Lakshmi Mishra and Swati Gupta*
and Swati Gupta*
Amrita School of Pharmacy, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, AIMS-Ponekkara PO, Kochi, Kerala - 682041, India
Corresponding Author E-mail: swatigupta@aims.amrita.edu
DOI : https://dx.doi.org/10.13005/bpj/2305
Abstract
Purpose: Topical nanoemulsion comprising of fluconazole and curcumin was developed to target multiple drug resistance dermatophytes infection and to facilitate cutaneous delivery of these poorly water soluble drugs. Methods: Almond oil, sesame oil and paraffin light were used to formulate nanoemulsions and screened for the stability. The solubility of fluconazole and curcumin in surfactants, co-surfactants and oils was screened to decide the various components of the nanoemulsion. The oil phase was light paraffin whereas tween 80 and span 80 were the surfactants and ethanol was used as a co-surfactant. To identify the area of nanoemulsion existence, a pseudoternary diagram was drawn and optimum systems were developed. Drug-loading efficiency was assessed and the developed nanoemulsions were characterized for globule size, stability, robustness to dilution and pH. The optimized nanoemulsion was further evaluated for drug content, viscosity, skin permeation study (ex vivo) and assay of antifungal activity. Results: The globule size was below 200 nm and uniform for the optimized nanoemulsion formulation. It showed enhanced skin permeation (ex vivo) and better antifungal efficacy as compared to the native form of fluconazole and curcumin suspensions. Antimicrobial assay confirmed the synergistic effect of fluconazole and curcumin combination against multiple drug resistance Trychophytum rubrum and Trichophyton metagrophytes as compared to the fluconazole alone. Conclusion: The results clearly indicate an optimized delivery of fluconazole and curcumin in a synergistic way from the nanoemulsion formulation. This resulted in better penetration of these poorly soluble molecules and overall enhanced antifungal activity as compared to these drugs as such against multiple drug resistance dermatophytes.
Keywords
Antifungal; Curcumin; Fluconazole; Multiple Drug Resistance Dermatophytes; Nanoemulsion
Download this article as:| Copy the following to cite this article: Mishra L, Gupta S. Fluconazole and Curcumin Loaded Nanoemulsion Against Multiple Drug Resistance Dermatophytes. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Mishra L, Gupta S. Fluconazole and Curcumin Loaded Nanoemulsion Against Multiple Drug Resistance Dermatophytes. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3yWSwUx |
Introduction
Dermatophytes cover three genera of pathogenic fungi namely, Epidermophyton, Microsporum, and Trichophyton, which illustrate a significant affinity for the keratinized structures present in skin, nails and hair, causing infections (superficial) identified to be dermatophytosis1-3. Keratin is metabolized by these organisms which causes a number of pathologic clinical presentations such as tinea corporis, tinea pedis, tinea cruris etc4,5. Trichophyton rubrum and Trichophyton mentagrophytes complex are a few common fungi responsible for dermatophytosis, which is endemic to India6,7. In spite of a plenty of antifungal drugs currently exist in the commercial pharmacies, the propagation of antifungal drug resistance and invasive type fungal infection, particularly caused by dermatophytes, has significantly increased worldwide and covers around 20‐25% of the world’s population8,9. Some identified reasons for resistant are misuse of fixed drug combination (FDC) creams containing very potent drugs like steroids, microbiological resistant of the causative fungi and a transformed immune response of host etc10.
The mechanism towards antifungal resistant consists of filamentation, formation of bio-film, genomic alterations and over expression of drug efflux pumps (which includes major facilitate or superfamily of few transporters and ATP-binding cassette transporters) 11. The dermatophyte showed reduced susceptibility to azole after a prolong use6,12,13. Drug-resistant can be minimized by advanced and better diagnostics for detection of infection at early stage, prophylaxis, multi drug therapy, pre-emptive treatment and identification of new antifungals14-17.
