Niladry Sekhar Ghosh1* , Ekta Pandey2, Madan Kaushik1
, Ekta Pandey2, Madan Kaushik1 , Jai Prakash Kadian1
, Jai Prakash Kadian1 , Bhupendra Chauhan1
, Bhupendra Chauhan1 , Ajeet yadav3 and Ranjit Singh1
, Ajeet yadav3 and Ranjit Singh1
1Shobhit University Gangoh, Saharanpur, UttarPradesh-247341, India.
2Bundelkhand Institute of Engineering and Technology Jhansi, UP- 284128.
3Department of Pharmaceutical Sciences. J.S University, UP.
Corresponding Author E-mail: niladry_chem@yahoo.co.in
DOI : https://dx.doi.org/10.13005/bpj/2301
Abstract
Nanoparticles can be synthesised using a variety of methods. These approaches, on the other hand, are connected with the development of undesired byproducts that are both harmful and expensive. As a result, several attempts are being undertaken to develop unique, cost-effective, safe, and dependable "green" techniques for producing desirable nanoparticles. To develop a novel, environment-friendly, economic, safe approach to the synthesis of gold nanoparticles via the biological entity. Addition of aqueous gold chloride solution to the microwave-exposed aqueous extracellular Cassia tora leaf extract yielded poly shaped gold nanoparticles. The UV-vis. spectroscopic investigations are led to notice and affirm the formation of nanoparticles. FTIR studies are performed to affirm the role of a biomolecule in stabilizing the nanoparticles. X-beam diffraction study is utilized to affirm the crystalline nature of nanoparticles. The elemental characterization of the samples is regulated by EDX studies. The size and morphology of the synthesized nanoparticles are explored using HR-TEM analysis and FESEM. It is seen that the flavonoids which are separated during microwave warming of extracellular solution of the cassia tora leaves are liable for the biosynthesis of gold nanoparticles. The nanoparticle was noted to be well dispersed and polyshaped with a 20-60 nm range. The leaf extract based preparation of AuNP is more gainful since leaf is used instead of microorganism as many of the issues like pathogenicity, procedural maintenance of hygiene of cell culture and labor efforts can be overcome. The presence of flavonoids in the leaf was discovered by the examination of produced nanoparticles, suggesting that they may have fulfilled both reduction and stabilisation activities. The presented approach can be inferred to be cost-effective, environmentally friendly, and capable of manufacturing nanoparticles with desired physical and pharmacological properties.
Keywords
Cassia tora leaf Extract; Environment Friendly; Gold Nanoparticles; Poly Shaped
Download this article as:| Copy the following to cite this article: Ghosh N. S, Pandey E, Kaushik M, Kadian J. P, Chauhan B, Yadav A, Singh R. Green Synthesis of Gold Nanoparticles: A Novel, Environment- Friendly, Economic, Safe Approach. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Ghosh N. S, Pandey E, Kaushik M, Kadian J. P, Chauhan B, Yadav A, Singh R. Green Synthesis of Gold Nanoparticles: A Novel, Environment- Friendly, Economic, Safe Approach. Biomed Pharmacol J 2021;14(4).Available from: https://bit.ly/3oVTP2F |
Introduction
Nano sized gold nanoparticles are extensively used in the fields of nanobiotechnology 1. The exceptional properties offered by them carry much potential for their widespread biomedical applications 2-4.These particles with different dimensions are used for the detection of biological molecules 5. Biological molecule & cancer cells can be easily adsorbed on the surface of gold nanoparticles and the surface plasmon resonance of bio capped AuNP causing them to have better photon capture cross- sections than those of photothermal dye 6 Colloidal solutions of AuNP are being used in a homogeneous form for DNA sequences detection and signal based mutations 7.The synthesis of the gold nanoparticles through the green process is a highly brought after area to develop systems for detection and destruction of the cancer cells in the early stage itself. The size and shape of the nanoparticles play a significant part in choosing the level of sensitive detection of nanoparticles. These nanoparticles can be synthesized with varying properties using a variety of methods via physical, chemical & biological. The advantages of biological methods include their environment, less time consumption, production of negligible waste & toxic substances and safety 8. The other methods are comparatively unsafe, expensive & hazardous. Thus there is more emphasis on green methods of synthesis which use a natural resources such as plants or microbes. To prepare metal nanoparticles with stability & controlled size and shape, there has been looking for a modest, safe, rapid and “green” approach. Plants give a superior stage to nanoparticles synthesis as they are nontoxic as well as contain natural reducing agents 9. Among various plants, we have chosen cassia tora leaves extract for the present study since it has several pharmacological effects such as anti-Cancer, anti-diabetic, anti-hyperlipidemia, antioxidant, and hypotensive activities 10-11. The plant-based synthesis of AuNP is more advantageous since parts of the plants are utilized rather than organisms as a significant number of the issues like pathogenicity, procedural support of cleanliness of cell culture and labor efforts can be overcome 12-13. This procedure is more suitable for industrial scaling as well. The main goal of this research was to come up with a simple, time-saving, cost-effective, and environmentally friendly green approach for synthesizing metal nanoparticles. As a result, efforts were made to investigate biological pathways for nanoparticle synthesis while keeping a close eye on composition and dimensions. Based on a thorough understanding of bioactive nanomaterials, efforts were made to develop novel green-technology-based nanoparticle synthesis methods. The nanoparticles were made in an aqueous solution by reducing metal ions with a natural catalyst.
