Ahmad Rohi Ghazali1* , Nor Fadilah Rajab2
, Nor Fadilah Rajab2 , Muhammad Firas Zainuddin1
, Muhammad Firas Zainuddin1 , Nazeha Ahmat1
, Nazeha Ahmat1 , Omchit Surien1
, Omchit Surien1
1Programme of Biomedical Science, Center for Toxicology and Health Risk Studies (CORE), Faculty of Health Sciences, The National University of Malaysia (UKM), Kuala Lumpur, Malaysia.
2Programme of Biomedical Science, Center for Healthy Aging and Wellness (H-CARE), Faculty of Health Sciences, The National University of Malaysia (UKM), Kuala Lumpur, Malaysia.
Corresponding Author E-mail: rohi@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2290
Abstract
Pterostilbene has dermal medicinal benefits such as anti-inflammatory, antioxidative effects and photoprotective properties against UVB radiation. The purpose of this study was to evaluate the dermal toxicity of pterostilbene via skin irritation and sensitisation. A skin irritation test was done according to the Organization Economic Co-operation and Development 404 guideline with the scoring of irritation based on erythema and oedema in 5 albino rabbits were observed up to 14 days. The sensitisation test using the Buehler Test in accordance with the ISO 10993-10 guideline was used to study the sensitisation effect of pterostilbene on the skin surface of albino guinea pigs. According to the primary dermal irritation index (PDII), the positive control group was classified with severe irritation (scorings of 7.71). No irritation was observed for the negative control and the 5% pterostilbene treated groups. But, a slight irritation reaction with PDII scorings of 0.86 was observed in the 10% pterostilbene treated group. The sensitisation study indicated that pterostilbene did not produce any sensitisation signs, thus classified as a non-sensitiser agent according to the Magnusson & Kligman classification. Pterostilbene-treated skin also did not indicate any signs of irritation and sensitisation. In conclusion, pterostilbene did not cause dermal toxicity upon application on the skin.
Keywords
Irritation; Skin; Sensitisation; Toxicity
Download this article as:| Copy the following to cite this article: Ghazali A. R, Rajab N. F, Zainuddin M. F, Ahmat N, Surien O. Assessment of Skin Irritation and Sensitisation Effects by Topical Pterostilbene. Biomed Pharmacol J 2021;14(4) |
| Copy the following to cite this URL: Ghazali A. R, Rajab N. F, Zainuddin M. F, Ahmat N, Surien O. Assessment of Skin Irritation and Sensitisation Effects by Topical Pterostilbene. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/30Ck04D |
Introduction
Pterostilbene (trans-3,5-dimethyl-4′-hydroxystilbene) is a natural compound classified under the group of polyphenols known as stilbenoids. Pterostilbene has a partially methylated structure due to the two methyl groups, which make up for its more desirable pharmacokinetics and bioavailability1,2. Pterostilbene has exhibited various pharmacological activities such as antibacterial activity, antibiotic adjuvant activity, chemoprevention activity, and high antioxidant capacity across experimental studies3-6. Moreover, pterostilbene has been proven to be a potential chemopreventive agent as its ability to decrease metabolic activation of procarcinogens and enhance detoxification7. Pterostilbene exhibited several promising medicinal benefits on the skin, primarily via its antioxidant activity. An in vivo study showed that pterostilbene improved the level of antioxidants in the plasma and blunt lipid molecule peroxidase activity. Pterostilbene demonstrated the capability to treat inflammatory dermatoses by inhibiting fungi such as Trichophyton sp and Candida albicans8. A study reported the photoprotective effects of pterostilbene, whereby pterostilbene significantly inhibited skin carcinogenesis induced by 12-O-tetradechamoylforbol-13-acetate (TPA)9. Those studies demonstrated a crucial potential for using pterostilbene in skin products, but the dermal toxicology and safety of pterostilbene have not been extensively studied. Evaluations of dermal toxicology and safety of a compound are essential before product development as these evaluations provide a fundamental characterisation of the potential hazards of a product10. Dermal irritation or irritant contact dermatitis (ICD) is a response displayed towards an external stimulus that may activate innate immunity, cellular changes and cause the appearance of severe eczematous lesions11,12. Conversely, skin sensitisation, also known as atopic contact dermatitis (ACD), is a delayed hypersensitivity response mediated by T-cells towards specific haptens. Haptens elicit an immune response when they covalently bind with amino acid residues13-15.
