Astrid Feinisa Khairani1* , Teuku Muhammad Adzdzikri2
, Teuku Muhammad Adzdzikri2 , Shafa Tasya Menggala2, Muhammad Hasan Bashari3
, Shafa Tasya Menggala2, Muhammad Hasan Bashari3 , Enny Rohmawaty3, Achadiyani Achadiyani1 and Nia Kania1
, Enny Rohmawaty3, Achadiyani Achadiyani1 and Nia Kania1
1Division of Cell Biology, Department of Biomedical Sciences, Universitas Padjadjaran, Sumedang, West Java, Indonesia.
2Undergraduate Program, Universitas Padjadjaran, Sumedang, West Java, Indonesia.
3Division of Pharmacology, Department of Biomedical Sciences, Universitas Padjadjaran, Sumedang, West Java, Indonesia.
Corresponding Author E-mail: astrid.khairani@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2262
Abstract
The World Health Assembly's target in exclusive breastfeeding has not been achieved. The most common factor contributing to this problem is the perceived insufficient production of breast milk, leading to the inability to give breastmilk to her child. Milk production can be increased using some ways, such as herbal galactagogue. This article aimed to review the effectiveness of some medicinal plants as galactagogues. This study uses a literature review approach by using several sources selected based on the criteria that have been set by researchers. Based on thirteen literature, herbs reviewed in this article showed positive effects as a galactagogue. Evidence regarding its efficacy and safety is scarce. Additionally, few clinical trials exist to justify its effectiveness. Further clinical trials are needed to support these findings.
Keywords
Breastfeeding; Galactagogue; Herbal; Lactation
Download this article as:| Copy the following to cite this article: Khairani A. F, Adzdzikri T. M, Menggala S. T, Bashari M. H, Rohmawaty E, Achadiyani A, Kania N. The Potential of Medicinal Plants as Galactagogue in Indonesia: A Review from Medical Perspective. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Khairani A. F, Adzdzikri T. M, Menggala S. T, Bashari M. H, Rohmawaty E, Achadiyani A, Kania N. The Potential of Medicinal Plants as Galactagogue in Indonesia: A Review from Medical Perspective. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3iuht2J |
Introduction
According to the World Health Organization, undernutrition is associated with 45% of child deaths globally. Infant and young child feeding is the key to ensure child survival and promote a child’s development and growth.1 Breast milk contains various essential nutrients needed for babies’ growth and development, such as carbohydrates, proteins, fats, vitamins, minerals, digestive enzymes, and hormones. Besides these nutrients, breast milk is also rich in immune cells, such as macrophages and stem cells, to protect the baby from infection.2 Giving optimal breastfeeding could save the lives of 820,000 children under the age of five years old.3 World Health Organization and The United Nations Children’s Fund (UNICEF) recommend that babies have to be breastfed for at least the first six months after birth, known as exclusive breastfeeding.4
Globally, only 41% of infants under six months are exclusively breastfed.5 Meanwhile, in Indonesia, only 37,3% are exclusively breastfed.6 The World Health Assembly’s global target of at least 50% exclusive breastfeeding by 2025. From these data, it can be seen that exclusive breastfeeding has not yet reached the specified target. Several factors contribute to this problem, and the most common factor is perceived insufficient production of breast milk with a prevalence between 30% and 80%.7 A reduced breast milk production is caused by some circumstances such as anxiety, fatigue, emotional stress, illness of the mother or child, separation of the mother and baby, and preterm birth.8
Milk production can be increased using some ways, such as improving the mother’s mood using relaxation techniques or psychological support. Oxytocin massage can also be an alternative way to increase milk production. However, many mothers ask for medication to their physician to increase their milk supply.9
Galactagogues are pharmaceutical agents, foods, or herbal supplements that can increase the production of breast milk. Women can use this galactagogue to induce, increase, or maintain milk production. Most of the galactagogues work by increasing prolactin through mechanisms such as direct stimulation of adenohypophysis or inhibition of prolactin inhibiting hormone.10 Prolactin is a peptide hormone that is synthesized and secreted by the anterior pituitary. This hormone serves to stimulate the production of milk in the mammary glands.11
The World Health Organization reported that approximately 80% of individuals worldwide primarily use herbal products.12 In Indonesia, people who have ever consumed traditional remedies drink known as Jamu are 59,12%.13 There have been many studies on herbs that have lactogenic activities, including the herbs reviewed in this article.14-19 Throughout history, many herbal remedies have been used to increase breast milk supply. Besides, the use of drugs and herbs when other non-pharmacological measures do not increase breast milk volume is widely recommended by breastfeeding medicine specialists and lactation consultants.20 It shows that there is a high recommendation for the use of herbal galactagogue. Using herbals as a galactagogue also gives beneficial effects such as practical, affordable, and more natural.21 Therefore, it is crucial for health care providers to know about herbal galactagogues.
Indonesia is a tropical archipelago with high diversity and complexity in the ecosystem, making Indonesia known as a megadiversity country. This variety of ecosystems shows the extraordinary richness of flora species in Indonesia. The number of flora species in Indonesia contributes 15.5% of the world’s total flora, which places Indonesia in the top five of the world with the highest plant species.22 Herbs reviewed in this article are part of Indonesian biodiversity.13,23-26 Based on the previous research, it shows that herbs reviewed in this article are potentially developed as a galactagogue in Indonesia. This article aims to review some medicinal plants’ effectiveness as galactagogues for health care providers and nursing mothers in Indonesia.
Material and Methods
Literature searching process was conducted through Google Scholar and PubMed from inception to September 2020 using search terms such as “breastfeeding”, “galactagogue”, “herbal”, and “lactation”. Using the same databases, the search was then narrowed to specific herbals by name, such as “Zingiber officinale + galactagogue”, “Sauropus androgynus + galactagogue”, “Musa paradisiaca + galactagogue”, “Trigonella foenum graecum + galactagogue”, “Cyperus rotundus + galactagogue” and “Pimpinella anisum + galactagogue”. All types of studies, including in vivo and in vitro, were included because of the lack of clinical trials of herbal galactagogues. The exclusion criteria consist of articles published over the past 15 years. Additional articles were obtained from article reference lists. Herbal galactagogues were evaluated for proposed mechanisms of action, effects, and safety.
