Kevin Tjoa1 , Kusmardi Kusmardi2,3,4
, Kusmardi Kusmardi2,3,4 and Yurnadi Hanafi Midoen5*
and Yurnadi Hanafi Midoen5*
1Faculty of Medicine, Universitas Indonesia, Jl Salemba Raya No 6, Senen, Central Jakarta 10430 Indonesia
2Departement of Anatomic Pathology, Faculty of Medicine, Universitas Indonesia, Jl Salemba Raya No 6, Senen, Central Jakarta 10430 Indonesia
3Drug Development Research Cluster, Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jl Salemba Raya No 6, Senen, Central Jakarta 10430 Indonesia
4Human Cancer Research Center, Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jl Salemba Raya No 6, Senen, Central Jakarta 10430 Indonesia
5Departement of Medical Biology, Faculty of Medicine, Universitas Indonesia, Jakarta Pusat, Indonesia
Corresponding Author E-mail: yurnadi.kes@ui.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2321
Abstract
Colorectal cancer (CRC) is the world’s third most cancer and the second highest mortality rate. The searching for new anti-inflammation substances with less adverse effects than aspirin for chemoprevention and adjuvant chemotherapy of CRC is running. The most notable one is fish oil containing omega 3. Kusmardi, et al. studied that industrial waste fish oil omega-3 level comes close enough to conventional fish oil industry. Study aims to reducing the level IL-6 on mice colon tissue being induced CRC using AOM/DSS by fish oil administration. Thirty male Swiss Webster mice are grouped into six treatments: positive control (aspirin), negative control (physiological saline), normal, high dose (fish oil 6mg/kgBW), medium dose (fish oil 3mg/kgBW), dan solvent control (corn oil). Colon tissue was stained using anti IL-6 antibody. Ten photos per slide were taken by microscope (400x), analyzed for the IL-6 expression by ImageJ®, and quantified for H-score. Data was analyzed using SPSS 24.0 (CI 95%) and p-value <0.05 is consider significant. Data are not normally distributed with median of 161.64 (119.4-260.67). Kruskal-Wallis test is significant in addition with Mann-Whitney test shows only high dose group has significant difference to negative control (p=0.008), medium dose (p=0.016) dan and solvent control (p=0.008). No significant difference reported between high dose and positive control group (p=0.69). High dose industrial waste fish oil can lower IL-6 expression in mice colon tissue induced CRC using AOM/DSS.
Keywords
Colorectal cancer; Colitis Associated Cancer; interleukin-6; Omega-3 Fish Oil
Download this article as:| Copy the following to cite this article: Tjoa K, Kusmard K, Midoen Y. H. Effects of Industrial Waste Fish Oil Administration on Interleukin-6 (Il-6) Expression at Mice Colon being Induced by Azoxymethane (AOM) and Dextran Sodium Sulphate (DSS). Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Tjoa K, Kusmard K, Midoen Y. H. Effects of Industrial Waste Fish Oil Administration on Interleukin-6 (Il-6) Expression at Mice Colon being Induced by Azoxymethane (AOM) and Dextran Sodium Sulphate (DSS). Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3ehfUCz |
Introduction
World Health Organization (WHO) in 2018 stated there are 9.6 million deaths, one-sixth of all death. Globally, colorectal cancer (CRC) is the third most (1.8 million cases) and second deadliest (862.000 deaths).1 In Indonesia, CRC is the second most in men and third most in women with mortality rate reaches 10.3%. Risk factors include age (>50 years old), unhealthy lifestyle: overconsumption of red meat, smoking and consuming alcohol, sedentary activity, and obesity. All risk factors contribute on exposure to free radicals which lead to tissue inflammation 2.