Several parameters can be used to determine skin irritation and sensitisation, and these could include skin scoring via observation, white blood cell count, skin histology and skin-fold thickness determination. Hazardous chemicals that may cause skin disease are mainly determined by characterising erythema and oedema formation in the skin. The severity of erythema present on the skin’s surface depends on certain factors such as moisture, pigmentation and skin age16. The general aim of this study was to assess skin irritation and sensitisation by pterostilbene applied topically.
Materials and Methods
Chemicals
Acetone 70 % System Chemical (USA), sodium lauryl sulphate (SLS) from Fisher Scientific (USA), 2, 4-dinitrochlorobenzene from Sigma-Aldrich (USA), cottonseed oil from Sigma Aldrich (USA), pterostilbene powder 99.8 % from Friendemann-Schmidt (USA), ethanol from System Chemicals (USA), ethyl acetate from Sigma-Aldrich (USA), KTX (Ketamine/Zoletil/Xylazine), formalin 10 % and H&E stain from Sigma-Aldrich (USA).
Animal Models
Albino rabbits weighing between 2-3 kg were used as the animal models for the skin irritation test. The rabbits were randomly divided into two groups, i.e. control and test group. A positive control agent, sodium lauryl sulfate (SLS) and the negative control agent, cottonseed oil, was applied on the same animal. Conversely, the animals in the test groups were applied with two different concentrations with 5% pterostilbene (n=2) and 10% pterostilbene (n=2). A day before the test was conducted, the dorsal part of the animal was shaved (approximately 6 cm²; 2 cm x 3 cm) using an electric shaver.
In the sensitisation study, a total of 31 albino guinea pigs weighing 300-500 g (17 females and 14 males) were used; whereby 3 guinea pigs were used in the pilot test and 28 guinea pigs were used in the Buehlers Test. Animals were obtained from the Faculty of Medicine, The National University of Malaysia (UKM) and were housed in Makmal Bioserasi, UKM Bangi, a week prior to the tests. All of the animals were given pellets and drinking water ad libitum in an adaptable environment with 12 hours light and 12 hours dark cycle. The temperature of the room was maintained at 25 ± 3°C days and nights. The research was approved by the Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC) with the approval code of FSK/2018/AHMADROHI/28NOV./973-NOV.-2018-NOV./2019.
Pterostilbene Preparation
Pterostilbene was prepared in three concentrations (2.5 %, 5% and 10%). The pterostilbene solution was prepared a day before the exposure on the test animal. Only 0.5 mL of pterostilbene solution was applied on each exposure site, with the exposure site for the irritation test being 6 cm² and 5 cm ² for the sensitisation test.
Control Group
Based on a previous study, SLS causes skin irritation when applied at a specific concentration17,18. As it is widely used in skin irritant research, 20% SLS was therefore chosen as a positive control agent in our study. 1 g of SLS powder was dissolved in 5 ml of phosphate-buffered solution (PBS). For the sensitisation test, 0.06% DNCB was used as the positive control agent as it was proven to be a potent sensitizer19,20. The 0.06 % DNCB was prepared by diluting 0.06 g of DNCB in 1.25 mL of ethyl alcohol. Cottonseed oil was used as the negative control agent. All control agents were applied on exposed skin at a fixed volume of 0.5 ml each.