Results and Discussion
Results
Thirteen studies were reviewed representing six herbs in this article. Only four clinical trials were found representing two herbs; three clinical trials for ginger and one clinical trial for fenugreek. Other than that, were pre-clinical trials. The summary of the trials can be seen in Table 1.
 |
Table 1: Summary of Experimental Trials of Herbal Galactagogue. |
Zingiber Officinale
Ginger (shown in figure 1) is widely used as a spice in cooking around the world. Apart from being used as a spice, ginger is also used in medicine.27 Ginger has a wide variety of constituents such as gingerol, shogaol, paradol, and zingerone.27,28 Gingerol and shogaol in ginger are reported to have a vasodilating effect caused by stimulating muscarinic receptors and blocking channels.27 This vasodilation effect is presumed to cause increased milk production by increasing blood flow to the breasts.28
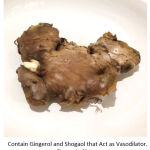 |
Figure 1: Ginger |
A randomized, controlled trial was conducted in Japan to evaluate Xiong-gui-tiao-xue-yin in increasing breast milk in the postpartum period. Xiong-gui-tiao-xue-yin is a traditional regimen consisting of 13 herbs, one of which is ginger. This study used 82 women who gave birth to normal delivery. These 82 women were randomly divided into two groups, namely those who were given Xiong-gui-tiao-xue-yin (Group X) at a dose of 6 g / day and those who were given ergometrine (Group E) at a dose of 0.375 mg/day. Breastmilk volume measurements were taken between 1 and 6 postpartum days by measuring the infant’s weight before and after breastfeeding. Serum prolactin and oxytocin concentrations were also measured on days 1 and 6 postpartum. The study results indicated that the volume of milk in group X was higher than group E between the fourth and sixth postpartum days. Group X had a higher concentration in serum prolactin than Group E on days 1 and 6 postpartum. Group E had a higher concentration in serum oxytocin than Group X on day 1, but there was no significant difference between them on day six. No adverse effects were observed in both groups. The author concludes that Xiong-gui-tiao-xue-yin can increase milk production after delivery without any adverse effects.14
A randomized, double-blind controlled trial was conducted in Thailand to assess ginger’s effect on breast milk volume in the early postpartum period. One hundred eight women aged 18 years and over who were healthy, gave birth spontaneously and at term, and aimed to breastfeed their children for at least six months were selected for this study. Women who had severe medical conditions, were allergic to ginger or had contraindications to breastfeeding were excluded from this study. Furthermore, these 108 women will be divided into two groups: the ginger group (GG) and the placebo group (PG). This study uses dry ginger capsules for the ginger group and corn starch capsules for the placebo group. The dose given to each group was the same, 500 g / capsule twice a day for seven days. The volume of breastmilk was measured at the 3rd and seventh day postpartum. On day 3, breast milk volume was measured by measuring the baby’s weight before and after breastfeeding. Maternal serum prolactin was also measured. On day 7, the measurement of breast milk volume used the 1-hour milk production method. Of the 108 women selected for the study, only 63 completed the study (ginger group=30 and placebo group=33). On day 3, the breastmilk’s mean volume in the ginger group was higher than the placebo group. The mean serum prolactin in the two groups was not different. On day 7, there was no significant difference in the mean volume of breastmilk for the two groups. The authors concluded that ginger could increase milk volume in the immediate postpartum period, and the mechanism for increasing breast milk volume may not be through stimulation of serum prolactin.28
A randomized, double-blind placebo-controlled trial was conducted in Thailand to assess the effect of a mixed herbal supplement (Fenugreek, Ginger, and Turmeric) on increased breast milk volume. This study used 50 mothers aged 20-40 years, one month postpartum with exclusive breastfeeding, and willing to participate in the study. These 50 mothers will be divided into the Herbal Group and the Placebo Group at random. The Herbal group (herbal capsules) and the placebo group (placebo capsules) will be asked to take the capsules three times a day. Breastmilk volume measurements were carried out for two days at the start, second week, and the fourth week using a breast pump. At baseline, there was no difference in the volume of milk between the two groups. At weeks two (49% vs. 11%) and fourth (103% vs. 24%), breastmilk volume in the herbal group increased significantly compared to the placebo group. No adverse effects were observed. The authors concluded that mixed herbal supplements could increase breast milk volume without any adverse effects over two weeks.29
There have been several reports regarding the safety of using ginger. The side effects of using ginger can cause headaches, diarrhea, abdominal discomfort, esophageal reflux, and drowsiness. Sedation can also occur with excessive ginger use. Based on patch testing, exposure to ginger is one of the most common contact allergens among spices.30 For its use in pregnancy, consumption of ginger is likely safe.30-36 Also, ginger may interact with anticoagulants and antiplatelet drugs, increasing bleeding risk.30,37-39
Sauropus Androgynous
Sauropus androgynus (figure 2) is a widespread shrub in Southeast Asia and South Asia—known as Daun Katuk, Sweet Shoot Leaf, Chakrmanis, and Phak wan ban. This plant is cultivated widely in several Asian countries. This plant can be used for food and medicine.25 There are many chemical constituents in this plant, such as flavonoids, essential oils, papaverine, and steroids.15,25,40 In the leaves, papaverine acts as a vasodilator and steroids, which increases estrogen in providing a lactogenic effect.15,40
 |
Figure 2: Katuk Leaves. Whole Plant (A) and Leaves (B) |
The study was conducted in Indonesia to assess the katuk leaf hexane fraction in improving milk production. This study used 80 rats which were divided into five groups of feed, namely the hexane fraction (0.87%), ethanol fraction (4.53%), ethyl acetate fraction (0.45%), water fraction (3.22%), and control. (without being given any extract). The feed was given according to the test group and was given up to 10 days postpartum. Milk production was measured using the pups’ difference in weight before and after breastfeeding, starting when the pups were four days old until ten days old. There was an increase observed in the hexane fraction group’s total milk production compared to the control. In the water fraction group, total milk production was comparable to that of the control group, whereas for the ethanol and ethyl acetate fraction group, the total milk production was smaller than the control group.40