Materials and methods
Chemical and Reagents
Fresh leaves of Cassia tora was collected from the Botanical garden Dr. YS Parmar University of Horticulture and Forestry, Solan ,India. All the chemicals and reagents utilized in this investigation were of analytical grade. Gold nanoparticles were obtained from HI media Chemicals.
Biosynthesis of Gold nanoparticles using Cassia tora leaf extract
Gold chloride solutions of a 10-3 M concentration analytical grade were prepared. Freshly collected & cut leaves of Cassia tora were taken in a flask and washed properly using deionized water. Then 200 mL of double distilled water was put into the flask containing the leaves and the flask was heated in a microwave for 3 minutes to inactivate the enzymes and proteins which may interfere in the process. The solution was then filtered in hot conditions to remove the solid fibrous residues. The clear filtrate was the extracellular extract of the leaf is used for the nanoparticles synthesis. 50 ml of gold chloride solution was made to interact with the10 ml extracellular extract of the leaf at 27°C in a rotary shaker at 120 rpm. Immediately after the addition of plant extract to gold chloride aqueous solution, a light golden color appeared which changed to dark purple color within 5 min. The color change indicated that the formation of gold nanoparticles has taken place.
Results
UV-Visible Spectra
The progression of the reaction of the mixture of leaf extract and gold chloride solution in water as a function of time is given in fig 1. A change in color of the reaction mixture from light golden to dark purple within few minutes indicates the formation of gold nanoparticles, which was further confirmed by UV-Vis spectroscopy. Further darkening of color to dark purple indicates the enhanced growth of gold nanoparticles. It is well supported by the fact that gold NPs exhibit dark purple color in water due to SPR. The spectrum of synthesized AuNPs was noted to have a maximum absorption band in the wavelength range of 550-575 nm, due to the SPR of AuNPs.
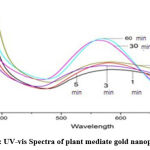 |
Figure 1: UV-vis Spectra of plant mediate gold nanoparticles |
X-ray powder diffraction (XRD) & Energy Dispersive X-Ray (EDX) Analysis
XRD studies carried out to assess crystalinity of the synthesized Gold nanoparticles and results are depicted in figure 2 a).Gold nanoparticles displayed four particular peaks at 2θ= 38.1, 44.3, 64.5, and 77.7. This indicates the presence of pure gold.
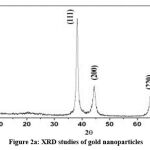 |
Figure 2a: XRD studies of gold nanoparticles |
The results of EDX analysis are shown in Fig2 b). The graph indicates presence of elemental Gold. This suggested the reduction of gold ions to elemental Gold. Carbon and Oxygen presence confirms that organic moiety is protecting on the surface of gold nanoparticle
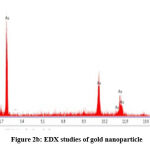 |
Figure 2b: EDX studies of gold nanoparticle |
Field Emission Scanning Electron Microscopy (FESEM)
FESEM images at different resolutions are shown in figure 3. A careful observation indicates the presence of bio moiety covering the metal surface of all nanoparticles (AuNP).The nanoparticle was noted to be well dispersed and poly shaped. Most of the particles are spherical in shape
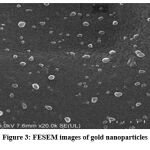 |
Figure 3: FESEM images of gold nanoparticles |
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR analysis of the nanoparticles is given in (Figure 4). The interpretation of IR spectra shows bands at 3433, 2921,2846, 1628, 1396, 1043 and 591cm-1 The peaks at 3433 cm-1 is cm−1 corresponds to O-H stretch vibration alcohol and The band at 2921 cm-1 showed O-H stretching in carboxylic acid group while adsorbed bond at 2846 cm-1can be assigned to C-H stretch vibration of aldehyde. The peak at 1628 cm-1 mention to amide group. The peak at 1396 cm-1 cm−1 refers to C-O stretch, which is due to stretching vibration of carboxylic acid group.
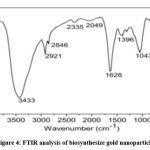 |
Figure 4: FTIR analysis of biosynthesize gold nanoparticles |
IR spectroscopic investigation affirmed that proteins along with carbohydrates bind the metal, and reduce gold ions to gold nanoparticles .These outcomes recommend that the biomolecule perform double action by formation of Gold nanoparticles and stabilized them in aqueous solution. We can assume that the proteins which are available in large quantities in cassia tora leaves show typical peaks which could be to be responsible for reduction and capping process The proteins present over the gold nanoparticle surface may played an important role in reduction of gold ions to Gold nanoparticles and stabilization of nanoparticles
High-resolution transmission electron microscopy (HRTEM) & Size distribution histogram
From Fig no 5(a) and (b) indicates that, it is observed that above 62 % of the particles are in 20-60 nm size range. The particles are generally spherical in shape with a few rod shapes, hexagonal and square. The rod shape particles are noted to be in the size range of 7-12 nm.