Experimental Procedures
The sensitisation test was carried out according to the ISO 10993-10 Guideline by implementing the Buehlers Test method (also known as the closed patch test method). A pilot test was carried out prior to the Buehlers test to determine the inducing and the challenge doses for the conformational Buehlers Test. A day before patching the test substances, the flank of the guinea pigs was shaved with an electronic shaver to ensure maximal contact and absorption of substances through the skin of the animals. The application of each compound was done by applying 0.5 mL of the compound on a piece of 2.5 cm x 2.5 cm cut of filter paper and cotton gauze before being patched on the marked surface of the skin. It was then wrapped with a semi-occlusive surgical tape to ensure the patch stayed in place.
Pilot test, the left and right flanks of the guinea pigs were shaved, and a 2.5 cm x 2.5 cm area was marked for the application site for each dose of pterostilbene tested. The left flank was applied with a 10% concentration of pterostilbene, while the right flank was applied with 2.5 % pterostilbene on the upper flank and 5% pterostilbene on the lower flank. The application patch was left for approximately 6 ± 30 hours. Upon removal of the patch, the area that was applied with pterostilbene was wiped with tap water and was observed for any signs of erythema or oedema and was scaled from 0-3 based on the Magnusson and Kligman scale at 0 hour, 24 hours and 48 hours after patch removal. The 10 % pterostilbene is the inducing dose as it was the minimum dose that produced minimal erythema on the surface skin, while 5 % pterostilbene was chosen as the challenge dose for being the maximum dose that produced no signs of erythema on the skin surface. After determination of the inducing and challenge doses, these doses were also used to further confirm any sensitisation effects on the surface on the skin.
Buehler’s Test with 28 guinea pigs was assigned into three groups, which were the positive control group, negative control group and the pterostilbene group. The positive control group consisted of 11 animals and was applied with 0.06 % DNCB. The negative control group consisted of 6 animals and was applied with cottonseed oil and the pterostilbene group consisted of 11 animals and was applied with the pterostilbene. All animals had to undergo two phases in the Buehler Test, which were the inducing and challenge phases. In the inducing phase, the left flank of the animals was shaved 24 hours prior to the application of the tested agent or control and each animal was patched with respective compounds. The animals in the pterostilbene group were patched with the 10% inducing dose. The animals were patched for 3 days a week and 3 weeks continuously, with each patch being applied for approximately 6 ± 30 hours. After the inducing phase, the animals were given 14 days rest period to allow any priming of the immune system towards any possible sensitiser. After 14 days, the animals entered the challenge phase and the right flank of the animal was used to patch respective compounds with shaving done 24 hours prior to the patching. The pterostilbene group was applied with the 5 % challenge dose, and all the patches were applied for 6 ±30 hours.
Skin morphology observation
In the irritation test, the exposed area was observed before the patch was applied to ensure that the reaction that appeared after patch removal was caused by the tested substances. Scoring and grading of reactions were carried out according to Draize’s test scoring system with a maximum grade of 4 for a severe reaction21. Erythema, oedema and eschar were major indicators for irritation reaction. For the sensitisation study, upon removal of the patch, the skin of the animals was rinsed with water and was observed for any signs of erythema or oedema. The sensitisation effect was scaled using the Magnusson and Kligman scale with 0 as an indication of no observed reaction and 3 being the most severe reaction observed at the time interval of 0 hours, 24 hours and 48 hours after patch removal.
Skin-fold thickness
Skin-fold thickness measurement is widely used nowadays to determine the presence of oedema in the irritation test22. (Kim, 2016). This non-invasive method was carried out using the Harpenden skinfold calliper by pinching the end-to-end exposure area and clipping the area with a calliper.
Differential white blood cell count
The blood obtained from cardiac skin puncture was used to perform a blood smear and smeared slide was then stained with Wright’s stain to allow a better observation of the blood cells on the slide. After smearing, a differential white blood cell count was done to observe any changes in the level of each white blood cells.