A study to determine the effect of katuk leaf extract on prolactin and oxytocin gene expression in lactating BALB / C rats was conducted in Indonesia. The study used 24 lactating BALB / C rats, divided into three groups based on feeding, namely young katuk leaf extract, mature katuk leaf extract, and control (not given katuk leaf extract). Katuk leaf extract was given to mice for 12 days of the breastfeeding period. After 12 days, euthanasia was performed in all mice, and the rat pituitary glands were collected. RNA from the rat pituitary gland was analyzed using qRT-PCR to see the prolactin and oxytocin coding genes’ expression. There was an increase in prolactin and oxytocin coding genes in mice given young katuk leaf extract (9.04- and 2.25-fold) compared to the control group. In mice given the mature katuk leaf extract, there was a significant increase in the gene coding for prolactin and oxytocin (15.75- and 25.77-fold) compared to the control group. The authors concluded that the papaverine present in mature katuk leaves increases prolactin and oxytocin genes’ expression.15
Sauropus androgynus is not a completely safe plant for consumption to some extent. Many studies have proved this. Previous studies have shown that excessive consumption of Sauropus androgynus can lead to drowsiness, constipation, and even respiratory failure. Test results on Sprague Dawley rats given dry powder Sauropus androgynus feed (5 g/kg) for 30 days showed that Sauropus androgynus dry powder can damage the liver, kidneys, and lungs depending on a specific dose. Besides, excessive consumption of Sauropus androgynus has been linked to pulmonary disease. In Taiwan in 1995, there was an outbreak of rapidly progressive respiratory dyspnea (Bronchiolitis obliterans) associated with excessive consumption of fresh Sauropus androgynus leaves.25
Musa Paradisiaca
Musa paradisiaca, commonly known as banana, is a herbaceous plant cultivated for fruits, especially in southern Asia.41-43 Also, Musa paradisiaca can be used in medicine.41 Musa paradisiaca is a hybrid of Musa accuminata and Musa balbisiana.44 The parts of Musa Paradisiaca such as fruit, flowers, stem juice, and roots have various health benefits. The part that has a role in increasing breast milk is flowers (figure 3). Musa paradisiaca flowers contain various constituents such as flavonoids, tannins, saponins, and alkaloids.16,45 These constituents play a role in providing the lactogenic effect of this plant by acting as antagonists to dopamine receptors.16
 |
Figure 3: Banana Flowers |
The study was conducted in Malaysia to assess the lactogenic effect of banana flower extract. This study used 20 Spraque Dawley rats divided into four feed groups: petroleum ether extract, ethanol extract, aqueous extract, and control (distilled water). The dose given was the same in each group, 500 mg/kg body weight. Banana flower extract was given to mice from day 5 to day 14 postpartum. Milk production was measured 12 hours after treatment, starting from day 5 to day 15 using the weight-suckle-weight method. This study indicated that the aqueous extract group had the highest average milk production and total milk production on day ten compared to other feed groups. Researchers concluded that the banana flower has the potential to be a galactagogue.16
Side effects of bananas are rare, but consumption in very high doses can cause high blood potassium levels.46 In addition, allergies to bananas are relatively common, especially in sensitive with latex people.47,48 It has been observed that 20-50% of patients allergic to natural rubber latex (NRL) develop symptoms after eating bananas.47
Trigonella Foenum-Graecum
Fenugreek is an annual plant belonging to the Fabaceae family that is cultivated all around the world.49-51 Fenugreek seeds (figure 4) are widely used as galactagogue through a mechanism of sweat production enhancement which is believed to increase milk production since mammary glands are modified apocrine sweat glands.10 Fenugreek contains alkaloids i.e trimethylamine, neurin, trigonelline, and choline; saponins i.e graecunins, trigofenosides A-G, fenugrin B; flavonoids; amino acids; and carbohydrates. Alkaloids, flavonoids, and saponins are the major phytochemicals in fenugreek’s seeds that plays a role in increasing milk production by acting as a dopamine antagonist.51,52
 |
Figure 4: Fenugreek Seeds |
An experiment towards lactating female rats was conducted in Indonesia to assess the lactogenic activity of fenugreek seeds. The female rats were divided into five groups. Group I, II, and III were given 30 mg/200g BW, 100 mg/200g BW, and 300 mg/200g BW dose of fenugreek and moringa extracts (1:1); Group IV was given 2 mL/200g BW of aquades; Group V was treated by 94 mg/200g BW of Maloco. Measurement was conducted on the sixth, eleventh, sixteenth, and twenty-first day of lactation. The final cumulative weight measurement results of groups I, II, III, IV, and V were 174,71; 159,01; 111,40; 139,34 and 116,45 grams, respectively. The highest increase in milk production was seen in the administration of fenugreek seeds extract at a dose of 30 mg/200g BW.53
Another experiment was conducted in Turkey towards twenty-four Anatolian Water Buffaloes that were divided into two groups. Group A was given a diet that contained 50 g/kg of ground fenugreek seeds; Group B was given a diet without ground fenugreek seeds. An electronic pulse-measuring device (Ex-endis, PT-V Pulsatortester) and pressure-measuring device (PT 100 vacuum meter) were used. A pressure of 45 kPa is used by the milking machine, and a pulse rate of 60 pulses per minute. The result shows that the mean daily milk production was higher in group A than group B. The increase in milk production in group A was 0.67 kg/day.54
A randomized, double-blind, placebo-controlled trial was conducted in a maternal hospital in Turkey. Herbal tea containing fenugreek was given to 66 women that were divided into three groups. Group I was given three cups of herbal tea with fenugreek each day; Group II was given 3 cups of herbal tea with apples each day as a placebo; group III with no tea as control. Measurement was conducted on the third postpartum day by measuring maternal milk volume following 15 minutes of electrical pumping. The result shows that Group I had almost double the mean volume of pumped milk (73 mL) compared to the placebo (39 mL) and control group (31 mL).17
Since fenugreek is from the pea family, fenugreek should not be consumed by those who are allergic to peas or other Fabaceae family plants such as soybean and peanuts.55 Fenugreek has been reported could stimulate uterine contractions; therefore, fenugreek should not be consumed during pregnancy.49 Fenugreek is categorized as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA).56 Although, some cases have been reported, such as nausea, diarrhea, heartburn, rhinitis, and fainting.57 Fenugreek should not be consumed along with anticoagulant drugs since it may increase the risk of bleeding. Fenugreek has been reported to have hypoglycemic properties; therefore, monitoring blood glucose level is recommended for people who are consuming hypoglycemic agents concomitantly.58
Cyperus Rotundus Linn.
