Nahla A. Mohamed 1 and Eman Refaat Youness2*
and Eman Refaat Youness2*
1Department of Pediatrics, El Galaa Teaching Hospital,, General Organization of Teaching Hospitals and Institutes. Egypt.
2 Department of Medical Biochemistry, National Research Centre, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2292
Abstract
Sepsis is a systemic inflammatory disorder that may be associated with higher rate of morbidity and mortality in pediatric patients admitted to intensive care unit with sepsis. Usage of different biomarkers may be helpful for early detection and appropriate management of sepsis. Our objectives was to investigate the role of serum lactate dehydrogenase in prediction of sepsis in critical pediatric patients, and its relation with prognostic scoring systems. A prospective cohort study was conducted at El Galaa teaching hospital between January 2020 and December 2020. A total of 168 pediatric patients were divided into the septic group (84) critically ill patients with sepsis from the pediatric intensive care unit (PICU)] and control group (84 stable patients admitted to the inpatient word). Demographic and clinical data were collected, routine laboratory investigation including LDH on admission and after 24 hours were performed. Pediatric Risk of Mortality III (PRISMIII) and Sequential Organ Failure Assessment (pSOFA) were assessed. Serum LDH level was significantly higher in septic group than control (P=0.000) and in non-survivor than survivor group (P=0.000). Also there was statistically significant correlation between survivor and non-survivor as regarding length of hospitality, pSOFA score and PRISMIII score. There was statistically significant positive correlation between LDH, PRISMIII (r=0.842, P<0.001) and pSOFA (r=0.785, P<0.001). We concluded that LDH is a useful marker in predicting of sepsis in critically ill pediatric patients especially when combined with prognostic scoring systems.
Keywords
Lactate Dehydrogenase; Pediatric Intensive Care Unit; Pediatric Risk Of Mortality III; Psofa; Sepsis
Download this article as:| Copy the following to cite this article: Mohamed N. A, Youness E. R. Clinical Role of Serum Lactate Dehydrogenase Assessment in Critically Ill Pediatric Patients with Sepsis. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Mohamed N. A, Youness E. R. Clinical Role of Serum Lactate Dehydrogenase Assessment in Critically Ill Pediatric Patients with Sepsis. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3mvexoG |
Introduction
Sepsis is a life-threatening health problem that may be associated with increased mortality in children and young adult even in developed countries. It has been defined as a systemic inflammatory response syndrome (SIRS) caused by blood stream infections or organ dysfunction caused by a host response deregulation to infection1.
Moreover, SIRS may be due to infectious and non-infectious causes. Pediatric SIRS is defined by abnormal temperature: hyperthermia or hypothermia (>38.5°C or <36°C); or abnormal leukocyte count: elevated or depressed leucocytic count for age, or >10% immature neutrophils, tachycardia or bradycardia, tachypnea 2. Abnormal temperature and leukocyte count are essential for diagnosis of SIRS, while abnormal respiratory rates and heart rate are common in pediatrics may occur in clinical conditions and unnecessarily indicate SIRS3.
Biomarkers can play an important role in providing a timely diagnosis of sepsis, helping in distinguishing between infectious and non-infectious SIRS and the decision-making in the initial management 4. In pediatrics, one of most commonly used biomarker to differentiate sepsis from non-infectious SIRS is serum lactic dehydrogenase (LDH) 5. It’s one of the enzyme involved in anaerobic metabolic pathway, its level increased in multiple clinical conditions associated with tissue damage 6. Many studies suggested that significant elevation in serum LDH levels early in sepsis can be useful as a marker for reflecting the extent of tissue damage7.
Elevated serum LDH in pediatric patients with sepsis reflect imbalance between lactate production and clearance 8. Increased serum lactate levels in sepsis may occur through several mechanisms, including an aerobic glycolysis as a result of impaired oxygen delivery to tissue as well as tissue hypoperfusion, stress as endogenous and exogenous catecholamines are highly associated with lactic acid production in sepsis, elevated bacterial load 9 and decreased lactate clearance that induced by hepatic and renal dysfunction 10.
Aim of the study
To investigate the role of serum lactate dehydrogenase in prediction of sepsis in critical pediatric patients, and its relation with prognostic scoring systems.
Patients and Methods
A prospective cohort study was conducted at El Galaa teaching hospital in Cairo between January 2020 and December 2020. The study was carried out on 168 ill children, who were divided into 2 groups: Cases group (1): 84 critically ill children who were admitted to the PICU with sepsis and Control group (2): 84 stable control admitted to the inpatient word. Aiming to assess serum LDH levels in predicting sepsis in pediatric critical patients, and also the relation between LDH and scoring systems (Pediatric Risk of Mortality (PRISMIII), Pediatric Sequential Organ Failure Assessment pSOFA). The study was conducted after obtaining informed consent from the caregivers of participants and the approval of the Ethics Committee of National Research Centre.