Skin histology changes
After 14 days of observation in the irritation study, the animals were sacrificed by an overdose of ketamine/zoletil/xylazine (KTX) i.e. 1.0 ml/g intramuscularly. The skin of the exposure area was obtained by using a surgical puncher whilst the euthanised animals in the sensitisation study underwent skin biopsy to obtain the skin tissue sample. The obtained skin tissue sample was immersed in 10% neutral buffered formalin, then embedded in paraffin. The tissues were sectioned at 3-5 µm prior to staining with Haematoxylin & Eosin (H&E) staining.
Results and Discussion
Skin morphology
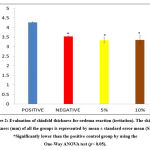
Figure 1 shows the irritation reaction observed in the positive control group on the exposed skin area at 1 hour, 24 hours, 48 hours and 72 hours after patch removal respectively. Application of 20% SLS as the positive control agent resulted in erythema and oedema reaction one hour after the patch removal. However, slight redness that appeared on the positive control group was graded with a score of one according to the skin irritation reaction23. No significant reaction appeared after one hour for the other groups. Thus they were graded with a score of zero. After 24 hours, 48 hours and 72 hours observations, skin sections in slides showed that the positive control group had developed a more obvious reaction of erythema and oedema. The grade for the reaction was also found to increase by fold. Eschar formation that started to appear after 72 hours in the positive control group indicated that the skin was irritated by 20% SLS. The skin was graded with a score of 4 for eschar formation and severe erythema. This reaction is classified as severe with a PDII score of 7.71 (severe = 5.0-8.0). Skin observation for both the negative control group and 5% of pterostilbene did not show any significant erythema or oedema reaction up to 72 hours. It thus was graded with a PDII score of 0 (not significant = 0.0-0.4) after 14 days of observation. Nonetheless, 10% of pterostilbene showed that a slight reaction appeared 72 hours after patch removal with slight erythema, and it was graded with a score of 2. After all the calculations and data recorded, 10% pterostilbene was classified as a slight reaction with a PDII score of 0.86. Table II shows the PDII score and the classification of the reaction up to 14 days of observation.
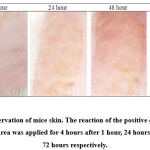 |
Figure 1: Gross observation of mice skin. The reaction of the positive control group on the exposed skin area was applied for 4 hours after 1 hour, 24 hours, 48 hours and 72 hours respectively. |
Table 1: Skin morphology (irritation) based on Primary Dermal Irritation Index (PDII) score and the classification of the reaction up to 14 days of observation.
| Group | PDII score | Classification of reaction |
| Positive | 7.71 | Severe |
| Negative | 0 | Not significant |
| 5% pterostilbene | 0 | Not significant |
| 10% pterostilbene | 0.86 | Slight |
Table 2: Skin morphology (sensitisation): Total number of guinea pigs exhibiting sensitisation reaction based on the Magnusson & Kligman scale
| Groups | Result | |
| Magnusson & Kligman scale | Total Guinea pig | |
| Positive control | 0: No visible change | 0 |
| 1: Discrete or patchy erythema | 2 | |
| 2: Moderate and confluent erythema | 8 | |
| 3: Intense erythema and swelling | 1 | |
| Negative Control | 0: No visible change | 5 |
| 1: Discrete or patchy erythema | 1 | |
| 2: Moderate and confluent erythema | 0 | |
| 3: Intense erythema and swelling | 0 | |
| Pterostilbene | 0: No visible change | 5 |
| 1: Discrete or patchy erythema | 1 | |
| 2: Moderate and confluent erythema | 0 | |
| 3: Intense erythema and swelling | 0 | |
In the sensitisation study, macroscopical observation of the skin for the positive control group applied with 0.06 % DNCB showed slight erythema at 0 hour and was scaled with a score of 1 according to the Magnusson & Kligman scale whilst both the negative control group that was applied with cottonseed oil and the pterostilbene group that was applied with pterostilbene solution did not exhibit any signs of erythema nor edema; thus, a scale of 0 was scored for both these groups at 0 hour. After 24 hours, the positive control group had a more intense, dispersed reaction, while the negative control group and the pterostilbene group still showed no indications of erythema and oedema. After 48 hours, the skin surface of the positive control group showed intense erythema with the presence of oedema whilst the negative control group and pterostilbene group remained with no signs of sensitisation reaction macroscopically. The results of the observation were scored according to the scale and are tabulated in Table II. The total number of animals that exhibited sensitisation reaction 48 hours after removal of the patch was tabulated with the percentage for each group. The sensitising potential was also scored based on the Magnusson & Kligman sensitiser classification, as shown in Tables III and IV.