Java grass’s rhizome (figure 5) is commonly used as a traditional medicine in different parts of the world.59 It belongs to the Cyperaceae family and one of the most popular among the Cyperus genus. Its rhizome’s essential oils contain alkaloids, i.e. rotundines A, B, and C; and flavonoids, i.e. afzelechin, catechin, and quercetin and lueolin; and other components like –sitosterol, cyperene, cyperol, sesquiterpenoids, and vitamin. Alkaloids and flavonoids are its major phytochemicals components, which has been discussed before that these two components contribute to the increase of the milk supply by acting as a dopamine antagonist.60,61
 |
Figure 5: Java Grass |
An experiment was performed in India to examine whether the aqueous extract of Cyperus rotundus rhizome (CRE) can stimulate milk production and mammary gland development in twenty-four lactating dams suckled six pups at the beginning of lactation. Histopathological analysis of pituitary gland and mammary gland were analyzed. Quantitatively, serum prolactin level was calculated. The female rats were divided into four groups. Group I was given 1 ml of 0.9% NaCl orally as control; Group II was given 300 mg/kg BW of CRE; Group III was given 600 mg/kg BW of CRE; Group IV was given 2.5 mg/kg BW of domperidone orally. Milk production was measured from day 3 to day 5 of lactation by weighing the pups daily three times a day. The result shows a higher increase in CRE-treated groups than for the control and domperidone treated group. The milk yield was 0.33, 0.37, 0.42, and 0.54 g/pup/5h for the controls, domperidone treated, those given 300 mg of CRE and 600 mg of CRE over the experimental period, respectively. The daily weight gain was 1.60, 1.79, 2.04, and 2.25 g/pup for the controls, domperidone treated, those given 300 mg of CRE and 600 mg of CRE, respectively. There was about a 67% increase of serum prolactin level in the experimental group of rats receiving 600 mg of CRE than the control group of animals. The rise in prolactin level is believed to contribute to the increase in milk yield in this study. The cortisol level of mother rats receiving 300 and 600 mg of CRE was relatively close with control and domperidone treated groups. Histological findings show differences between CRE-treated mammary tissue, which has more active alveoli than the control group. Moreover, CRE-treated mammary tissue consists of many distended alveoli filled with milk secretion compared to the control group. Administration of 2000 mg/kg BW of aqueous extract does not cause any behavioral alterations or deaths in rate. The author suggests that aqueous extract of Cyperus rotundus is safe and non-toxic.18
Another experiment was conducted in India to evaluate herbal galactagogue ethanol extract’s lactogenic activity towards 24 female Wistar rats divided into six groups based on their feeding. Group I was the control group; Group II was given 100 mg/kg of Asparagus racemosus (AR) extract; Group III was given 100 mg/kg of Musa paradisiaca (MP) extract; Group IV was given 100 mg/kg of Cyperus rotundus (CR) extract; Group V was given 100 mg/kg of Psidium guava (PG) extract; Group VI was given 400 mg/kg of Galactagogue formulation (consist of Asparagus racemosus, Cyperus rotundus, Musa paradisiaca, Psidium guava, Microcrystalline cellulose, Polyvinyl pyrrolidone, sodium benzoate, starch, sodium starch glycolate, talc, and magnesium stearate). Milk production estimation was measured from day 3 to day 15 of lactation. Milk production was estimated 15 hours after gavage. Pups body weight estimation were weighed daily from day 1 to day 15 using an electronic balance (Sartorius basic plus). Milk yield increased from 0.312, 0.300, 0.399, 0.311, 0.349, 0.340 g/pup per day to 2.123, 3.900, 2.770, 2.900, 2.986, 4.900 g/pup per day on the 15th day for the group I (control), group II (AR), group III (MP), group IV (CR), group V (PG), and group VI (Galactagogue formulation) respectively. Bodyweight gain was 8.3454 ± 0.6676, 11.343 ± 1.342, 9.985 ± 1.354, 8.643 ± 1.398, 11.7652 ± 0.7223, 12.899 ± 2.590 gm/pup up to 15 days treatment with the group I, group II, group III, group IV, group V, and group VI respectively. The possibility of increased milk production is due to prolactin stimulation.62
The safety profile of Java grass has not been clearly defined. Adverse effects are rare. In China, the use of this herbal while exercising in patients with “yin” deficiency is warned.63
Pimpinella Anisum
Anise is an annual herb belonging to the Umbelliferae family and is one of the oldest medicinal plants. Apart from Indonesia, it is cultivated in Iran, India, Europe, the Mediterranean region, and many other warm regions globally. About 1.5-5% of essential oils are contained in aniseeds. Aniseeds (figure 6) are used as antibacterial, antifungal, anticonvulsant, digestive, and relief of gastrointestinal spasms. The essential oils of aniseeds contain trans-anethole (93.9%), methyl chavicol, anisaldehyde, coumarins, and polyenes. Trans-anethole as its major component is believed to increase milk supply by acting as a dopamine antagonist.64
 |
Figure 6: Anise Seeds |
A study was conducted in Iran to assess the effect of aqueous and ethanolic extracts of anise seeds on milk production towards thirty lactating dams that suckled four to six pups were divided into five groups with six animals each. Group I was given 0.5 mL of saline orally as the control group; group II was given 0.5 g/kg of aqueous extracts per 0.5 mL saline orally; group III was given 1 g/kg of aqueous extracts per 0.5 mL saline orally; group IV was given 0.5 g/kg of ethanolic extracts per 0.5 saline orally; group V was given 1 g/kg of ethanolic extracts per 0.5 saline orally. The milk production was measured by measuring the pup’s weights from day 3 to day 15 of lactation and was measured 23 hours after gavage. The milk yield changed from 8.09 ± 0.2, 14.25 ± 0.4, 14.87 ± 0.35, 11.1 ± 0.4, and 15.89 ± 0.35 g/pup/day to 7.3 ± 0.71, 15.4 ± 0.7, 13.1 ± 0.29, 12.1 ± 0.7, and 12.41 ± 0.26 g/pup/day for group I, II, III, IV, and V respectively. The pups gained weight during the study period with the aqueous (0.5 and 1 g/kg, p <0.05) and ethanolic (0.5 and 1 g/kg, p <0.01) extracts. Trans-anethole, the major component of anise seeds, is believed to increase milk production since anethole is similar to dopamine and acts as a dopamine antagonist increasing milk production. The authors concluded that anise aqueous and ethanolic extracts could increase milk production.19