Inclusion criteria
Age:1 month-14 years old
Sex: male or female
Patients with sepsis (defined as SIRS in the presence of or as a result of suspected or documented infection) Goldstein et al. 11 admitted to the PICU.
Exclusion criteria
Patients on steroids
Patients known with metabolic disorders, chronic liver and kidney disease.
Death in less than48 hours.
Patients with acute hemolytic anemia.
Post-operative patients
Ethical considerations:
Informed consent was obtained willingly from all patients, control and/or their legal guardians before enrollment in the study. The ethics committee of General Organization of Teaching Hospital and Institutes approved the study design and conducted according to Helsinki declaration.
All studied cases were subjected to the following:
Full history and data including sex, age, primary diagnosis, history of chronic illness and chronic medication use and current medications.
Complete clinical and systemic examinations including vital signs especially heart rate, blood pressure and temperature, respiratory rate, conscious level of patients, presence of infection or sepsis
Laboratory investigations on admission including: Complete Blood Counts(CBC),C-reactive protein (CRP), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), potassium (K), sodium (Na), Blood Urea Nitrogen (BUN), serum creatinine (Cr), alanine transaminase (ALT), aspartate transaminase (AST), LDH (day1) and after 24 hour(day2).
System failure assessment (pSOFA score and PRISMIII score).Use of mechanical ventilation.
Evaluation of patients outcome (death or improved) and duration of hospital stay.
Samples collection, LDH assay
About 5 ml of whole blood were collected from cases and controls by aseptic vein puncture for LDH assay. Samples were immediately centrifuged and the serum was used for analysis on blood chemistry analyzer Dimension RXLMAX integrated chemistry system from Siemens Healthcare S.A.E, Germany.
Statistical analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBMSPSS) version 23. The quantitative data with parametric distribution were presented as mean, standard deviations and ranges while with nonparametric distribution were presented as median with inter-quartile range (IQR). Also qualitative variables were presented as number and percentages. The comparison between groups regarding qualitative data was done by using Chi-square test and/or Fisher exact test when the expected count in any cell found less than 5. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and with nonparametric distribution were done by using Mann-Whitney test. Comparison between two paired groups regarding nonparametric data was done by using Wilcoxon Rank test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Univariate and multivariate logistic regression analysis was used to assess the predictors of cases group and their outcome. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the level of <0.05.
Results
Table 1: Demographic and clinical data of cases and control groups
|
Variable |
Control group | Cases group | P-value | |
| No.=84 | No.=84 | |||
| Age in months | Median(IQR) | 13(6–34) | 13(6–26) | 0.722 |
| Range |
1–90 |
1–122 | ||
| Sex | Male | 35(41.7%) | 42(50.0%) | 0.278 |
| Female | 49(58.3%) | 42(50.0%) | ||
| Length of hospital stay in days |
Median(IQR) | 8(7–10) | 10(8–16) | 0.000 |
| Range | 5–18 | 5–34 | ||
| Diagnosis | Neurological disease | 4(4.8%) | 12(14.3%) | – |
| Cardiovascular disease | 0(0.0%) | 16(19.0%) | ||
| Respiratory disease | 28(33.3%) | 34(40.5%) | ||
| Blood born infection | 0(0.0%) | 14(16.7%) | ||
| Gastrointestinal disease | 39(46.4%) | 8(9.5%) | ||
| Renal infection | 8(9.5%) | 0(0.0%) | ||
| Others | 5(6.0%) | 0(0.0%) | ||
| Outcome | Survival | 84(100.0%) | 50(59.5%) | 0.000 |
| Non-survival | 0(0.0%) | 34(40.5%) | ||
| Mechanical ventilation |
No | 84(100.0%) | 62(73.8%) | 0.000 |
| Yes | 0(0.0%) | 22(26.2%) | ||
| SOFA | Median(IQR) | 5.5(4–7) | 10(7–17) | 0.000 |
| Range | 2–11 | 4–22 | ||
| PRISMIII | Median(IQR) | 22.5(18–28) | 44.5(23–62) | 0.000 |
| Range | 3–48 | 10–71 | ||
P-value>0.05: Non-significant; P-value<0.05: significant; P-value<0.01: highly significant
In the cases group, median age was 13 (6–26) months, 50.0% were males, and 50.0 % were females. In the control group, mean age was 13 (6–34) months, 41.7% were males, and 58.3% were females. There was significant difference in both groups regarding length of hospital stay, use of mechanical ventilation and outcome, pSOFA score and PRISMIII score (p-value=0.000).