Table 3: Magnusson & Kligman sensitiser classification table
| Sensitization presence
(>60 %) |
Concentration at induction phase (%) | Sensitization presence
(15-60 %) |
| Extreme | <0.2 | Strong |
| Strong | > 0.2 – < 20 | Moderate |
| Moderate | > 20 | Moderate |
Table IV: Sensitising potential of each compound
| Groups | 24 hours (%) | 48 hours (%) | Reaction Class |
| Positive Control | 100 | 100 | Strong sensitiser |
| Negative Control | 0 | 0 | Non-sensitizer |
| Pterostilbene 10% | 0 | 0 | Non-sensitizer |
A single application of 5% pterostilbene for 4 hours did not produce any severe reaction of erythema. However, 10% pterostilbene caused a slight reaction. We also found similar results in the sensitisation test whereby repeated 10% of pterostilbene application did not produce any sensitiser reaction. Our results agree with a previous study that reported Resvida ᴛᴍ did not cause any irritation and sensitisation effects towards rats24. (Williams et al., 2009). The macroscopical observation in the sensitisation study showed that the positive control group alone exhibited intense erythema along with the presence of oedema. However, no reactions were seen in the pterostilbene-treated groups.
The topical treatment of pterostilbene exhibits photoprotection and skin anti-aging properties on the UVB radiation induced skin damage in mouse model25. (Sirerol et al., 2015). Although a previous study evaluated the safety of orally administered pterostilbene at doses of 0, 30, 300 and 3000 mg/kg body weight/ day in vivo26, there remains a paucity for data on the dermal toxicity of pterostilbene. Evaluation of skin sensitisation is considered one of the five toxicological endpoints in evaluating the safety and risk of certain substances or ingredients in a product that will be applied on the skin surface27.
Sensitisation of the skin is categorised as a Type IV Hypersensitivity or delayed hypersensitivity, and it refers to the accelerated response and intense reactivity towards an antigen or sensitiser that was previously exposed to an individual28. (Eloy et al., 2001). According to the United Nations Globally Harmonized System of Classification and Labelling of 79 Chemicals in 2017, a skin sensitiser is a substance or mixture that can cause an allergic response when exposed to the surface of the skin. Generally, Type IV Hypersensitivity occurs in the presence of two phases, which are the induction phase and the challenge phase. The induction phase involves activation and maturation of dendritic cells following contact towards any sensitiser or hapten. A dendritic cell or also known as the antigen-presenting cell will then migrate to the lymph nodes to present the antigen of sensitiser that it has come into contact with within the skin to stimulate specific T cell proliferation29. Subsequent exposure towards the same sensitiser will cause the second phase of sensitisation, which is the challenge phase involving the migration to primed T cells and production of a localised immune response induced by CD8+ T Cell30,31. These events result in the manifestation of clinical symptoms such as rashes, urticarial and oedema, usually between 24 to 72 hours after exposure at the challenge phase.
To assess the sensitisation potential of a specific compound, the Buehlers Test was conducted in accordance with the ISO 10993-10 guideline. In our study, three groups of animals underwent two phases of sensitisation: the induction and the challenge phases. Upon removing the challenge patch, the animals were evaluated for the safety of pterostilbenes via macroscopical evaluation, differential white blood cell count and histological observation.