Numerous pathogeneses of CRC have been proposed. While no single pathway contributes solely to the development of CRC most of them (85%) are due to chromosomal instability. Epigenetic changes such as DNA methylation, histone modification, and dysregulation of miRNA also contribute to CRC pathogenesis. However, there is one important aspect in cancer development: microenvironment interaction. The last mentioned is regarded as supporting factor that can promote genomic instability and epigenetic changes or maintain a suitable condition for cancer to sustain. The most well-known microenvironment interaction that contribute to carcinogenesis is inflammation.3,4
Virchow, in 1863 stated the link of inflammation to carcinogenesis. In colitis associated cancer model, which account for 20% of all CRC cases, chronic inflammations such as inflammatory bowel disease (IBD) and ulcerative colitis (UC) lead to carcinogenesis.3 There are various pathway of inflammation and pro-inflammatory cytokine involved in CAC. One of the most prominent cytokines involved is IL-6. While working through many pathways, IL-6 works mainly via JAK/STAT3 signaling which activates transcription of proliferative and antiapoptotic genes. The signaling process also known to trigger migration and invasion of CRC cell.5
Anti-inflammatory substances are used to lower inflammatory cytokine such as IL-6 and further progression of inflammation and cancer. Aspirin, even though has been widely used as chemoprevention and adjuvant therapy for CRC (and other cancer), has some side effects as NSAID that irreversibly inhibit COX enzyme which results in increment of bleeding risk and gastrointestinal ulcer.6,7 Studies show that fish oil has anti-inflammatory activities (lowering inflammatory cytokine) which are mediated by ω-3 poly-unsaturated fatty acid (PUFA): EPA and DHA and potentially inhibit CRC progressiom.8,9 Fish oil works as anti-inflammatory substances through many mechanisms that results in lowering inflammatory mediators like prostaglandin and leukotriene, produces low pro-inflammatory potential mediator, and also increasing anti-inflammatory mediator such as resolving E and D. It also inhibits the NF-kB transcription, COX-2, and adhesion molecules by activating PPAR-γ. Due to its wide range of anti-inflammatory mechanism, fish oil containing PUFA ω-3 is a potential agent.
Regarding the resources of fish oil, Indonesia marine potential is estimated up to 1.2 billion USD (65 million tons) per year.10 However, only half has been optimized, included fish oil which still depend on import for domestic demand. Kusmardi et al. studied that fish oil from industrial waste of fish canning has ω-3 level close to conventional industry.11 As commodity value can increase 1000% and its merit on health as anti-inflammatory agent, intensifying fish oil production is crucial.10
In this study we are aimed to investigate the effect of industrial waste fish oil in lowering the expression of IL-6 in mice colon cancer model that has been induced by azoxymethane/dextran sodium sulfate (AOM/DSS) which act as genomic instability inducer and inflammatory mediator respectively.12
Methods
Pre-clinical experimental study was done using preserved colon tissue of Swiss-Webster mice. This study has pass ethical review by Health Research Ethical Committee Faculty of Medicine Universitas Indonesia. Thirty male Swiss Webster mice (mean weight 25 grams) were taken as sample and grouped to six groups: positive control/PC (AOM/DSS + aspirin), negative control/NC (AOM/DSS + physiological saline), normal/N (physiological saline), higher dose/D1 (AOM/DSS + fish oil 6mg/kgBW), lower dose/D2 (AOM/DSS + fish oil 3mg/kgBW), dan solvent control/SC (AOM/DSS + corn oil). AOM/DSS are used for tumorigenesis induction with AOM is genetic instability inductor and DSS as inflammation agent. AOM in NaCl 0.9% solution was injected intraperitoneally (IP) as single dose of 10mg/kg BW. AOM injection is given at the first week to each group except normal group. DSS 2% was dissolve in drinking water and administrated per oral for seven days. Treatment with aspirin, physiological saline, high dose fish oil, medium dose fish oil, and corn oil were given daily for four weeks by IP injection. Fish oils are obtained from remnant fish canning material of Maya Food company. Ketamine anesthesia and neck dislocation were done for termination. Visualization of experiment’s timeline is provided on Figure 1.
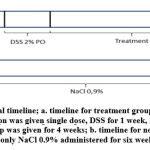 |
Figure 1: Experimental timeline; a. timeline for treatment group (PC, NC, D1,D2, SC) where AOM injection was given single dose, DSS for 1 week |
Microscopical preparations for colon histology were made in several steps and stained immunohistochemically with anti-IL-6 antibody. Tissue preparations were first stained for HE and deparaffinized in xylol I, II, and III for 5 minutes each. Dehydration was done by ethanol 96% and 70% for 5 minutes each. Retrieval microwave was used for immunostaining amplification. Hydrogen peroxide 3% and sheep serum 10% are used for blocker and incubated for whole night in temperature of 4oC by anti-IL-6. Secondary antibody embedded by horseradish peroxidase (HRP) was given and incubated for 30 minutes in 37oC and diaminobenzidine (DAB) was used as substrate. Counterstaining with hematoxylin was done prior to tissue mounting. Five slides for each group were made. Ten photos were taken with light microscope (400x) for each slide and expression of IL-6 was analyzed by ImageJ® in addition of IHC profiler plugin.