Table 2: Laboratory data of cases and control groups
| Variable | Control group | Cases group | P-value | |
| No.=84 | No.=84 | |||
| Hemoglobin | Mean±SD | 9.18±1.72 | 8.80±1.49 | 0.128 |
| Range | 5.2–12 | 5.7–12 | ||
| Neutrophil /Lymphocyte count
ratio |
Median(IQR) | 4(3.2–6) | 9(5–12) | 0.000 |
| Range | 2–11.2 | 3–17 | ||
| TLC | Median(IQR) | 8.2(7.2–10.5) | 11.9(7–21) | 0.000 |
| Range | 2.1–22 | 2.1–35 | ||
| Platelet | Median(IQR) | 203(167–260.5) | 207(113–294) | 0.263 |
| Range | 131–653 | 33–567 | ||
| Cr | Median(IQR) | 0.5(0.5–0.6) | 0.6(0.5–0.8) | 0.001 |
| Range | 0.3–1.1 | 0.3–3.3 | ||
| Urea | Mean±SD | 21.77±3.77 | 31.19±14.50 | 0.000 |
| Range | 11–30 | 16–72 | ||
| AST | Median(IQR) | 38(32–45) | 47.5(34–87) | 0.000 |
| Range | 21–103 | 22–254 | ||
| ALT | Median(IQR) | 31(26–38) | 36.5(23–67) | 0.009 |
| Range | 16–98 | 16–201 | ||
| PT | Mean±SD | 12.80±0.94 | 13.14±1.31 | 0.051 |
| Range | 12–15 | 12–16 | ||
| PTT | Mean±SD | 35.68±4.34 | 39.26±10.42 | 0.004 |
| Range | 32–52 | 32–67 | ||
| INR | Mean±SD | 1.18±0.20 | 1.36±0.50 | 0.002 |
| Range | 1–1.8 | 1–3.1 | ||
| CRP | Median(IQR) | 12(0–24) | 48(12–96) | 0.000 |
| Range | 0–96 | 0–212 | ||
| LDH at admission (day1) | Median(IQR) | 243(201–302) | 498(312–786) | 0.000 |
| Range | 173–457 | 214–2102 | ||
| LDH after 24 hour (day2) | Median(IQR) | 230.5(201–301) | 415(243–834) | 0.000 |
| Range | 168–422 | 201–2134 | ||
| Na+ | Mean±SD | 139.52±5.64 | 138.93±9.37 | 0.619 |
| Range | 130–152 | 124–170 | ||
| K+ | Mean±SD | 3.70±0.73 | 3.77±0.81 | 0.561 |
| Range | 2.1–5.2 | 2.1–5.2 | ||
There was significant difference between both groups regarding granulocyte/lymphocyte ratio, total leucocytic count(TLC), creatinine(Cr), Urea, aspartate transaminase (AST), alanine transaminase (ALT), partial thromboplastintime (PTT), international normalized ratio(INR), C- reactive protein (CRP), lactate dehydrogenase (LDH) on day1 and 2.
Table 3: Correlation of LDH at day1 with the other studied parameters in Cases group
| Variable | LDH at admission(day1) | |
| r | P-value | |
| Age in months | 0.246* | 0.024 |
| Length of hospital stay in days | 0.548** | 0.000 |
| Hb | -0.494** | 0.000 |
| Neutrophil / Lymphocyte count ratio | 0.774** | 0.000 |
| TLC | 0.483** | 0.000 |
| Platelet | -0.593** | 0.000 |
| Cr | 0.462** | 0.000 |
| Urea | 0.623** | 0.000 |
| AST | 0.754** | 0.000 |
| ALT | 0.771** | 0.000 |
| PT | 0.366** | 0.001 |
| PTT | 0.415** | 0.000 |
| INR | 0.403** | 0.000 |
| CRP | 0.818** | 0.000 |
| pSOFA | 0.785** | 0.000 |
| PRISMIII | 0.842** | 0.000 |
| Na | 0.064 | 0.565 |
| K | 0.320** | 0.003 |
P-value>0.05: Non- significant; P-value<0.05: Significant; P-value<0.01: highly significant Spearman correlation coefficient
There was statistically significant correlation between lactate dehydrogenase at admission and hemoglobin, granulocyte/lymphocyte ratio, total leucocytic count (TLC), creatinine (Cr), Urea, aspartate transaminase (AST), alanine transaminase (ALT), partial thromboplastin time (PTT), international normalized ratio (INR), C-reactive protein (CRP), serum potassium in cases group.