Evaluation of skin fold thickness for oedema reaction
Observation of oedema through physical changes is qualitative, and therefore the results are supported with the quantitative measurement known as skinfold thickness. Based on the data, skinfold thickness for the positive control group (4.27 ± 0.02 mm) was significantly higher than the negative control group (3.53 ± 0.03 mm). It was noted that skinfold thickness for pterostilbene treatment groups, i.e. 5% (3.33 ± 0.14 mm) and 10% (3.35 ± 0.21 mm), also showed significant differences compared to the positive control group (p< 0.05). Figure 2 shows the skinfold thickness of all the groups (data is shown in mean ± standard error mean).
Oedema was measured quantitatively via skinfold thickness measurement using Harpenden skinfold calliper. As shown by the data obtained, the skinfold thickness between the positive control group and the pterostilbene-treated group was significantly different, with lower skinfold thickness noticed in the pterostilbene-treated group. Thus, our study demonstrated that 5% and 10% of pterostilbene did not cause any oedema.
Differential white blood cell count
On analysis of differential white blood cell count, it was observed that the lymphocyte count for the positive control group (53 ± 5.2) was significantly higher (p<0.05) than the negative control group (37 ± 5.7) and the pterostilbene-treated group (30 ± 3.8) (Figure 3). Similarly, the monocyte count for the positive control group (12 ± 1.8) was also significantly higher (p<0.05) than the negative control group (4 ± 0) and pterostilbene-treated group (4 ± 1.2).
Differential white blood cell count showed a significant increase in the levels of lymphocyte and monocyte of the positive control group compared to the pterostilbene-treated group. The increase in the level of lymphocytes can be explained based on the observations from a study done by Albanesi (2010), which explained that the increase in lymphocytes was due to the repeated exposure towards an allergen or sensitiser over the period of 29 days32. (Albensi, 2010). Monocytes would then migrate and proliferate at the site of challenge exposure when an area of the skin was exposed to the challenge phase of a sensitizer33. (Gerberick, Robinson, 2000).
Skin Histological Observation
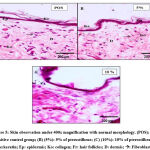
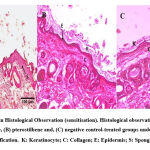
The negative control group showed normal morphology of skin tissue with three layers of the skin; epidermis, dermis and basal. Figure 3 shows the skin histology of the negative control group under 400x magnification. At the top of the epidermis layer, the keratin was found intact, with the epidermis arranged neatly. The epidermis did not show any spongiosis formation scattered, and some fibroblast, glands and follicle hairs were found on the epidermis and dermis layer. The groups with 5% and 10% of pterostilbene treatment were found to almost resemble the skin morphology of the negative control group. Observations showed that neither 5% nor 10% pterostilbene treated groups had any abnormal changes in skin morphology under the microscope (Figure 4). The positive control group also showed normal morphology of skin after 14 days of observation. It could indicate that there was a reversibility effect occurred (Figure 4). In addition, the positive control showed thinning of the epidermis and damaged keratinocytes. Spongiocytes were present in the epidermal layer. The negative control and pterostilbene treated groups did not indicate any sensitisation effects that could be seen histologically. The histological findings are shown in Figure 5.
