periodontal examination prior to and after nonsurgical treatment in chronic periodontitis patients. Biomedical Research., 2016; 27 (2): 406-412.