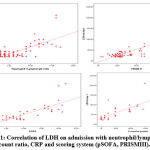 |
Figure 1: Correlation of LDH on admission with neutrophil/ lymphocyte count ratio,CRP and scoring system (pSOFA, PRISMIII). |
Table 4: Relation of outcome with demographic and clinical data in cases group
|
Variable |
Survival | Non-survival | P-value | |
| No.=50 | No.=34 | |||
| Age in months | Median(IQR) | 13(6–27) | 13(9–25) | 0.584 |
| Range | 1–122 | 2–65 | ||
| Sex | Male | 20(40.0%) | 22(64.7%) |
0.026 |
| Female | 30(60.0%) | 12(35.3%) | ||
| Length of hospital stay in days |
Median(IQR) | 9(7–12) | 16(10–25) | 0.000 |
| Range | 5–18 | 8–34 | ||
| Diagnosis | Neurological disease | 8(16.0%) | 4(11.8%) | 0.792 |
| Cardiovascular disease | 8(16.0%) | 8(23.5%) | ||
| Respiratory disease | 20(40.0%) | 14(41.2%) | ||
| Blood born infection | 8(16.0%) | 6(17.6%) | ||
| Mechanical ventilation | No | 44(88.0%) | 18(52.9%) | 0.000 |
| Yes | 6(12.0%) | 16(47.1%) | ||
| SOFA | Median(IQR) | 8(6–9) | 18(17–20) | 0.000 |
| Range | 4–14 | 16–22 | ||
| PRISMIII | Median(IQR) | 31(22‒34) | 63(59‒67) | 0.000 |
| Range | 10–65 | 48–71 | ||
P-value>0.05:Non-significant; P-value<0.05: significant; P-value<0.01: highly significant
There was statistically significance between survivor and non-survivor as regarding length of hospital stay, mechanical ventilation, pSOFA score and PRISMIII score.
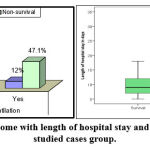 |
Figure 2: Relation of outcome with length of hospital stay and mechanical ventilation in studied cases group. |
Table 5: Relation of outcome with laboratory data in cases group
| Variable | Survival | Non-survival | P-value | |
| No.=50 | No.=34 | |||
| Hemoglobin | Mean±SD | 9.27±1.47 | 8.10±1.25 | 0.000 |
| Range | 6.3–12 | 5.7–10.2 | ||
| Neutrophil/Lymphocyte count ratio |
Median(IQR) | 6(4.2–8) | 13(11–15) | 0.000 |
| Range | 3–12 | 10–17 | ||
| Total leukocytic count | Median(IQR) | 9.5(6.2–12) | 21(18–25) | 0.000 |
| Range | 2.1–21 | 3.2–35 | ||
| Platelet | Median(IQR) | 234(201–432) | 101(68–151) | 0.000 |
| Range | 42–567 | 33–534 | ||
| Cr | Median(IQR) | 0.6(0.5–0.6) | 0.7(0.6–1.7) | 0.000 |
| Range | 0.3–1.9 | 0.5–3.3 | ||
| Urea | Mean±SD | 25.28±9.06 | 39.88±16.61 | 0.000 |
| Range | 16–57 | 19–72 | ||
| AST | Median(IQR) | 43(33–48) | 102(67–133) | 0.000 |
| Range | 22–125 | 33–254 | ||
| ALT | Median(IQR) | 27(21–35) | 67(48–98) | 0.000 |
| Range | 16–98 | 27–201 | ||
| PT | Mean±SD | 12.88±1.12 | 13.53±1.48 | 0.025 |
| Range | 12–16 | 12–16 | ||
| PTT | Mean±SD | 38.24±9.55 | 40.76±11.57 | 0.278 |
| Range | 32–67 | 33–67 | ||
| INR | Mean±SD | 1.25±0.30 | 1.53±0.67 | 0.011 |
| Range | 1–2.1 | 1–3.1 | ||
| C-reactive protein | Median(IQR) | 12(0–24) | 96(96–124) | 0.000 |
| Range | 0–96 | 24–212 | ||
| LDH at day1 | Median(IQR) | 312(245–432) | 834(745–980) | 0.000 |
| Range | 214–765 | 629–2102 | ||
| LDH at day2 | Median(IQR) | 256(209–387) | 856(754–1267) | 0.000 |
| Range | 201–701 | 627–2134 | ||
| Na+ | Mean±SD | 140.92±11.24 | 136.00±4.29 | 0.017 |
| Range | 133–170 | 124–145 | ||
| K+ | Mean±SD | 3.47±0.70 | 4.20±0.76 | 0.000 |
| Range | 2.2–4.5 | 2.1–5.2 | ||
P-value>0.05: Non- significant; P-value<0.05: significant; P-value<0.01: highly significant
: Independent t-test; ≠: Mann-Whitney test
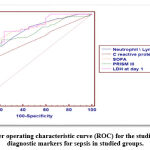 |
Figure 3: Receiver operating characteristic curve (ROC) for the studied parameters as diagnostic markers for sepsis in studied groups. |
| Variables | Cutoff point | AUC | Sensitivity | Specificity | +PV | -PV |
| Neutrophil/Lymphocyte ratio | >7.5 | 0.818 | 61.90 | 90.48 | 86.7 | 70.4 |
| CRP | >24 | 0.700 | 52.38 | 82.14 | 74.6 | 63.3 |
| SOFA | >8 | 0.840 | 66.67 | 88.10 | 84.8 | 72.5 |
| PRISM3 | >28 | 0.787 | 71.43 | 78.57 | 76.9 | 73.3 |
| LDH at day1 | >302 | 0.845 | 80.95 | 76.19 | 80.95 | 76.19 |
The previous ROC curve shows that the best cutoff point between cases and controls regarding granulocyte/lymphocyte ratio was found>7.5 with sensitivity of 61.90%, specificity of 90.48% and AUC of 81.8%, regarding C-reactive protein was found>24 with sensitivity of 52.38%, specificity of 82.14% and AUC of 70.0%, regarding SOFA score was found>8 with sensitivity of 66.67%, specificity of 88.10% and AUC of 84.0%, regarding PRISM3 was found>28 with sensitivity of 71.43%, specificity of 78.57% and AUC of 78.7% while regarding LDH at day1 the best cutoff point was found>302 with sensitivity 80.95%, specificity 76.19% and AUC 84.5%.