Another study was conducted in Egypt to assess the effect of anise seeds and active dry yeast supplements in Egyptian buffaloes on milk yield and milk composition. Twelve pregnant buffaloes at 6 weeks before parturition were divided into four equal groups. Group I was given food mixture without supplements as control group; Group II was given the basal diet plus 50 g/head/day of anise seeds; Group III was given the basal diet plus 20 g/head/day of active dry yeast; Group IV was given the basal diet plus 10 g of active dry yeast plus 25 g of anise seeds/head/day. Buffaloes were hand milked twice daily at 6.00 AM and 4.00 PM from the 5th day of calving until the end of the 15th week. The total milk yields were 540.01, 621.20, 641.88, and 670.86 for group I, II, III, and IV, respectively. The overall daily milk means were 5.14, 2.93, 6.11, and 3.39 kg/hour/day, respectively. Milk composition for group I, II, III, and IV for fat were 5.90%, 6.78%, 6.31%, and 6.62% respectively; for protein were 3.52%, 3.48%, 3.34%, and 3.61%, respectively; and for lactose 4.34%, 4.79%, 4.59%, 4.92%, respectively. The authors concluded that anise seeds could be used to increase milk yield through a mechanism of prolactin induction.65
Evidence from historical use reported that aniseeds had caused stomatitis and cheilitis. Repeated nocturnal tongue angioedema is reported to occur in a hypersensitive Spanish patient after consumption of aniseed liqueur.66 People who are allergic to aniseed may cause occupational rhinoconjunctivitis and food allergy.67 Anethole, as the major component of aniseed, has been reported to cause dermatitis (scaling, erythema, and vesiculation).63 Anise oil may cause interactions with many prescription drugs, pretreatment with aniseed oil may diminish the antidepressant effect of imipramine and fluoxetine. Pretreatment with anise oil may also enhance midazolam-induced motor impairment, increase the analgesic effect of codeine, and augment diazepam’s effect on motor activity.68 Five days pretreatment of mice with 0.3 mg/kg/day of aniseed essential oils significantly decreased the peak plasma of acetaminophen and decreased caffeine’s bioavailability.69
Discussion
Galactagogues are pharmaceutical, food, or herbal supplement agents that can increase milk production. Increasing prolactin through direct stimulation of adenohypophysis or inhibition of prolactin inhibiting hormone is the mechanism of action of most galactagogues.10 However, other mechanisms can occur. In herbal galactagogue, chemical components play a role in providing lactogenic effects. This article has explored six herbal galactagogues such as ginger, katuk leaves, banana, fenugreek, Java grass, and anise. There are several differences in the mechanism of action of the herbal galactagogue (as seen in figure 7). Banana flowers, fenugreek, Java grass, and anise contain saponins, tannins, alkaloids, flavonoids, and anethole, acting as dopamine receptor antagonists, causing an increase in serum prolactin and induce the production of milk.19,70-74 In ginger, the mechanism that occurs is thought to be due to the vasodilator effect of gingerol and shogaol so that the supply of hormones and nutrients for breast milk formation increases.27,28 In katuk leaves, the mechanism of action is to increase the mammary glands’ secretory cells’ population because of their steroid content.40,45 Also, katuk leaves also contain papaverine, which causes vasodilation and inhibits dopamine receptors, increasing milk production.15 Fenugreek is also known to increase milk production through a mechanism of sweat production enhancement since mammary glands are modified apocrine sweat glands.49
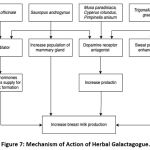 |
Figure 7: Mechanism of Action of Herbal Galactagogue. |
Many health care providers have recommended various types of drugs and herbs to increase milk production when other non-pharmacological methods are insufficient to increase milk production.20,64 However, some providers may inappropriately recommend using galactagogue before evaluating the efficacy and other medical factors involved.10 Moreover, pregnant and breastfeeding women often use herbal galactagogue based on historical use and anecdotal data.75,76 Although the herbal galactagogue uses are widely used by breastfeeding women, our review found that herbal galactagogue has limited reports regarding safety and efficacy data, particularly on the herbs we reviewed. From thirteen13 trials we reviewed, the outcome of these studies assessed breast milk volume, infant weight, serum prolactin and oxytocin, prolactin and oxytocin genes, and mammary gland histology. Thirteen trials showed a positive lactogenic activity of six herbs based on different outcome measurements. However, nine out of thirteen trials were pre-clinical studies. While the gold standard for assessing efficacy is a randomized trial.77 In addition, most clinical trials are herbal combinations, so it is not easy to determine the herbs responsible for galactagogue activity. Therefore, most of the herbs we reviewed cannot be said to be efficacious.
The same as studies on its efficacy, most of the studies we used in this review were pre-clinical studies, so the safety and tolerability data cannot be used as a reference for humans’ safety. Studies for other indications are used to get information about the safety and adverse effects of these herbals. This is done because of the wide potential use of these herbs for both lactation and non-lactation indications. Some data states that ginger consumption during pregnancy is safe. However, it remains controversial because studies say ginger consumption in pregnancy can cause death in infants.30-36 Fenugreek is not recommended to be used during pregnancy because it can cause uterine contraction.49 Therefore, it is important for women who want to use the herbal galactagogue during pregnancy to know the herbal galactagogue’s toxicity, which will be used. Ginger and fenugreek have been reported to increase bleeding risk, so it is important for those taking anticoagulants and antiplatelet drugs to be cautious.30,37-39,58 Excessive consumption of katuk leaves can lead to lung disease, so it is crucial for people suffering from lung disease to be careful.25 Aniseed may decrease the antidepressant effect of imipramine and fluoxetine and decrease acetaminophen and caffeine’s therapeutic efficacy.69 In addition, it is necessary to be careful in consuming ginger because it can irritate the stomach in high doses.30 Therefore, it is necessary to be cautious in using herbs reviewed in this article because of its side effects.