ImageJ® automatically quantified the percentage of cell with high positive, positive, low positive, and negative expression of IL-6. Histological score (H-score) was counted by following formula.
H-score = 4×% high positive + 3×%positive + 2×%low positive + 1×%negative
Average of H-score was counted and analyzed statistically in SPSS 24.0. Normality test was done by Saphiro-Wilk test and homogenity test was done by Levene test. Non-normal data are logarithmically transformed. ANOVA and Post hoc are used when data are normally distributed. Otherwise, Kruskal-Wallis and Mann-Whitney test are used. P-value less than 0.05 are considered significant.
Results
Data are not normally distributed pre and post transformation (p<0.05). Box plot diagram are shown for data visualization on Figure 2. Median IL-6 expression is 161.64% with minimum expression of 119.4% (N group) and maximum expression 260.67% (D2 group). Further descriptive IL-6 expressions are shown on Table 1. Kruskal-Wallis test shown significant p-value, thus Mann-Whitney test was done. Negative control differs significantly from N, PC, and D1 group. Aspirin treatment and D1 group shows similar result. Furthermore, D2 and SC differs significantly to PC and D1 group but comparable result to NC group. Complete p-value from Mann-Whitney analysis for each group are shown on Table 2. For simplicity, intergroup difference p-value only written once.
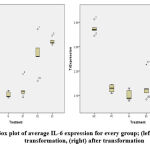 |
Figure 2: Box plot of average IL-6 expression for every group; (left) before transformation, (right) after transformation |
Table 1: Descriptive data of IL-6 expression
| Group | Median (Min-Max) | IQR | p-valuea |
| Normal (N) | 122.61 (119.40 – 134.86) | 12.8 | <0.05 |
| Positive control (PC) | 134.76 (128.22 – 139.58) | 11.36 | |
| Negative control (NC) | 234.93 (221.38 – 258.65) | 21.82 | |
| Fish oil 6 mg/kgBW (D1) | 133.69 (121.21 – 168.52) | 26.24 | |
| Fish oil 3 mg/kgBW (D2) | 204.44 (154.76 – 260.67) | 61.66 | |
| Solvent control (SC) | 232.62 (228.00 – 247.33) | 12.76 |
ap-value from Kruskal-Wallis test
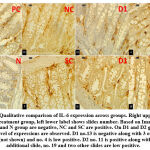
Qualitatively there are 12 positive, 4 low positive, and 14 negative slides. All PC and N group show negative expression of IL-6. Meanwhile all NC and SC group slides show positive expression. Low positive expressions are observed on one D1 slide and three D2 slides. Higher dose group accompanied for four negative slides and D2 group has 2 slides with positive expression of IL-6. Comparison of qualitative expressions are shown on Figure 3.
Table 2: Mann-Whitney test for IL-6 expression between groups
| N | PC | NC | D1 | D2 | SC | |
| N | 1 | – | – | – | – | – |
| PC | 0.151 | 1 | – | – | – | – |
| NC | 0.008 | 0.008 | 1 | – | – | – |
| D1 | 0.421 | 0.69 | 0.008 | 1 | – | – |
| D2 | 0.008 | 0.008 | 0.222 | 0.016 | 1 | – |
| SC | 0.008 | 0.008 | 0.841 | 0.008 | 0.151 | 1 |
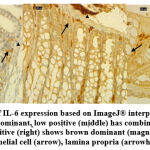 |
Figure 3: Comparison of IL-6 expression based on ImageJ® interpretation (400x): negative (left) shows blue dominant |
Discussion
Carcinogenesis, AOM/DSS, and IL-6 expression
In mice model, AOM induce genomic instability by becoming a substrate for CYP2E1 which metabolize to methyazomethanol and lead to DNA mutation on K-ras, APC, PI3K-Akt, MAPK, and Wnt/β-katenin gene. While DSS induve inflammation (colitis) by forming nanolipid complex and disrupt phospolipid membrane integrity.12,13 This condition iduce activation of inflammatory pathway and synthesis of inflammatory cytokine, such as IL-6. IL-6 stimulates more inflammatory response by NF-kB activation via JAK/STAT-3, PI3K/Akt, and SH2/SHP2/Ras/MAPK. This will lead not only to further synthesis of more IL-6 which makes inflammation cycle continuously happened, but also lead to cell proliferation which promore carcinogenesis.14 Another pathway involving miRNA miR-21 and miR-29b also promote carcinogenesis via suppresion of tumor supressor gen by activates TLR8.15 However, according to Pan et al. our models are on colitis stage, had not been progressing to dysplasia. They observed dysplasia at 7th week, which this study was done for 6 week 13. The increasing of IL-6 expression supported by Eichele et al. which state that IL-6 is a better cytokine to represent chronic inflammation together with IL-4, IL-10, and IFNγ.16
Aspirin therapy and IL-6 expression
Aspirin has been used as chemoprevention and recommended by US Preventive Task Force.5 In this study aspirin was confirmed to lower IL-6 expression even though higher dose was used in this study compared to Guillem-Llobat et al. that used 20mg/kgBB/day.17 Aspirin works as irreversible inhibitory of COX-1 and COX-2 (slightly more selective to COX-1). This inhibition results in lower prostaglandin and leukotriene which further leads to reduce TLR activity and NF-kB. The outcome of IL-6 is observed in correlation to lower NF-kB activity.14,18 The PC group shows all negative expression of IL-6, indicates that aspirin anti-inflammation effect is homogeny compared to fish oil therapy that less homogeny (not all D1 group have negative expression).