Table 6: Univariate and multivariate logistic regression analysis for predictors of cases group
| Variable | Univariate | Multivariate | ||||||
| P-value | OR | 95%C.I.for OR | P-value | OR | 95%C.I.forOR | |||
| Lower | Upper | Lower | Upper | |||||
| Length of hospital stay in days>11 | 0.000 | 7.848 | 3.368 | 18.284 | – | – | – | – |
| Neutrophil/Lymphocyte ratio>7.5 | 0.000 | 15.437 | 6.590 | 36.164 | 0.011 | 5.072 | 1.454 | 17.697 |
| Total leucocytic count>11.4 | 0.000 | 6.667 | 3.250 | 13.673 | 0.094 | 2.532 | 0.854 | 7.504 |
| Creatinine>0.6 | 0.003 | 2.750 | 1.397 | 5.412 | – | – | – | – |
| Urea>24 | 0.000 | 6.906 | 3.428 | 13.912 | – | – | – | – |
| AST>49 | 0.000 | 6.727 | 3.062 | 14.779 | – | – | – | – |
| ALT>44 | 0.000 | 6.113 | 2.780 | 13.440 | – | – | – | – |
| PTT>42 | 0.002 | 4.613 | 1.762 | 12.076 | – | – | – | – |
| INR>1.6 | 0.001 | 6.250 | 2.034 | 19.207 | 0.030 | 0.139 | 0.023 | 0.828 |
| C-reactive protein>24 | 0.000 | 5.060 | 2.504 | 10.227 | – | – | – | – |
| SOFA>8 | 0.000 | 14.800 | 6.642 | 32.976 | 0.001 | 6.871 | 2.274 | 20.763 |
| PRISMIII>28 | 0.000 | 9.167 | 4.534 | 18.535 | – | – | – | – |
| LDH at day 1>302 | 0.000 | 13.600 | 6.484 | 28.526 | 0.000 | 8.600 | 3.358 | 22.028 |
The previous univariate logistic regression analysis shows that all the previous parameters were associated with sepsis with p-value<0.001; also the multivariate analysis shows that the most important predictors for sepsis was found LDH at day1>302 with OR (95%CI) of 8.600(3.358–22.028) followed by SOFA>8 with OR(95%CI)6.871(2.274–20.763) followed by total leucocytes count>11.4 with OR (95%CI)of5.072(1.454–17.697) and lastly INR>1.6 with OR(95%CI) of 0.139(0.023–0.828).
Table 7: Univariate logistic regression analysis for predictors of outcome in cases group
| Variable | B | S.E. | Wald | P-value | Odds ratio (OR) |
95%C.I. for OR | |
| Lower | Upper | ||||||
| Sex | -1.012 | 0.461 | 4.824 | 0.028 | 0.364 | 0.147 | 0.897 |
| Length of hospital stay in days>9 | 2.420 | 0.606 | 15.977 | 0.000 | 11.250 | 3.433 | 36.862 |
| Mechanical ventilation | 1.875 | 0.554 | 11.431 | 0.001 | 6.519 | 2.199 | 19.325 |
| Hemoglobin<=7.8 | 1.776 | 0.517 | 11.820 | 0.001 | 5.906 | 2.146 | 16.257 |
| Neutrophil \ lymphocyte ratio > 9 | 1.525 | 0.420 | 13.176 | 0.000 | 4.597 | 2.017 | 10.474 |
| Total leucocytic count>13.2 | 3.533 | 0.626 | 31.858 | 0.000 | 34.222 | 10.035 | 116.706 |
| Platelet<=151 | 3.621 | 0.660 | 30.128 | 0.000 | 37.375 | 10.258 | 136.181 |
| Creatinine>0.6 | 2.028 | 0.501 | 16.367 | 0.000 | 7.600 | 2.845 | 20.302 |
| Urea>28 | 2.534 | 0.539 | 22.100 | 0.000 | 12.600 | 4.381 | 36.235 |
| AST>65 | 3.171 | 0.594 | 28.498 | 0.000 | 23.833 | 7.440 | 76.351 |
| ALT>35 | 3.168 | 0.627 | 25.532 | 0.000 | 23.750 | 6.951 | 81.146 |
| PT>14 | 1.386 | 0.564 | 6.040 | 0.014 | 4.000 | 1.324 | 12.084 |
| INR>1.4 | 1.052 | 0.527 | 3.987 | 0.046 | 2.864 | 1.020 | 8.043 |
| C-reactive protein>24 | 3.925 | 0.801 | 24.041 | 0.000 | 50.667 | 10.551 | 243.311 |
| PRISMIII>48 | 5.951 | 1.026 | 33.657 | 0.000 | 384.000 | 51.433 | 2866.937 |
| K>3.9 | 2.028 | 0.501 | 16.367 | 0.000 | 7.600 | 2.845 | 20.302 |
The previous table shows that the outcome of the studied patients was associated with male gender with p-value = 0.028 and OR (95%CI) of 2.750 (1.115–6.782)….