Fluconazole (FLZ) is a triazole antifungal drug of third generation with broad spectrum activity against superficial and systemic fungal infections. It acts by inhibiting the production of vital elements in the membrane of fungi as ergosterol by limiting the fungal cytochrome P450 enzyme. After prolong exposure of fluconazole, major facilitator superfamily (MFS) of proteins are over expressed in dermetophytes resultant enhanced resistant18-22. The efflux pump inhibitors can be used to sensitize the resistant type of C. albicans strains to azoles, which can lower the dose of required antifungal for desired pharmacological benefits and potentially limiting side-effects22.
The solubility of FLZ in water is around 5 mg/mL at 37°C (slightly soluble), with 306.3 Da molecular weight and has a pKa value of weak base 3.723. It is presented as parenteral and oral dosage forms and may exhibit serious side effects as diarrhoea, vomiting, stomach upset, feeling sick, rashes, and reduction in the count of red blood cells. Incidence of hepatotoxicity in patients receiving triazoles has also been reported. Therefore, topical application of FLZ is highly indicated to prevent such adverse effects25,26. Due to the large molecular size of FLZ and hydrophobicity, the development of topical dosage form is challenging. Recently, nano-based formulation strategies are valuable to reduce the adverse effects of FLZ and increase the efficiency27.
The concomitant use of natural chemical compounds co-administered with the conventional chemotherapeutic agents has been recommended as a valuable strategy to overcome multi drug resistance (MDR) in fungi and thus, restoring the susceptibility to anti-fungals28. One natural polyphenol Curcumin, which is found in the roots and rhizomes of Curcuma longa. A recent study has reported that it could be used as an important modulator of MDR in Saccharomyces cerevisiae strain carrying mutations that has an MDR phenotype29. Curcumin is reported to enhances the activity of fluconazole in many study30,31. Curcumin is poorly water soluble, meaning that it is restricted to the superficial stratum corneum (SC) after topical delivery32,33. Nanoemulsion system improved curcumin permeability34. Nanoemulsions are kinetically stable and isotropic emulsions in which the drug containing oil droplets are stabilized by a very thin layer of emulsifier35. Dissolution rates and bioavailability of poorly water soluble drugs can be improved in the form of nanoemulsions with added advantage of reduced side effects. The high drug loading capacity, enhanced drug solubility, better thermodynamic stability and permeation enhancement through skin without skin irritation makes the nanoemulsion as one of the suitable system for topical delivery36,37.
In this study, we proposed that curcumin can sensitize fluconazole resistant strains of dermatophytes (T. rubrium and T. mentagrophytes) and potentiate the effect of fluconazole against resistant strains. Further development of this combination into a nanoemulsion formulation may facilitate deep penetration of these poorly soluble drugs into the skin layers.
Materials and Methods
Materials
Fluconazole was gifted from Virupakasha organics Ltd, Hyderabad and curcumin (95% pure) from Elixir extract Pvt Ltd, Cochin. Tween 80 and Span 80 were purchased from Nice chemicals Pvt Ltd, Cochin. Ethanol and paraffin light were purchased from Spectrum reagents and chemicals Pvt Ltd, Kochi. Remaining chemicals used were of analytical grade.
Selection of various components of the nanoemulsion
To select the components of nanoemulsion, the solubility of fluconazole and curcumin was determined in various oils and surfactants, such as light paraffin, almond oil and sesame oil and Span 80, Tween 80, ethanol. Slightly excess quantity of fluconazole and curcumin (separately) was mixed to 10 mL of each oils and surfactants. The tubes were shaken for 24 h at 20°C. The supernatant was collected, filtered and assayed spectrophotometrically at 260 and 421 nm respectively for fluconazole and curcumin.
Creation of pseudoternary phase diagram
The pseudo tertiary diagram was constructed using Chemix school software. Paraffin was chosen as oil phase, ethanol and Tween 80 were chosen as co-surfactant and surfactant, respectively. The aqueous phase was distilled water. The surfactant and co-surfactant were used at various mass ratios. These ratios were selected in ascending concentration of co-surfactant with respect to surfactant and an exhaustive study of phase diagram was carried out. Aqueous filtration method was used in order to develop the ternary phase diagram for oil, Surfactant and aqueous phase. To oil and surfactant mixture, aqueous phase is added slowly and titrated. Visual observations were made for emulsions. The physical state of water, oil and a blend of surfactant and co-surfactant at particular set mass ratio were marked on the pseudo ternary phase diagram.