Clinical decisions cannot be made based on this report’s findings regarding which herbs may be used as galactagogues due to limited scientific data on galactagogue herbs. Furthermore, the research on herbs that we reviewed is still in pre-clinical trials and has different measurement units, making it difficult to compare their efficacy. Therefore, more clinical trials are needed, and further studies should use the same measurement units to make it easier for comparison, evaluating effectiveness, and extension into generalizable findings.
The protocol for using pharmaceutical and herbal galactagogues has been published by the Academic of Breastfeeding Medicines (ABM). Due to the lack of scientific evidence supporting the use of herbal agents, several things must be considered before deciding to give galactagogues such as evaluation of the cause of low milk production and maximizing non-pharmacological techniques to increase breast milk supply. Current research on pharmaceutical and herbal galactagogues are still relatively inconclusive and all agents have potential side effects so ABM can’t recommend a specific galactagogue at this time. Suppose a healthcare provider chooses to prescribe a galactagogue after considering the potential risks versus potential benefits of this agent, they should inform the women who will be using the galactagogue about the efficacy data, time of use, duration of therapy, and side effects. Besides, it is necessary to screen the mother for contraindications, allergies, or drug interactions with selected drugs or other substances.20
Conclusion
Six herbs (Zingiber officinale, Sauropus androgynus, Musa paradisiaca, Trigonella foenum-graecum, Cyperus rotundus, and Pimpinella anisum) reviewed in this article have a positive effect as herbs as a galactagogue. However, the trials assessing their efficacy and safety as herbal galactagogues are limited, and the widespread use of herbal galactagogue without strong evidence-based in lactating women. Therefore, more clinical studies are needed to evaluate their effectiveness and safety as galactagogues because of their potential.
Acknowledgement
The authors would like to thank all colleagues in the Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran for all discussion and technical assistance in publishing this review.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
This work was supported by Universitas Padjadjaran, West Java, Indonesia.
References
- Promoting proper feeding for infants and young children. Nutrition [Internet]. 2015;(November 2016):7–9. Available from: http://www.who.int/nutrition/topics/infantfeeding/en/
- Martin C. R, Ling P. R and Blackburn G. L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients. 2016;8(5):1–11.
CrossRef - Victora C. G, Bahl R., Barros AJdD, França GVA, Horton S., Krasevec J., et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet [Internet]. 2016;387(10017):475–90. Available from: http://dx.doi.org/10.1016/S0140-6736(15)01024-7.
CrossRef - Bhandari N., Chowdhury R. Infant and young child feeding. Proc Indian Natl Sci Acad. 2016;82(5):1507–17.
CrossRef - info DATIN (Pusat Data dan Informasi Kementrian RI). Kementeri Kesehat RI. 2018;1–7.
- Increasing Commitment To Breastfeeding Through Funding and Improved Policies and Programmes. Glob Breastfeed Scorec [Internet]. 2019;(3):4. Available from: https://www.who.int/nutrition/publications/infantfeeding/global-bf-scorecard-2019/en/
- Mannion C., Mansell D. Breastfeeding Self-Efficacy and the Use of Prescription Medication: A Pilot Study. Obstet Gynecol Int. 2012;2012:1–8.
CrossRef - Zuppa A.A, Sindico P., Orchi C., Carducci C., Cardiello V., Romagnoli C., et al. Safety and efficacy of galactogogues: Substances that induce, maintain and increase breast milk production. J Pharm Pharm Sci. 2010;13(2):162–74.
CrossRef - Sjölin S., Hofvander Y. and Hillervik C. Factors related to early termination of breast feeding. A retrospective study in Acta Paediatr Scand. 1977 Jul;66(4):505–11.
CrossRef - Gabay M.P. Galactogogues: Medications That Induce Lactation. J Hum Lact. 2002;18(3):274–9.
CrossRef - Horseman N.D, Gregerson K.A. Prolactin [Internet]. Seventh Ed. Vols. 1–2, Endocrinology: Adult and Pediatric. Elsevier; 2015. 91-103.e4 p. Available from: http://dx.doi.org/10.1016/B978-0-323-18907-1.00006-8.
CrossRef - WHO Global report on traditional and complementary medicine 2019 [Internet]. World Health Organization. 2019. 1–228 p. Available from: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?ua=1
- Kemenkes RI. Formularium Ramuan Obat Tradisional Indonesia. 2017;7(1):45–56.
- Ushiroyama T., Sakuma K., Souen H., Nakai G., Morishima S., Yamashita Y., et al. Xiong-gui-tiao-xue-yin (Kyuki-chouketsu-in), a traditional herbal medicine, stimulates lactation with increase in secretion of prolactin but not oxytocin in the postpartum period. Am J Chin Med. 2007;35(2):195–202.
CrossRef - Soka S., Alam H., Boenjamin N., Agustina T. W, Suhartono M. T. Effect of Sauropus androgynus leaf extracts on the expression of prolactin and oxytocin genes in lactating BALB/C Mice. J Nutrigenet Nutrigenomics. 2010;3(1):31–6.
CrossRef - Mahmood A., Omar M. N, Ngah N. Galactagogue effects of Musa x paradisiaca flower extract on lactating rats. Asian Pac J Trop Med [Internet]. 2012;5(11):882–6. Available from: http://dx.doi.org/10.1016/S1995-7645(12)60164-3.
CrossRef - Turkyilmaz C., Onal E., Hirfanoglu I. M, Turan O., Koç E., Ergenekon E., et al. The effect of galactagogue herbal tea on breast milk production and short-term catch-up of birth weight in the first week of life. J Altern Complement Med. 2011;17(2):139–42.
CrossRef - Badgujar S. B, Bandivdekar A. H. Evaluation of a lactogenic activity of an aqueous extract of Cyperus rotundus Linn. J Ethnopharmacol [Internet]. 2015;163:39–42. Available from: http://dx.doi.org/10.1016/j.jep.2015.01.019.
CrossRef - Hosseinzadeh H., Tafaghodi M., Abedzadeh S., Taghiabadi E. Effect of aqueous and ethanolic extracts of Pimpinella anisum L. seeds on milk production in rats. JAMS J Acupunct Meridian Stud [Internet]. 2014;7(4):211–6. Available from: http://dx.doi.org/10.1016/j.jams.2013.10.004.