Discussion
Many potential biomarkers and scores come into focus in the last decade for early diagnosis, risk stratification and evaluation of critically ill patient’s prognosis in the Emergency Department 12. Diagnosis of critically ill patients with suspected sepsis is challenging and complex, early identification and immediate management are crucial to increase the chances of favorable outcome of septic patients, depending on clinical evaluation alone is often insufficient for an early diagnosis of sepsis 13.
Serum lactate Dehydrogenase is a cytoplasmic enzyme that is present in different body tissues especially muscle, liver and kidney contain high concentration of LDH as well as red blood cells also contain moderate concentrations of this enzyme. This differential expression of LDH is the basis of its importance as a clinical diagnostic biomarker 14. Elevated serum LDH is associated with tissue breakdown. Consequently, present in several clinical conditions, such as hemolysis, cancers, severe infections and sepsis 15. Measuring the LDH level for critically ill patients with suspected sepsis, provides useful information on the severity of the condition and enables monitoring progression of disease 4.
No single biomarkers of sepsis can be used to distinguish sepsis from other inflammatory conditions 16. The most widely used biomarkers in critically ill patients with suspected sepsis are (CRP), procalcitonin (PCT), lactate another biological simple inexpensive marker as well as granulocyte and lymphocyte count ratio 17.
The present study demonstrated that the LDH level was significantly increased in case than control as well as in non-surviving critically ill patients with sepsis .The cutoff value of> 302 μL was a predictor for sepsis with a sensitivity of 80.95% and specificity of 76.19%
This is in agreement with Aharon et al. 15 study reported a significant increase in serum level of LDH at the onset of sepsis symptoms and suggested that presence of high serum LDH at admission required through investigations for sever underlying disease especially cancer and severe infections and can be consider as independent predictor factor of morbidity and mortality . Also Wacharasint et al. 18 assumed that patients with LDH levels in the normal-range (between 1.4 and 2.3 mmol/L) had markedly increasing risk of organ failure and higher mortality compared with patients who had LDH levels less than 1.4 mmol/L
Wasserman et al. 19 demonstrated that the finding of very high isolated LDH in admitted medical patients is a marker of unfavorable outcome and very high isolated LDH is an important distinguishing marker for the presence of a limited list of underlying diseases, mostly infections, particularly pneumonia, cancer (27% vs. 4%, in the LDH group and controls respectively, P < 0.0001), liver metastases (14% vs. 3%,P < 0.0001), and hematologic malignancies (5% vs. 0%, P=0.00019). Also Hendya et al. 20 study reported that LDH, albumin, CRP, and neutrophils% are important serum markers in determining community acquired pneumonia prognosis and they should be performed on admission to predict probable complications and outcome of patients with community acquired pneumonia. This can be explained by serum lactate dehydrogenase is present in almost all tissues So, during tissue damage LDH will released from most of this tissues and lead to elevated serum LDH level as well as decreased clearance in some cases such as septic conditions 21.
But in contrary Helliksson et al. 22 suggested that presence of LDH in all most cell types, making it an unspecific biomarker of cell damage anywhere in the body, and its level increases within minutes of a cell’s entering a hypoxic-ischemic state. LDH has proven more valuable as prognostic biomarker for sepsis as elevated LDH levels have been associated with high mortality in several studies 23, 24. While study by Zein et al. 25 reported increased serum LDH levels are commonly occurred in patients with severe sepsis and consider as a marker of cell injury that reflects the degree of tissue damage also Lu et al. 26 revealed elevated LDH was associated with 28-day mortality in patients with sepsis.
The present study showed positive correlation between serum and duration of hospital stay that in agreement with study by Halden et al. 4 that suggested early elevated LDH levels in children with suspected sepsis are associated with mortality, organ dysfunction and prolonged length of hospital stay.
Our study showed statistically significant correlation between lactate dehydrogenase (LDH) at admission and hemoglobin, granulocyte/ lymphocyte ratio, total leucocytic count (TLC), creatinine (Cr), Urea, aspartate transaminase (AST), alanine transaminase (ALT), C reactive protein (CRP) in cases group.