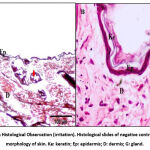 |
Figure 4: Skin Histological Observation (irritation). Histological slides of negative control with normal morphology of skin. Ke: keratin; Ep: epidermis; D: dermis; G: gland. |
In the irritation study, the histological observation of the positive control, negative control, and pterostilbene-treated groups showed the characteristics of normal skin histology. The positive control showed normal histology due to the fact that resolution had probably occurred over the course of 14 days. The appearance of normal histology in the 5 % and 10 % pterostilbene treated skin indicated that no irritation occurred. In the sensitisation study, the positive control group showed a thickening in the epidermal layer suggesting infiltration of inflammatory cells due to the exposure towards a sensitizer33. Spongiocytes were also present in the epidermal layer, leading to the observation of oedema in the positive control group34. On the other hand, the pterostilbene treated groups showed the appearance of normal skin histology, indicating no sensitisation had occurred, and no inflammatory cells were present in the epidermis layer, as demonstrated in Figure 6.
Conclusion
A single dermal application of pterostilbene did not cause any significant skin irritation. The slight erythema effect reported was not reliable since the application of the repeated exposure of pterostilbene in the skin sensitisation test did not indicate any signs of erythema nor oedema. Besides, the absence of significant changes in the white blood cell, skinfold thickness and normal skin histological observation proved that topical application of pterostilbene did not cause skin irritation and sensitisation.
Acknowledgement
This work was supported financially by the GUP-2016-081 grant funded by The National University of Malaysia (UKM).
Conflict of Interest
We declare that there are no conflicts of interest to declare regarding the publication of this manuscript.
Funding Sources
There are no funding source.
References
- Remsberg C. M, Yáñez J. A, Ohgami Y, Vega‐Villa K. R, Rimando A. M and Davies N. M. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytotherapy Res., 2008; 22(2): 169-179.
CrossRef - Yeo S. C. M., Ho P. C and Lin H. S. Pharmacokinetics of pterostilbene in Sprague‐Dawley rats: The impacts of aqueous solubility, fasting, dose-escalation, and dosing route on bioavailability. Mol Nutr Food Res., 2013; 57(6):1015-1025.
CrossRef - Harun Z and Ghazali A. R. Potential chemoprevention activity of pterostilbene by enhancing the detoxifying enzymes in the HT-29 cell line. Asian Pac J Cancer Prev., 2012; 13(12): 6403-6407.
CrossRef - Hasiah, A. H, Ghazali A. R, Weber J. F. F, Velu S, Thomas N. F and Inayat Hussain S. H. Cytotoxic and antioxidant effects of methoxylated stilbene analogues on HepG2 hepatoma and Chang liver cells: Implications for structure-activity relationship. Hum Exp Toxicol., 2011; 30(2): 138-144.
CrossRef - Ishak S. F, Ghazali A. R, Zin N. M and Basri D. F. Pterostilbene enhanced anti-methicillin resistant Staphylococcus aureus (MRSA) activity of oxacillin. Am. J. Infect., 2016; (1):1-10.
CrossRef - Lee W. X, Basri D. F and Ghazali A.R. Bactericidal effect of pterostilbene alone and in combination with gentamicin against human pathogenic bacteria. Molecules., 2017; 22(3): 463.
CrossRef - Ghazali A. R, Lee W. X, Cheng X. Y, Ahmad A and Nagapan T. S. Effects of Pterostilbene on Activities and Protein Expression of Cytochrome P450 1A1 (CYP1A1) and Glutathione S-Transferase (GST) in Benzo [a] pyrene-Induced HT-29 Colorectal Cancer Cell Line. Jurnal Sains Kesihatan Malaysia (Malaysian Journal of Health Sciences)., 2018; 16.
CrossRef - Masaki H, Atsumi T and Sakurai H. Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biophys. Res. Commun., 1995; 206(2): 474-479.
CrossRef - Estrela J. M, Ortega A, Mena S and Rodriguez M. L, Asensi M. Pterostilbene: biomedical applications. Rev. Clin. Lab. Sci., 2013; 50(3).
CrossRef - Wang J, Li Z, Sun F, Tang S, Zhang S, Lv P, et al. Evaluation of dermal irritation and skin sensitisation due to vitacoxib. Toxicology Reports., 2017; (4):287-290.