Microbiological assay of Fluconazole and curcumin
In vitro antifungal studies were performed against multiple drug resistance Tricophyton Rubrum and Tricophyton Metagrophytes. These dermatophytes were developed at 37°C and maintained on Sabouraud dextrose agar (SDA) slants at a temperature of 4°C. Dermatophytes were preserved at −20°C in 20% glycerol. The fluconazole’s breakpoint value for dermatophytes resistant was calculated in mg/l. The combinatorial effect of curcumin and fluconazole was evaluated by checkerboard experiments using PRI (protein rich isolate). In brief, BHI (brain heart infusion) medium was used to grow the cells overnight at 37°C with constant shaking. After the incubation period, the cells were harvested, washed in PBS and then suspended at a concentration of 4 × 103 cells/ml. This cell suspension (100 μl aliquot in each well) was placed in a 96-well microtitre plate containing sabouraud without the glutamine medium in the presence and absence various concentrations of curcumin and fluconazole, in combination or alone. The concentration of Fluconazole was 0.5–128 mg/l within the vertical cells whereas curcumin was used at 2–32 mg/l in horizontal lines wells. Curcumin and fluconazole’s interaction was determined by calculating the FICI (fractional inhibitory concentration index). The FICI value ≤ 0.5 represents synergistic interactions whereas the FICI value >4.0 represents antagonistic effect and values in between 0.5 and 4.0 represent no interaction.
Where
FICI= Fractional inhibitory concentration index useful
FIC= Fractional inhibitory concentration
MIC= Minimum inhibitory concentration
Preparation of O/W nanoemulsion
Different nanoemulsion (NE) formulations were developed by ultrasonication as suggested by Paniwnyk et al., 2016 with some modifications using different concentration of selected oils, surfactants, co-surfactants and distilled water. Tween 80/SDS was dissolved in water in a beaker (solution A) and ethanol, drug and Span 80 were mixed together in another beaker then added to selected oil phase (solution B). Then aqueous phase (solution A) was kept under magnetic stirrer and oil phase (solution B) was added drop by drop and stirred till 30 min until emulsion was formed. Then the emulsion was sonicated using probe sonicator for 20 min continuously at 24 kHz at power of 400 W. Eleven Different formulations were developed (Table 1).
Table 1: Composition of various nanoemulsion formulations
| Material used | Formulations | ||||||||||
| BNE1 | BNE2 | BNE3 | BNE4 | BNE5 | BNE6 | BNE7 | BNE8 | BNE9 | BNE10 | BNE 11 | |
| Paraffin light (ml) | 16 | 16 | 16 | 14 | 3 | 12 | 12 | 12 | – | – | – |
| Almond oil (ml) | – | – | – | – | – | – | – | – | 16 | 21 | – |
| Sesame oil (ml) | – | – | – | – | – | – | – | – | – | – | 16 |
| Span 80 (ml) | 0.5 | – | 1 | – | – | – | 4 | – | – | – | 1 |
| Tween 80 (ml) | 0.5 | 0.5 | 1 | 0.8 | 7 | 8 | – | 4 | 2 | 8 | 1 |
| SDS (g) | – | 0.5 | 0.5 | 0.5 | – | – | – | – | 0.5 | – | – |
| Ethanol (ml) | 3 | – | – | – | 2 | 2 | 2 | 2 | – | 3 | 3 |
| Distilled Water (ml) | 80 | 80 | 80 | 86 | 30 | 80 | 84 | 84 | 80 | 80 | 80 |
Characterization of nanoemulsion systems
Visual appearance
The final formulation visually inspected for clarity, colour and transparency. The formulation evaluated for the presence of any gritty particles.
Homogeneity
Homogeneity was tested by visual inspection. Small quantity of formulation was pressed between thumb and the index finger to find out the homogeneity of formulation.
Measurement of pH
A digital pH meter was used to determine the pH of the formulations which were previously standardized by adjusting the pH in three different buffers (pH 4, 4.5 and 5). After standardization the probe of pH meter dipped in the formulation and the pH was noted38.
Grittiness
Smears of formulation were prepared in glass slide and observed under optical microscope for the presence of any gritty particles.