This can be explained by the level of inflammatory biomarker (CRP) is increasing with the severity of illness, so inflammatory biomarkers can be used as a diagnostic and prognostic factors, level of SGOT which is one of liver enzyme which increase with hepatic dysfunction &inflammatory cells as staff cell also increase with the severity of illness.
This is in agreement with Hussain and Kim 27 study concluded that CRP is used as one of the markers of choice in monitoring the acute phase response & McWilliam and Riordan 28 study showed that Serial CRP measurement can be used as a diagnostic tool for finding clinical infections, monitoring effects of treatment, outcome, and early detection of relapse of the disease. Also study by Pradhan et al. 29 revealed the value of CRP in predication of patients with suspected sepsis especially who present with the SIRS manifestation. Also, CRP could be very helpful in resource‑limited places, where recent biomarkers such as procalcitonin or interleukins unavailable.
Koozi et al. 30 suggested that high CRP level at admission (>100 mg/L) was associated with an high risk of 30-day ICU mortality as well as prolonged hospital stay in survivors
Huang et al. 31 showed that: amount of AST and ALT in the blood is directly related to the extent of the tissue damage. After severe damage, AST levels rise 10 to 20 times and greater than normal, whereas ALT can reach higher levels (up to 50 times greater than normal).
Our study showed statistically significantly elevation in NLR in case as compared with control as well as in non-surviving critically ill patients with sepsis and significant positive correlation with LDH. The NLR is a common inflammatory marker, calculated from complete blood cell counts. Zahorec et al. 32 who first used NLR as marker of systemic inflammation and a predictor of critical infections such as bacteremia and sepsis as well as severity of disease
This is in agreement with Gozdas et al. 33 that suggested higher NLR ratio may be useful in estimating nosocomial sepsis in hospitalized patients also found correlation between increased NLR and CRP elevation at the time of nosocomial sepsis.
Also Naess et al. 34 concluded role of NLR in distinguishing between patients with suspected septicemic bacterial infections from patients with other bacterial infections,as NLR higher in septicemic than non-septicemic patients. Zhang et al. 35 studied the diagnostic role of different hematological parameters in sepsis and suggested that value of NLR in predicting sepsis superior to CRP. Also the predictive value of the combination of NLR, platelet distribution width (PDW) and red cell distribution width (RDW) was almost equal to that of procalcitonin. In contrast study by Lowsby et al. 36 that found NLR alone was insufficient in predicting bacteremia as blood cultures were positive in 13.8% of patients.
Our study showed positive correlation between LDH, pSOFA, (r=0.785, P=0.000) and. PRISM III (r=0.842, P=0.000). Similarly, García-Gigorro et al. 37 concluded that SOFA widely used for daily assessing acute morbidity and follow up critically ill patients in critical care units. This is in agreement with Chkhaidze et al. 38 who observed that pSOFA scores is an excellent tool to assess the extent of organ dysfunction in critically ill patients while PRISM III gives a good rank for diagnosis risk rather than specific organ involvement. This in agreement with study Zhou et al. 39 concluded pSOFA has better predictive value in the outcome of patients with suspected sepsis than PRISM III but studies by suggested that the PRISM III score had good sensitivity and specificity in prediction of mortality in septic patients.
Conclusion
Sepsis is one of most common cause of morbidity and mortality in pediatric ICU unless early detected and properly managed. The study suggests that serum LDH a simple and early marker can be a useful in diagnosis and prognosis of patients with suspected sepsis. A future studies on large sample size are required to confirm the precise role of serum LDH in early predication of sepsis especially in limited laboratory facilities hospitals.
Acknowledgment
The authors thank all participants and their parents.
Conflicts of interest
The authors declare no conflicts of interest.
Funding Sources
This research did not receive any fund.
Reference
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. The Third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016; 315: 801–810.
CrossRef - Goldstein B, Giroir B, Randolph A, and the Members of the International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 1.
CrossRef - Javed Ismail and Jhuma Sankar. System inflammatory response syndome (SIRS) and sepsis. An Ever-evolving Paradigm Indian J Pediatr 82(8):675-676,
CrossRef - Halden SF, Brou L, Deakyne SJ, Kempe A, Fairclough DL, Bajaj L. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA pediatrics. 2017; 171(3):249-55.
CrossRef - Singer AJ, Taylor M, Domingo A, Ghazipura S, Khorasonchi A, Thode Jr HC, Shapiro NI. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Academic Emergency Medicine. 2014; 21(8):853-7.
CrossRef - Vincent JL, e Silva AQ, Couto L, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Critical care. 2016; 20(1):1-4.
CrossRef - Long B and Koyfman A. Ready for Prime Time? Biomarkers in Sepsis. Emergency Medicine Clinics of North America 35.1 (2017): 109-122.
CrossRef - Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 2013;3:12.
CrossRef - Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010;182:752–61.
CrossRef - Marik PE and Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care 2013;1:3–8.
CrossRef - Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, Bunchman TE, Baker C, Mottes T, Mcafee N, Barnett J. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney international. 2005; 67(2):653-8.