CrossRef - Brodribb W. ABM Clinical Protocol #9: Use of Galactogogues in Initiating or Augmenting Maternal Milk Production, Second Revision 2018. Breastfeed Med. 2018;13(5):307–14.
CrossRef - Lynch N., Berry D. Differences in perceived risks and benefits of herbal, over-the-counter conventional, and prescribed conventional, medicines, and the implications of this for the safe and effective use of herbal products. Complement Ther Med. 2007;15(2):84–91.
CrossRef - McNeely J. A. The convention on biological diversity. Encycl Anthr. 2017;1–5:321–6.
CrossRef - Erwin, Nur M., Panggabean A., Tantalum P. K, Oksida N., Bangka T., et al. Info Komoditi. GANENDRA Maj IPTEK Nukl. 2016;7(1):41–50.
- Indonesia BPS. Statistics of Medicinal Plants Indonesia. 2017;81.
- Zhang B dou, Cheng J xin, Zhang C feng, Bai Y dan, Liu W yuan, Li W, et al. Sauropus androgynus L. Merr.-A phytochemical, pharmacological and toxicological review. J Ethnopharmacol [Internet]. 2020;257. Available from: https://doi.org/10.1016/j.jep.2020.112778.
CrossRef - Outlook Komoditas Pisang. Komod Pertan Sub Sekt Hortik. 2016;19(7):28.
- Ali B. H, Blunden G., Tanira M. O and Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46(2):409–20.
CrossRef - Paritakul P., Ruangrongmorakot K., Laosooksathit W., Suksamarnwong M. and Puapornpong P. The effect of ginger on breast milk volume in the early postpartum period: A randomized, double-blind controlled trial. Breastfeed Med. 2016;11(7):361–5.
CrossRef - Bumrungpert A., Somboonpanyakul P., Pavadhgul P. and Thaninthranon S. Effects of fenugreek, ginger, and turmeric supplementation on human milk volume and nutrient content in breastfeeding mothers: A randomized double-blind controlled trial. Breastfeed Med. 2018;13(10):645–50.
CrossRef - Twain M. Chapter 68. Roughing It. 2020;466–74.
CrossRef - Borrelli F., Capasso R., Aviello G., Pittler M. H and Izzo A. A. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;105(4):849–56.
CrossRef - Stanisiere J., Mousset P. Y, Lafay S. How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods. 2018;7(4).
CrossRef - Ensiyeh J., Sakineh MAC. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery [Internet]. 2009;25(6):649–53. Available from: http://dx.doi.org/10.1016/j.midw.2007.10.013.
CrossRef - Haji Seid Javadi E, Salehi F. and Mashrabi O. Comparing the Effectiveness of Vitamin B6 and Ginger in Treatment of Pregnancy-Induced Nausea and Vomiting. Obstet Gynecol Int. 2013;2013:1–4.
CrossRef - Matthews A., Haas D.M, O’Mathúna D.P and Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2015;2015(9).
CrossRef - Heitmann K., Nordeng H. and Holst L. Safety of ginger use in pregnancy: Results from a large population-based cohort study. Eur J Clin Pharmacol. 2013;69(2):269–77.
CrossRef - Jiang X, Blair EYL and McLachlan A. J. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: A population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol. 2006;46(11):1370–8.
CrossRef - Marx W., McKavanagh D., McCarthy A. L, Bird R., Ried K., Chan A., et al. The effect of ginger (Zingiber officinale) on platelet aggregation: A systematic literature review. PLoS One. 2015;10(10):1–13.
CrossRef - Jiang X, Williams K. M, Liauw W. S, Ammit A. J, Roufogalis B. D, Duke C. C, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59(4):425–32.
CrossRef - Agik, Kusumorini N. and Arita SED. Fraksi Heksan Daun Katuk Sebagai Obat Untuk Memperbaiki Produksi Susu, Penampilan Induk, dan Anak Tikus (HEXANE. J Vet [Internet]. 2015;16(1):88–95. Available from: https://ojs.unud.ac.id/index.php/jvet/article/view/13326
- Imam M. Z, Akter S. Musa paradisiaca l. and musa sapientum l. : A phytochemical and pharmacological review. J Appl Pharm Sci. 2011;1(5):14–20.
- Vilhena R. O, Figueiredo I. D, Baviera A. M, Silva D. B, Marson B. M, Oliveira J. A, et al. Antidiabetic activity of Musa x paradisiaca extracts in streptozotocin-induced diabetic rats and chemical characterization by HPLC-DAD-MS. J Ethnopharmacol [Internet]. 2020;254:112666. Available from: https://doi.org/10.1016/j.jep.2020.112666.
CrossRef - Arun K. B, Madhavan A., Reshmitha T. R, Thomas S. and Nisha P. Musa paradisiaca inflorescence induces human colon cancer cell death by modulating cascades of transcriptional events. Food Funct. 2018;9(1):511–24.
CrossRef - Jang D. S, Park E. J, Hawthorne M. E, Vigo J. S, Graham J. G, Cabieses F., et al. Constituents of Musa × paradisiaca Cultivar with the Potential To Induce the Phase II Enzyme, Quinone Reductase – Journal of Agricultural and Food Chemistry (ACS Publications). 2002;6330–4.
CrossRef - Mahmood A., Ngah N. and Omar M. N. Phytochemicals constituent and antioxidant activities in Musa x paradisiaca flower. Eur J Sci Res. 2011;66(2):311–8.
- Tazoe M., Narita M., Sakuta R., Nagai T., Narita N. Hyperkalemia and hyperdopaminemia induced by an obsessive eating of banana in an anorexia nervosa adolescent. Brain Dev. 2007;29(6):369–72.
CrossRef - Hassan AKG, Venkatesh Y. P. An overview of fruit allergy and the causative allergens. Eur Ann Allergy Clin Immunol. 2015;47(6):180–7.
- Egger M., Mutschlechner S., Wopfner N., Gadermaier G., Briza P., Ferreira F. Pollen-food syndromes associated with weed pollinosis: An update from the molecular point of view. Allergy Eur J Allergy Clin Immunol. 2006;61(4):461–76.
CrossRef - Zapantis A., Steinberg J. G and Schilit L. Use of herbals as galactagogues. J Pharm Pract. 2012;25(2):222–31.