Stability studies
The nanoemulsions were centrifuged at 4000 g for 30 min to determine the dispersion stability tests. The phase separation was evaluated and the nanoemulsions which didn’t show any phase separations were further evaluated by freeze–thaw cycle. Only the surviving formulations in the stability test were selected for further studies39.
Determination of droplet size and zeta potential
Dynamic Light Scattering method at 90º angle was used to determine the polydispersity index and average size and of the nanoemulsion (NICOMP TM 380 ZLS, USA Particles Sizing Systems, Santa Barbara, CA). 100 mg nanoemulsion was diluted with methanol to 10 ml shaking. The instrumental settings were set at viscosity 0.01 poise, temperature 20°C and refractive index 1.333. Along with size, zeta potential of nanoemulsion formulation was also recorded40-42.
Measurement of Viscosity
Viscosity assessment is one of the important physicochemical parameter in the characterization of nanoemulsions. It affirms the type of nanoemulsion system (O/W or W/O). Generally, O/W type systems show low viscosity whereas W/O systems show high viscosity. Viscosity of formulation was determined by rheometer at the temperature 25±2°C43.
Fluconazole and curcumin loaded in optimized nanoemulsion
Optimized formulation was selected drug loading. Fluconazole and curcumin was dissolved in light paraffin by slow addition of surfactants and co-surfactants and distil water using magnetic stirrer for 30 min followed by probe sonication for 20 in continuation min 24 kHz at power of 400 W44. (Table 2)
Table 2: Composition of optimized nanoemulsion
|
Ingredients |
Quantity (% w/w) |
| Fluconazole | 5 |
| Curcumin | 5 |
| Paraffin light | 10 |
| Span 80 | 2 |
| Tween 80 | 2 |
| Ethanol |
1 |
| Water | 75 |
Estimation of drug content
Nanoemulsion formulation (5 mg fluconazole and curcumin equivalent) was stirred with 15 ml methanol in a volumetric flask for 30 min. More quantity of methanol was added subsequently to make up the volume to 50 ml. 2 mL of this solution was then diluted with 5 ml of 0.1 N HCl and 3 ml of methanol to achieve a concentration of 20 µg/ml. 0.45 µm membrane filter was used to filter the solution before measuring the absorbance spectrophotometrically at 260 and 421 nm respectively (UV-1800 UV–Vis Spectrophotometer, Shimadzu Corporation, Japan).
Skin permeation study (ex vivo)
Porcine ear skin was collected from a local slaughter house. An electric shaver was used to remove the hairs and a scalpel along with surgical scissors was used to remove the adherent subcutaneous fatty layer. The skin surface was thoroughly cleaned with freshly prepared Ringer’s solution and kept it for drying. The skin piece of suitable size was mounted on Franz diffusion cell. Phosphate buffer pH 5.5 was filled in the receptor compartment. The donor compartment was the epidermal side of the skin whereas the dermal side was facing the receptor solution. The temperature of diffusion cells was maintained as 37°C using an external constant water circulator. The receiver medium was continuously kept on stirring using magnetic bar.
Fluconazole and curcumin loaded nanoemulsion (the composition shown in Table 2), and Fluconazole and curcumin suspensions in distilled water were studied for skin permeation. The nanoemulsion formulation and the drug suspentions were applied on the skin at donor compartment. 3 ml of samples were withdrawn at a predetermined time intervals and same volume of fresh receptor medium was added each time. The samples were analyzed at 260 and 421nm respectively for fluconazole and curcumin using a spectrophotometer. The experiment was performed in triplicate and the steady-state flux (Jss, lg/cm 324 2/h) was calculated from the slope of the linear portion of the plots drawn for cumulative permeated amount of drug versus time in the steady state conditions. The flux was divided with initial concentration of the drug in the donor compartment to calculate the permeability coefficient (Kp, cm/h). The enhancement ratios were calculated by dividing the flux (Jss) of the nanoemulsion with the flux of the control drug suspensions38,45
Results and Discussion
Solubility studies
The solubility profile of fluconazole and curcumin in the selected oil and surfactants has been presented in Table 3. The solubility data along with physicochemical properties of fluconazole and curcumin indicated that it has promising potential for development as topical drug delivery system.