CrossRef - Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for Sepsis: What is and What Might Be? Biomarker Insights. SAGE J. 2015; 10s4:7-17.
CrossRef - Freund Y, Delerme S, Goulet H, Bernard M, Riou B, Hausfater P. Serum lactate and procalcitonin measurements in emergency room for the diagnosis and risk-stratification of patients with suspected infection. Biomarkers. 2012; 17(7):590-6.
CrossRef - Alkhatib AJ and Alrakaf NA. Lactate Dehydrogenase: Physiological Roles and Clinical Implications. Clin Med. 2019; 137(5):363-9.
CrossRef - Aharon E, Shental O, Tchebiner JZ, Laufer-Perl M, Wasserman A, Sella T, Guzner-Gur H. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J. 2014; 16(7):439-
- Pierrakos C and Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010; 14(1):R15.
CrossRef - Sankar V and Webster NR. Clinical application of sepsis biomarkers. J Anesth. 2013; 27(2):269–83.
CrossRef - Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012; 38(1):4–10.
CrossRef - Wasserman A, Shnell M, Boursi B. Prognostic significance of serum uric acid in patients admitted to the department of medicine. AMJ Med Sci. 2010; 339:5-21.
CrossRef - Hendya RM, Elawadyb MA, Abd EL Kareemc HM.Role of lactate dehydrogenase and other biomarkers in predicting prognosis of community-acquired pneumoniaEgyptian JofBroncholo .2020; 13(4):539-544.
CrossRef - Tapia P, Soto D, Bruhn A, Alegría L, Jarufe N, et al. Impairment of exogenous lactate clearance in experimental hyperdynamic septic shock is not related to total liver hypoperfusion. 2015;Crit Care 19: 188
CrossRef - Helliksson F, Wernerman J, Wiklund L, Rosell J, Karlsson M. The combined use of three widely available biochemical markers as predictor of organ failure in critically ill patients Scandinavian J of clinical and laboratory investigation. 2016: 1-7.
CrossRef - Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Annals of emergency medicine. 2005; 45(5):524–8.
CrossRef - Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009; 37 (5):1670–7.
CrossRef - Zein JG, Lee GL, Tawk M, Dabaja M, Kinasewitz GT. Prognostic significance of elevated serum lactate dehydrogenase (LDH) in patients with severe sepsis. Chest. 2004; 126(4): 873S
CrossRef - Lu J, Wei ZH, Jiang H, Cheng L, Chen QH, Chen MQ, et al. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: a retrospective observational study. J Surg Res 2018; 228: 314-321.
CrossRef - Hussain TM and Kim DH. C-reactive protein and erythrocyte sedimentation rate in Spring. 2002; 15:13–6.
- McWilliam S and Riordan A. How to use: C-reactive protein. Arch Dis Child Educ Pract Ed. 2010; 95(2):55–58.
CrossRef - Pradhan S, Ghimire A, Bhattarai B, Khanal B, Pokharel K, Lamsal M, Koirala S. The role of C-reactive protein as a diagnostic predictor of sepsis in a multidisciplinary Intensive Care Unit of a tertiary care center in Nepal. Indian J Crit Care Med 2016; 20:417-20.
CrossRef - Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis; A Swedish multicenter study .Journal of Critical Care, 2020; 56:73-79
CrossRef - Huang XJ, Choi YK, Im HS, Yarimaga O, Yoon E, Kim HS. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors (Basel). 2006; 6(7):756-782.
CrossRef - Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske lekarske listy. 2001; 102(1):5– 14
- Gozdas H, Gel K, Turken E, Yasayacak A, Kesgin M, Akdeniz H. The role of hematological parameters in estimating nosocomial sepsis Electron J Gen Med 2019; 16(3): 139.
CrossRef - Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection 2017;45:299-307.
CrossRef - Zhang HB, Chen J, Lan QF, Ma XJ, Zhang SY. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Exp Ther Med 2016; 12:2215-9.
CrossRef - Lowsby R, Gomes C, Jarman I, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J 2015;32:531-4.
CrossRef - García-Gigorro R, Sáez-de la Fuente I, Marín Mateos H, Andrés-Esteban EM, Sanchez-Izquierdo JA, Montejo-González JC. Utility of SOFA and -SOFA scores for predicting outcome in critically ill patients from the emergency department. Eur J Emerg Med 2019; 26(4): 309-310.
- Chkhaidze MG, Kheladze ZS, Pruidze DR, Abelashvili DI, Gvetadze PR. Comparison of PIM and SOFA scoring systems for mortality risk prognosis in critically ill children with sepsis. Georgian Med News 2006; (131): 66-68.
- Zhou LB, Chen J, DU XC, Wu SY, Bai ZJ, Lyu HT. Value of three scoring systems in evaluating the prognosis of children with severe sepsis. Zhongguo Dang Dai Er Ke Za Zhi. 2019; 21(9):898-903.