Fish oil administration effect on IL-6 expression
Dose dependent manner of fish oil administration was observed in this study. Using 6mg/kgBW/d there is no significant difference compared to aspirin therapy as positive control. While lower dose of 3mg/kgBW/d differs significantly to positive control and D1 group. This result implies that D1 has achieved therapeutic dose, while D2 does not. Optimal dose in human of 2g/day has been widely accepted. On the other hand, there are no consensus about optimal dose in mice. Irun et al. in their systematic review stated that 8mg/kgBW of fish oil has given anti-inflammatory effect, confirmed by lipid profile and inflammation markers.19 However, optimum dose depends on many factors: sources, method of production and processing, method of administration, and the ω-6: ω-3 ratio. This later property are critical to determine the anti-inflammatory or pro-inflammatory effect as ω-6 is proinflammatory substance and ω-3 has anti-inflammation effect.20
The balance ratio is differs throughout researches, depends on target of prevention/threapy. Ratio as much as 4:1 effectively prevent cardiovascular diseases. Lower ratio of 2.5:1 is neded for colorectal cell proliferation [20]. Lower ratio give higher anti-inflammatory effect and vice versa. This ratio also explain why solvent control group that are given corn oil shows no IL-6 expression difference to negative control and all of the has positive IL-6 expression. Corn oil has 84% unsaturated fatty acid consist of 52% linoleic acid (ω-6), 31% oleic acid (ω-9), and around 1% linolenic acid (ω-3).21 Omega-9 is also a proinflammatory substance. While fish oil ω-3 are consist of EPA and DHA, corn oil has no EPA and DHA. Study shows that only high dose linolenic acid (>1mM) can suppress cell proliferation. Furthermore, linolenic acid does not work via COX modification which EPA and DHA modify to give anti-inflammatory effect.22 EPA and DHA also results in activation of PPAR-γ which inhibits NF-kB action and results in less cytokine, adhesion molecules, and COX production. Corn oil does not exhibit this anti-inflammatory action, makes it less potent.8,9
Our study use corn oil as diluent because of high viscosity of fish oil which will make it is hard to be administered. However, the use of corn oil may disrupt the potential anti-inflammatory effect of fish oil as suggested from D2 group. Our fish oil has more potent anti-inflammatory effect compare to other studies according to Irun et al. systematic review. Our optimal dose for is 6mg/kgBW compare to 8mg/kgBW19 even though we use residual materials.
Conclusion
High dose of industrial waste fish oils as high as 6mg/kgBW/d results in lower IL-6 expression on colon epithelium significantly in CRC mice model induced by AOM/DSS with comparable effect to aspirin therapy. IL-6 lowering trend by fish oil administration shows dose dependent manner. Further studies for longer induction period and various dosage are needed. We also suggest to compares our fish oil with other industrial fish oil available.
Acknowledgement
The authors would like to thank Universitas Indonesia for funding this research through PUTI Grant Universitas Indonesia 2020.
Conflict of Interest
There are no conflicts of interest to be declared
Funding Source
Funding was provided by the PUTI Grant with contract number NKB-2236/UN2.RST/HKP.05.00/2020.