Table 3: Solubility profile of fluconazole and curcumin
| Solvents | Fluconazole | Curcumin |
| Paraffin light | ++++ | ++++ |
| Sesame oil | +++ | +++ |
| Almond oil | +++ | +++ |
| Tween 80 | ++ | ++ |
| Span 80 | ++ | ++ |
++++ = Very soluble; +++ = Freely soluble; ++ = Soluble
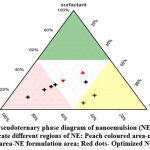
Pseudoternary phase diagram
The pseudoternary phase diagram with various weight ratios of water, surfactant, co-surfactant and oil has been presented as Figure 1. This phase study indicated that the maximum proportion of oil was incorporated in nanoemulsion systems when the ratio of surfactant to co-surfactant was equal (1:1) and formulation of nanoemulsion ratio for water, oil and surfactant was 6:4:6 and 6:5:4. The composition of optimized nanoemulsion system is shown in Figure 1.
Microbiological assay of Fluconazole and curcumin
Garcia-Gomes et al., (2012) has reported that curcumin exhibit beneficial effects against resistant type of isolated C. albicans when combined with fluconazole30. Sharma et al., 2020 has also depicted the role of curcumin in modulating efflux mediated by yeast ABC multidrug transporters and it was synergistic with antifungals29. In the same line, we studied the combined effect of curcumin with fluconazole against two strains of dermatophytes (T. rubrum and T. metagrophytes) and reporting it first time. The result of antimicrobial assay is presented in Table 4. The FICI value represents synergistic interactions of curcumin and fluconazole against dermatophytes.
Table 4: FICI for fluconazole and curcumin for dermetophytes
| Dermetophytes | FIC of fluconazole | FIC of curcumin | FICI |
| T. rubrum | 0.0031 ± 0.0016 | 0.0239 ± 0.0079 | 0.0270 ± 0.0095 |
| T. metagrophytes | 0.0053 ± 0.0075 | 0.0143 ± 0.0051 | 0.0196 ± 0.0126 |
Characterization of nanoemulsion
The stable nanoemulsion formulation was further characterized for polydispersity index, average droplet size and zeta potential. Polydispersity is determined by calculating the ratio of standard deviation to the average droplet size, which is directly proportional to the uniformity of the formulation droplet size. Average globule size of nanoemulsion was found as 149.23 nm with the lowest value of polydispersity index of 0.125. In the present study, low polydispersity index indicated the uniformity of nanoemulsion droplets size within the formulation.
The potential stability of any colloidal system depends on the magnitude of zeta potential. When the globules have a large positive or negative charge on that, the globules/droplets repels each other and hence achieve the dispersion stability. Scientifically, zeta potential value beyond +30 or -30 mV is considered optimum to keep the globules away from each other. The Zeta potential was -45 mV for the optimized formulation which confirms that formulation was stable. The pH of the formulation was in the range of 6.5–7.1 as depicted in the Table 5. This pH range was within the acceptable limits for products meant for skin application. The drug content of the nanoemulsion was in the range of 88.7–93.9 % and 86.5–91.8% for fluconazole and curcumin respectively for the optimized formulation. The viscosity of the formulation was found to be 11.32±1.3 mPa.s.
Table 5: Characterization of nanoemulsion formulation
| Parameters | Observations |
| Clarity | Optically clear |
| Colour | Slight off white |
| Transparency | Translucent |
| Grittiness | No gritty particle |
| Homogeneity | Homogenous in nature |
| pH | 6.5-7.1 |
| Viscosity | 11.32±1.3 mPa.s |
Ex vivo skin permeation study
The ex vivo skin permeation study of the optimized fluconazole and curcumin loaded nanoemulsion formulation and fluconazole and curcumin suspension was performed and permeation of developed nanoemulsion was found better than the respective suspensions of individual drugs. The flux, permeability coefficient and enhancement ratio are shown in the Table 6. The data represents mean value of three replicates. These results clearly indicate that the fluconazole and curcumin loaded nanoemulsion (BNE1) has shown maximum ex vivo drug permeation of the fluconazole and curcumin.