CrossRef - Lee S. H. Evaluation of Acute Skin Irritation and Phototoxicity by Aqueous and Ethanol Fractions of Angelica Keiskei. Exp. Ther. Med., 2014; 5(1):45 50.
CrossRef - Shrotriya S. N, Ranpise N. S and Vidhate B. V. Skin targeting of resveratrol utilising solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Transl. Res.,2017; 7(1): 37-52.
CrossRef - Bonneville M, Chavagnac C, Vocanson M, Rozieres A, Benetiere J, Pernet I, et al. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J. Invest. Dermatol., 2007; 127(6): 1430-1435.
CrossRef - Chew A. L and Maibach H. I. Irritant Dermatitis, Springer Science & Business Media., 2006.
CrossRef - Smith Pease C. K, White I. B and Basketter D. A. Skin as a route of exposure to protein allergens. Clin. Exp. Dermatol., 2002; 27(4): 296-300.
CrossRef - Hönigsmann H. Erythema and Pigmentation, PHOTODERMATOL PHOTO., 2002; 18(2): 75-81.
CrossRef - Smith H. R, Basketter D. A and McFadden J. P. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol., 2002; 27(2): 138-146.
CrossRef - Tupker R. A, Willis C, Berardksca E, Lee C. H, Fartasch M, Atinrat T, et al. Guidelines on sodium lauryl sulfate (SLS) exposure tests: A report from the Standardization Group* of the European Society of Contact Dermatitis. Contact Dermatitis., 1997; 37(2): 53-69.
CrossRef - Lauerma, A. I. Immunomodulation of Contact Dermatitis. (pnyt.), Exogenous Dermatology., 1995; 22:51-53.
CrossRef - Newell L, Polak M. E, Perera J, Owen C, Boyd P, Pickard C, et al. Sensitisation via healthy skin programs Th2 responses in individuals with atopic dermatitis. J. Invest Dermatol., 2013; 133(10): 2372-2380.
CrossRef - Draize J, Woodard G, Calvery H, Draize JH , Calvery Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther.,1944; 82:377-390.
- Kim H. K. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules., 2016; 21(1):70.
CrossRef - Draize J. H. Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics, The Association of Food and Drug Officials of the United States, Austin, TX. 1959: 46-59.
- Williams L. D, Burdock G. A, Edwards J. A, Beck M and Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol., 2009;47(9).
CrossRef - Sirerol J. A, Feddi F, Mena S, Rodriguez M. L, Sirera P, Aupí M, et al. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Biol. Med., 2015; 85:1-11.
CrossRef - Kosuru R, Rai U, Prakash S, Singh A and Singh S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. J. Pharmacol., 2016; 789:229-243.
CrossRef - CAESAR project. Available from: http://www.caesar.-eu >.
- Eloy R, Charton-Picard F, Delubac C and Kergozien S. Current and future issues in sensitisation testing. Med. Device Technol..,2001; 12(7): 12-15.
- Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas J. F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy., 2009; 64(12): 1699-1714.
CrossRef - Cavani A, De Luca A. Allergic contact dermatitis: novel mechanisms and therapeutic perspectives. Curr Drug Metab., 2010; 11(3): 228-233.
CrossRef - Kimber I, Maxwell G, Gilmour N, Dearman RJ, Friedmann PS, Martin SF. Allergic contact dermatitis: a commentary on the relationship between T lymphocytes and skin sensitising potency. Toxicology., 2012;291(1-3):18-24.
CrossRef - Gerberick G. F and Robinson M. K. A skin sensitisation risk assessment approach for evaluation of new ingredients and products. Am J Contact Dermatitis., 2000;11(2):65-73.
CrossRef - Albanesi C. Keratinocytes in allergic skin diseases. Curr Opin Allergy C Immunol., 2010; 10(5): 452-456.
CrossRef - Delves P. J and Roitt I. M. The immune system. N. Engl. J. Med. 2000;343(1):37-49.
CrossRef