Refernces
- World Health Organization. Cancer [Internet] (2020). http://www.who.int/news-room/fact-sheets/detail/cancer. Accessed on 2020 Jul 21. (Geneva)
- Arnold M, Sierra M. S, Laversanne M, Soerjomataram I, Jemal A and Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut., 2016; 27 (0): 1-9. DOI: 1136/gutjnl-2015-310912
CrossRef - Grady W. M and Markowitz S. D. The molecular pathogenesis and potential of colorectal cancer and its potential application to colorectal cancer screening. Dig. Dis. Sci., 2015; 60 (3): 762-72. DOI: 1007/s10620-014-3444-4
CrossRef - Cui G, Yuan A, Sun Z, Zheng W and Pang Z. IL-1β/IL-6 network in the tumor microenvironment of human colorectal cancer. Res. Pract., 2018; 214: 986-92. DOI: 10.1016/j.prp.2018.05.011
CrossRef - Lu C. C, Kuo H. C, Wang, F. S, Jou M. H, Lee K. C and Chuang J. H. Upregulation TLRs and IL-6 as marker in human colorectal cancer. Int. J. Mol. Sci., 2015; 16: 159-77. DOI: 10.3390/ijms16010159
CrossRef - Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med., 2016; 164 (12): 836-45. DOI: 7326/M16-0577
CrossRef - Coyle C, Cafferty F. H and Langley R. E. Aspirin and colorectal cancer prevention and treatment: is it for everyone?. Curr. Colorectal. Cancer. Rep., 2016; (12): 27-34. DOI:1007/s11888-016-0306-9
CrossRef - Calder P. C. Omega-3 fatty acids and inflammatory processes. Nutrients., 2010; 18(2): 355-74.DOI: 3390/nu2030355
CrossRef - Calviello G, Serini S and Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Med. Chem., 2007; 14: 3059-69. DOI: 10.2174/092986707782793934
CrossRef - Ministry of Industry. The government encourages the fish oil industry [Internet] (2015). http://www.kemenperin.go.id/ article/15592/Government-Dorong-Industri-Oil-Ikan. Accessed on 22 Jul 2020. (Jakarta)
- Kusmardi K and Tedjo A. Fish oil from fish canning waste has the potential to reduce the expression of inflammatory markers in the colonic malignancy of mice induced by azoximethane and dextran sodium sulfate. M Patol. Indon (MPI)., 2016; 25 (3): 1-
- Chen J and Huang F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer. Biol. Ther., 2009; 14 (8): 1313-17. DOI.org/10.4161/cbt.8.14.8983
CrossRef - Pan Q, Lou X, Zhu Y, Li F, Shan Q and Chen X. Genomic variants in mouse model induced by azoxymethane and dextran sodium sulfate improperly mimic human colorectal cancer. Rep., 2017; 7(25): 1-12. DOI: 10.1038/s41598-017-00057-3
CrossRef - Wang W and Sun Y. M. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (review). Int. J. Oncol., 2014; 7 (44): 1032-40. DOI: org/10.3892/ijo.2014.2259
CrossRef - Patel S. A and Gooderham N. J. IL6 mediates immune and colorectal cancer cell crosstalk via miR-21 and miR-29b. Mol. Cancer., 2015; 13(11): 1502-8. DOI: 10.1158/1541-7786.MCR-15-01
CrossRef - Eichele D. D and Kharbanda K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing out understanding of inflammatory bowel diseases pathogenesis. World. J. Gastroenterol., 2017; 23(33): 6016-29. DOI: 3748/wjg.v23.i33.6016
CrossRef - Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotargets. Ther., 2016; 7(22): 32462-77. DOI: 18632/oncotarget.8655
CrossRef - Garcia-Albeniz X and Chan A. T. Aspirin for the prevention of colorectal cancer: Best Pract. Res. Clin. Gastroenterol., 2011; 25(0): 461-472. DOI: 1016/j.bpg.2011.10.015
CrossRef - Irun P, Lanas A and Piazuelo E. Omega-3 polysaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation a review. Front. Pharmacol., 2019; 2(10): 852. DOI: 10.3389/fphar.2019.00852
CrossRef - Simopoulos A. P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med., 2008; 233(6):674-88. DOI: 10.3181/0711-MR-311
CrossRef - Gunstone P. Fatty acid and lipid chemistry. Blackie: London. 1996
CrossRef - Volpato M and Hull M. A. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer. Metastasis. Rev., 2018; 3(37): 545-55. DOI: 10.1007/s10555-018-9744-y
CrossRef