The presence of surfactant and co-surfactant in the nanoemulsion formulation might have worked as penetration enhancers and thus affected the structure of stratum corneum and reduced the diffusion barrier. As a thumb rule, nanoemulsions are expected to cross the stratum corneum in its intact form and exist in the whole horny layer without damage to the nanoemulsion system. The nanoemulsion formulation may alter both the lipid and the polar pathways simultaneously after entering into the stratum corneum layer. There are many ways in which interaction occurs between the stratum corneum and the lipophilic domain of the nanoemulsion formulation. The fluconazole and curcumin are present in dissolved form in the lipid domain of a nanoemulsion formulation which helps in increase permeability through lipid layer of stratum corneum. At the same time, the stratum corneum gets hydrated by the hydrophilic domain of the nanoemulsion formulation up to a certain extent.
The percutaneous uptake of poorly soluble drugs largely depends on the degree of hydration of the skin. The interlamellar volume of the lipid bilayer of stratum corneum increases once the aqueous fluid present in the nanoemulsion system enters the polar pathway. This increases the permeation of hydrophobic molecules like fluconazole and curcumin. The combined effects of both the hydrophilic and lipophilic domains of nanroemulsions attributed to the greater drug penetration enhancing activity.
In vitro antifungal activity study against multiple drug resistance Tricophyton Rubrum and Tricophyton Metagrophytes revealed that the FICI is higher for combination of fluconazole and curcumin as compared to fluconazole and curcumin alone. This may be due to the combination of fluconazole with curcumin is effective in MRD dermatophyte species. Curcumin has the potential to block “Major Facilitator Superfamily (MFS) transporters” system of fungal cell wall. MFS transporters system is responsible for active efflux of fluconazole from fungal cell. Blocking of MFS transporters system, helps to retain fluconazole in fungal cell and available for action. Nanoemulsion helps in enhancement of deep penetration of drug.
Table 6: Skin permeation parameters
| Formulations | Permeability coefficient (cm/h) | Flux (lg/cm2/h) | Enhancement ratio
|
| BNE1 | 4.72×10-5 | 23.49 | 1.7 |
| BNE6 | 4.39×10-5 | 22.37 | 1.4 |
| Fluconazole and curcumin suspension | 2.56×10-5 | 13.78 | – |
Conclusion
In the present study, fluconazole and curcumin were successfully incorporated into a nanoemulsion system under the optimized conditions and adequate use of surfactants, co-surfactants and oil phase which are topically acceptable. The results clearly indicate an optimized delivery of fluconazole and curcumin in a synergistic way from the nanoemulsion formulation. The improved skin penetration of these molecules from the nanoemulsion formulation as compared to the respective drug suspensions can be taken up further for design and development of nanoemulsion based delivery system of these lipophilic drugs for the enhanced antifungal activity against multiple drug resistant dermatophytes, in future.
Acknowledgment
The authors thank Virupakasha organics Ltd, Hyderabad for the generous gift of Fluconazole and Elixir extract Pvt Ltd, Cochin for Curcumin.
Conflict of Interest
The author has no conflict of interest in this research.
Funding Source
There is no funding source.
References
- Mercer DK, Stewart CS. Keratin hydrolysis by dermatophytes. Med Mycol 2019;57(1):13-22. doi: 10.1093/mmy/myx160
CrossRef - Nweze EI, Eke IE. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med Mycol 2018;56(1):13-28. doi: 10.1093/mmy/myx025
CrossRef - Martinez-Rossi NM, Bitencourt TA, Peres NTA, Lang EAS, Gomes EV, Quaresemin NR, et al. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front Microbiol 2018;9:1108. doi: 10.3389/fmicb.2018.01108
CrossRef - Monod M, Feuermann M, Salamin K, Fratti M, Makino M, Alshahni MM, et al. Trichophyton rubrum Azole Resistance Mediated by a New ABC Transporter, TruMDR3. Antimicrob Agents Chemother 2019;63(11):e00863-19. doi: 10.1128/AAC.00863-19
CrossRef - Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J 2016;7(2):77-86. doi:10.4103/2229-5178.178099
CrossRef - Ebert A, Monod M, Salamin K, Burmester A, Uhrlaß S, Wiegand C, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses 2020;63(7):717-728. doi: 10.1111/myc.13091
CrossRef - Ates A, Ozcan K, Ilkit M. Diagnostic value of morphological, physiological and biochemical tests in distinguishing Trichophyton rubrum from Trichophyton mentagrophytes complex. Med Mycol 2008;46(8):811-822. doi: 10.1080/13693780802108458.