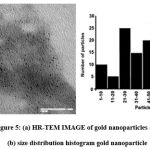 |
Figure 5: (a) HR-TEM IMAGE of gold nanoparticles and (b) size distribution histogram gold nanoparticle |
Discussion
A new, simple, cost-effective microwave-assisted rapid extracellular manufacturing technique of spherical gold nanoparticles was proposed and developed using plant extract. The flavonoids in Cassia tora leaf extract were found to cover the surface of gold nanoparticles. Chemical and physical processes, which typically produce harmful byproducts, have an advantage with this new revolutionary technology. Plant-mediated silver nanoparticle synthesis is industrially practicable, as opposed to microbe-mediated procedures, which require strict aseptic conditions.
Conclusion
The green synthesis method for the preparation of gold nanoparticles using plant leaf extract was found to be rapid, environment -friendly and cost effective. The nanoparticle was noted to be well dispersed and polyshaped with 20-60 nm range. The analysis of synthesized nanoparticles indicated presence of flavonoids in the leaf which possibly performed dual functions of formation and stabilization. It can be concluded that the developed method is cost effective, eco-friendly and capable of producing nanoparticles with desire physical & pharmacological properties.
Conflicts of Interests
There is no Conflict of Interest.
Funding Source
There is no Funding Source.
Authors Contributions
All authors have contributed equally in this work.
References
- Yakimovich NO, Ezhevskii AA, Guseinov DV, Smirnova LA, Gracheva TA, Klychkov KS. Antioxidant properties of gold nanoparticles studied by ESR spectroscopy. Russian Chemical Bulletin.2008; 57: 520–523. https://doi.org/10.1007/s11172-008-0080-1
CrossRef - El-Sayed M. Small is different: shape-, size-, and composition- dependent properties of some colloidal semiconductor nano- Acc Chem Res. 2004;37(5):326-333. DOI: 10.1021/ar020204f
CrossRef - Ali, S.W., Rajendran, S., Joshi, M.: Synthesis and characteri- zation of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym.2011; 83: 438–446. DOI: 1016/j.carbpol.2010.08.004
CrossRef - Huang, X., Qian, W., El-Sayed, I.H., El-Sayed, M.A.: The poten- tial use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg. Med. 2007;39: 747–753. DOI: 1002/lsm.20577
CrossRef - Chen, X.J., Sanche Gaytan, B.L., Qian, Z., Park, S.J.: Noble metal nanoparticles in DNA detection and delivery. Wiley Inter- discip. Rev. Nanomed. Nanobiotechnol.2012; 4: 273–290. doi: 10.1002/wnan.1159.
CrossRef - Zambre, A Silva, F, Upendran ,A Afrasiabi ,Z Xin ,Y Paulo A and Kannan, R. Synthesis and characterization of functional multicomponent nanosized gallium chelated gold crystals. Chem. Commun., 2014; 50; 3281-3284. https://doi.org/10.1039/C3CC47308B
CrossRef - Yeh, P., Perricaudet, M.I.: Advances in adenoviral vectors: from genetic engineering to their biology. FASEB J. 1997;1: 615–623 Raveendran, P., Fu, J., Wallen, S.L.: Completely “green” synthe- sis and stabilization of metal nanoparticles. J. Am. Chem. Soc.2003; 125: 13940–13941. doi/10.1021/ja029267j
CrossRef - Gurunathan S., Park J.H., Han J.W., Kim J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed.; 2015, 10:4203–4222. DOI: 2147/IJN.S83953
CrossRef - Zhao, X., Wang, Qian, Y.,Pang ,L,. Cassia tora L. has anticancer activity in TCA8113 cells in-vitro and exerts anti-metastatic effects. Oncology Letter.2012; 5: 1036-1042. https://doi.org/10.3892/ol.2012.1097
CrossRef - Rejiya, C.S.,Cibin, T.R., Annie Abraham Leaves of Cassia tora as a novel cancer therapeutic – An in vitro study. Toxicology in Vitro .2009; 23: 1034-1038. https://doi.org/10.1016/j.tiv.2009.06.010
CrossRef - 11 .Shankar, S.S., Rai, A., Ahmad, A., Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004;275: 496–502. DOI: 1016/j.jcis.2004.03.003
CrossRef - Dhuper, S., Panda, D., Nayak, P.L. Green synthesis and charac- terization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends J. Nanotech. App.2012; 13:16–22.
- Dwivedi, A.D,.Gopal, K,. Biosynthesis of Silver and Gold Nanoparticles Using Chenopodium album Leaf Extract. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010; 369: 27-33. https://doi.org/10.1016/j.colsurfa.2010.07.020
CrossRef