CrossRef - Khan T. M, Wu DBC and Dolzhenko A. V. Effectiveness of fenugreek as a galactagogue: A network meta-analysis. Phyther Res. 2018;32(3):402–12.
CrossRef - Mandegary A., Pournamdari M., Sharififar F., Pournourmohammadi S., Fardiar R. and Shooli S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem Toxicol [Internet]. 2012;50(7):2503–7. Available from: http://dx.doi.org/10.1016/j.fct.2012.04.020.
CrossRef - Yadav R., Kaushik R. A study of phytochemical constituents and pharmacological actions of trigonella foenum-graecum: A review. Int J Pharm Technol. 2011;3(2):1022–8.
- Widowati L., Isnawati A., Alegantina S. and Retiaty F. Potensi Ramuan Ekstrak Biji Klabet dan Daun Kelor sebagai Laktagogum dengan Nilai Gizi Tinggi. Media Penelit dan Pengemb Kesehat. 2019;29(2):143–52.
CrossRef - Degirmencioglu T., Unal H., Ozbilgin S. and Kuraloglu H. Effect of ground fenugreek seeds (Trigonella foenum-graecum) on feed consumption and milk performance in Anatolian water buffaloes. Arch Anim Breed. 2016;59(3):345–9.
CrossRef - Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US); 2006-. Fenugreek. [Updated 2021 Feb 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501779/
- Food and Drug Administration. Code of Federal Regulations Title 21 [Internet]. 2014. p. 5–6. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20
- Şahin B., Kaymaz N., Yıldırım Ş. Herbal remedies for perceived inadequate milk supply are perhaps not as safe as women think: A brief case report. Women and Birth. 2016;29(6):e133.
CrossRef - Anderson P. O. Herbal Use during Breastfeeding. Breastfeed Med. 2017;12(9):507–9.
CrossRef - Mannarreddy P., Denis M., Munireddy D., Pandurangan R., Thangavelu K. P and Venkatesan K. Cytotoxic effect of Cyperus rotundus rhizome extract on human cancer cell lines. Biomed Pharmacother [Internet]. 2017;95(April):1375–87. Available from: http://dx.doi.org/10.1016/j.biopha.2017.09.051.
CrossRef - Pirzada A. M, Ali H. H, Naeem M., Latif M., Bukhari A. H and Tanveer A. Cyperus rotundus L.: Traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol [Internet]. 2015;174:540–60. Available from: http://dx.doi.org/10.1016/j.jep.2015.08.012.
CrossRef - Sonwa M. M, König W. A. Chemical study of the essential oil of Cyperus rotundus. Phytochemistry. 2001;58(5):799–810.
CrossRef - Shanish Antony A, Nehru Sai Suresh C, Vijaya Sreedhar S, Yalla BR and Kosaraju J. Formulation and evaluation of a polyherbal formulation (lacto-4) for its galactogogue activity in wistar rats. Int J PharmTech Res. 2012;4(2):554–60.
- Akbar S. Handbook of 200 Medicinal Plants. Handbook of 200 Medicinal Plants. 2020.
- Shojaii A, Abdollahi Fard M. Review of Pharmacological Properties and Chemical Constituents of Pimpinella anisum. ISRN Pharm. 2012;2012:1–8.
CrossRef - Sallam M. T, El Barody M. A, Abd-Allah M. and Tawfik M. H. Effect of Anise Seeds (Pimpinella anisum L) and Active Dry Yeast (Saccharomyces cerevisiae) Supplements as Feed Additives on the Productive Performance of Lactating Egyptian Buffaloes. Egyptian Journal of Nutrition and Feeds. 2018;21:583–92.
CrossRef - Gázquez García V, Gaig Jané Pand Bartolomé Zavala B. Aniseed-induced nocturnal tongue angioedema. J Investig Allergol Clin Immunol. 2007;17(6):406–8.
- García-González JJ, Bartolomé-Zavala B, Fernández-Meléndez S, Barceló-Muñoz J. M, Miranda Páez A, Carmona-Bueno M. J, et al. Occupational rhinoconjunctivitis and food allergy because of aniseed sensitization. Ann allergy, asthma Immunol Off Publ Am Coll Allergy, Asthma, Immunol. 2002 May;88(5):518–22.
CrossRef - Samojlik I, Mijatović V, Petković S, Škrbić B and Božin B. The influence of essential oil of aniseed (Pimpinella anisum, L.) on drug effects on the central nervous system. Fitoterapia. 2012;83(8):1466–73.
CrossRef - Samojlik I, Petković S, Stilinović N, Vukmirović S, Mijatović V and Božin B. Pharmacokinetic Herb-Drug Interaction between Essential Oil of Aniseed (Pimpinella anisum L., Apiaceae) and Acetaminophen and Caffeine: A Potential Risk for Clinical Practice. Phytother Res. 2016 Feb;30(2):253–9.
CrossRef - Javan R., Javadi B. and Feyzabadi Z. Breastfeeding: A review of its physiology and galactogogue plants in view of traditional Persian medicine. Breastfeed Med. 2017;12(7).
CrossRef - Buntuchai G., Pavadhgul P., Kittipichai W. and Satheannoppakao W. Traditional Galactagogue Foods and Their Connection to Human Milk Volume in Thai Breastfeeding Mothers. J Hum Lact. 2017;33(3):552–9.
CrossRef - Sahoo H., Mandal .P, Sagar R. and Bhattamisra S. Evaluation of lactogenic activity of Triumfetta rhomboidea L. root: Validating its traditional usage. J Exp Integr Med. 2016;6(1):26.
CrossRef - Majeedi S. F, Roqaiya M., Begum W. andKhan A. A. A Review on Galactogogue Herbs of Unani Medicine. Int J Herb Med. 2015;4(2):112–5.
- Badgujar S. B, Patel V. V. and Bandivdekar A. H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014.
CrossRef - Low Dog T. The use of botanicals during pregnancy and lactation. Altern Ther Health Med. 2009;15(1):54–8.
- Forinash A. B, Yancey A. M, Barnes K. N and Myles T. D. The Use of Galactagogues in the Breastfeeding Mother. Ann Pharmacother. 2012;46(10):1392–404.
CrossRef - Hariton E., Locascio J. J. Randomised controlled trials—the gold standard for effectiveness. HHS Public Access. 2018;125(13):1–4.
CrossRef