CrossRef - Pai V, Ganavalli A, Kikkeri NN. Antifungal Resistance in Dermatology. Indian J Dermatol 2018;63(5):361-368. doi: 10.4103/ijd.IJD_131_17
CrossRef - Martinez-Rossi NM, Peres NT, Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia 2008;166(5-6):369-383. doi: 10.1007/s11046-008-9110-7
CrossRef - Verma S, Madhu R. The Great Indian Epidemic of Superficial Dermatophytosis: An Appraisal. Indian J Dermatol 2017;62(3):227-236. doi:10.4103/ijd.IJD_206_17
- Zheng YH, Ma YY, Ding Y, Chen XQ, Gao GX. An insight into new strategies to combat antifungal drug resistance. Drug Des Devel Ther 2018;12:3807-3816. doi: 10.2147/DDDT.S185833
CrossRef - Hryncewicz-Gwóźdź A, Kalinowska K, Plomer-Niezgoda E, Bielecki J, Jagielski T. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 2013;176(1-2):49-55. doi: 10.1007/s11046-013-9655-y
CrossRef - Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is Antifungal Resistance a Cause for Treatment Failure in Dermatophytosis: A Study Focused on Tinea Corporis and Cruris from a Tertiary Centre?. Indian Dermatol Online J 2018;9(2):90-95. doi:10.4103/idoj.IDOJ_137_17
CrossRef - Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK. Overcoming antifungal resistance. Drug Discov Today Technol 2014;11:65-71. doi: 10.1016/j.ddtec.2014.02.005
CrossRef - Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist 2017;10:249-259. doi: 10.2147/IDR.S124918
CrossRef - Ogawara H. Comparison of Strategies to Overcome Drug Resistance: Learning from Various Kingdoms. Molecules 2018;23(6):1476. doi: 10.3390/molecules23061476
CrossRef - Annunziato G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int J Mol Sci 2019;20(23):5844. doi: 10.3390/ijms20235844
CrossRef - Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12(4):501-517.
CrossRef - Sharma M, Prasad R. The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob Agents Chemother 2011;55(10):4834-4843. doi: 10.1128/AAC.00344-11
CrossRef - Coleman JJ, Mylonakis E. Efflux in fungi: la pièce de résistance. PLoS Pathog 2009;5(6):e1000486. doi:10.1371/journal.ppat.1000486
CrossRef - Bandara HMHN, Wood DLA, Vanwonterghem I, Hugenholtz P, Cheung BPK, Samaranayake LP. Fluconazole resistance in Candida albicans is induced by Pseudomonas aeruginosa quorum sensing. Sci Rep 2020;10(1):7769. doi: 10.1038/s41598-020-64761-3
CrossRef - Keniya MV, Fleischer E, Klinger A, Cannon RD, Monk BC. Inhibitors of the Candida albicans Major Facilitator Superfamily Transporter Mdr1p Responsible for Fluconazole Resistance. PLoS One 2015;10(5):e0126350. doi: 10.1371/journal.pone.0126350
CrossRef - Alekha KD, William FE. Fluconazole. In: Florey K, editor. Analytical profiles of drug substances and excipients. London, UK: Academic Press; 2006. P. 57–113.
- Song JC, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs 2005;6(2):170-177.
- Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B. Development and characterization of effective topical liposomal system for localized treatment of cutaneous candidiasis. J Liposome Res 2010;20(4):341-350. doi: 10.3109/08982101003596125
CrossRef - El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv 2018;25(1):78-90. doi: 10.1080/10717544.2017.1413444
CrossRef - Behtash A, Nafisi S, Maibach HI. New Generation of Fluconazole: A Review on Existing Researches and Technologies. Curr Drug Deliv 2017;14(1):2-15. doi: 10.2174/1567201813666160502125620
CrossRef - Petrikkos G, Skiada A. Recent advances in antifungal chemotherapy. Int J Antimicrob Agents 2007;30(2):108-117. doi: 10.1016/j.ijantimicag.2007.03.009
CrossRef - Sharma M, Manoharlal R, Shukla S, Puri N, Prasad T, Ambudkar SV, et al. Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrob Agents Chemother 2009;53(8):3256-3265. doi:10.1128/AAC.01497-08
CrossRef - Garcia-Gomes AS, Curvelo JA, Soares RM, Ferreira-Pereira A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med Mycol 2012;50(1):26-32. doi: 10.3109/13693786.2011.578156
CrossRef - Da Silva DL, Magalhães TF, Dos Santos JR, de Paula TP, Modolo LV, de Fátima A, et al. Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice. J Appl Microbiol 2016;120(1):41-48. doi: 10.1111/jam.12966
CrossRef - Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, et al. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv2019,9(35):20192-20206. doi:10.1039/C9RA03102B.
CrossRef - Mangalathillam S, Rejinold NS, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012;4(1):239-250. doi: 10.1039/c1nr11271f
CrossRef - Rachmawati H, Budiputra DK, Mauludin R. Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug Dev Ind Pharm 2015;41(4):560-566. doi: 10.3109/03639045.2014.884127
CrossRef - Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, et al. Formulation development and optimization using nanoemulsion technique: a technical note. AAPS PharmSciTech 2007;8(2):E12-28. doi:10.1208/pt0802028
CrossRef - Rajitha P, Shammika P, Aiswarya S, Gopikrishnan A, Jayakumar R, Sabitha M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis, J Drug Deliv Sci Tech 2019;49:463-476. doi: 10.1016/j.jddst.2018.12.020
CrossRef - Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today 2011;16(7-8):354-360. doi:10.1016/j.drudis.2010.02.009
CrossRef - Pillai AB, Nair JV, Gupta NK, Gupta S. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Dermatol Res 2015;307(7):625-633. doi: 10.1007/s00403-015-1573-z
CrossRef - Arianto A, Cindy C. Preparation and Evaluation of Sunflower Oil Nanoemulsion as a Sunscreen. Open Access Maced J Med Sci 2019;7(22):3757-3761. doi:10.3889/oamjms.2019.497
CrossRef - Zhu Z, Wen Y, Yi J, Cao Y, Liu F, McClements DJ. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J Colloid Interface Sci 2019;536:80-87. doi: 10.1016/j.jcis.2018.10.024
CrossRef - Pillai DV, Sabitha M, Gupta S. BrinzolaJmide-2-hydroxypropyl beta cyclodextrin complex loaded chitosan nanogel for ocular drug delivery. Int J Pharm Res 2019;11(2):350-362. doi: 10.31838/ijpr/2019.11.02.002
CrossRef - Mohammadi G, Fathian-Kolahkaj M, Mohammadi P, Adibkia K, Fattahi A. Preparation, Physicochemical Characterization and Anti-Fungal Evaluation of Amphotericin B-Loaded PLGA-PEG-Galactosamine Nanoparticles. Adv Pharm Bull 2021;11(2):311-317. doi: 10.34172/apb.2021.044
CrossRef - Pongsumpun P, Iwamoto S, Siripatrawan U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason Sonochem 2020;60:104604. doi: 10.1016/j.ultsonch.2019.05.021
CrossRef - Schultze E, Coradini K, Dos Santos Chaves P, da Silva LP, Buss J, Guterres SS, et al. Drug-loaded nanoemulsion as positive control is an alternative to DMSO solutions for in vitro evaluation of curcumin delivery to MCF-7 cells. Pharmacol Rep 2017;69(6):1408-1412. doi: 10.1016/j.pharep.2017.05.003
CrossRef - Murali M, Thayyilakandy S, Shafi MPA, Venu A, Surendran SA, Nair SC. Propranolol hydrochloride topical gel for the treatment of infantile hemangioma. Int J App Pharmaceutics2020;12(6):143-150. doi: 10.22159/ijap.2020v12i6.34373
CrossRef