Fatina I Fadel1 , Abeer M Nour ElDin Abd ElBaky2
, Abeer M Nour ElDin Abd ElBaky2 , Mohamed A Abdel Mawla2
, Mohamed A Abdel Mawla2 , Wesam I Moustafa3
, Wesam I Moustafa3 , Gamal Eldin Saadi4
, Gamal Eldin Saadi4 and Doaa M Salah1
and Doaa M Salah1 ,
,
1Department of Pediatrics, Cairo University, Cairo, Egypt
2Department of Pediatrics, National Research Center, Giza, Egypt
3Department of Pathology, Bani Suef University, BaniSuef, Egypt
4Department of Internal Medicine, Cairo University, Cairo, Egypt
Corresponding Author E-mail: drmohamedahmed85@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2218
Abstract
Background: By the time of histological confirmation of rejection is achieved, renal scarring may for treatment as a realistic option . This study aims to study the subclinical pathological graft data and to evaluate the histopathological impact of different immunosuppression protocols in pediatric renal transplant recipients. Methods: This is a case series that included twenty living donor renal transplant recipients. All included cases received the classic triple immunotherapy for at least one month post-transplantation [Steroids, calconurine inhibitors (CNI), and mycofenlolic mofetile (MMF)]. Based on their immunological risk stratification; included cases were divided into 2 groups: group (A) continued on CNI based triple therapy protocol; group (B) shifted to evirolimus /low dose CNI protocol. Surveillance biopsies were done for all cases at one and four month post-transplantation. Results: One and four month biopsies revealed subclinical rejection (including borderline changes) in 4 (20%) cases and 6 (30%) cases respectively. The number of patients received tacrolimus/MMF therapy significantly increased (p=0.02) while that of patients on everloimus/low dose CNI significantly decreased (p=0.014) due to drug modifications based on four month surveillance biopsy data. Conclusion: Subclinical rejection is not uncommon in pediatric renal graft recipients which makes surveillance biopsy might be of help. Early usage of evirolimus/low CNI protocol is associated with higher rejection rate than triple therapy.
Keywords
Immunosuppression; Pediatric Transplantation; Surveillance biopsy
Download this article as:| Copy the following to cite this article: Fadel F. I, ElBaky A. M. N. E. A, Mawla M. A. A. , Moustafa W. I, Saadi G. E, Salah D. M. Subclinical Rejection and Immunosuppression in Pediatric Kidney Transplant Recipients : Single Centre Study. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Fadel F. I, ElBaky A. M. N. E. A, Mawla M. A. A. , Moustafa W. I, Saadi G. E, Salah D. M. Subclinical Rejection and Immunosuppression in Pediatric Kidney Transplant Recipients : Single Centre Study. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3nZTagL |
Introduction
Kidney transplantation (KT) is the gold standard treatment for end stage renal disease (ESRD) pediatric patients 1. Despite the progression in the field of immunosuppressive (IS) treatment, renal allograft dysfunction is still common after KT which may be due to acute rejection, chronic rejection, CNI toxicity, infections and recurrence of original renal disease 2.
The identification of an ideal IS regimen should safely prolong graft survival and minimizes side effects. This is very important in pediatrics as the IS regimens have an additional critical impact on normal growth and development 3. The mammalian target of the rapamycin inhibitors (m-TORI) offers an alternative intriguing therapeutic option to CNI, as prophylaxis of acute and chronic rejection 4]. m- TORI act synergistically and facilitate the use of lower CNI doses. This not only reduces the risk of the potential side effects of CNI but may also improve long-term graft outcome because, unlike other IS drugs, m-TORI can prevent the proliferation of vascular muscle and cancer cells 5,6
Surveillance biopsy ‘protocol biopsy,’ is defined as the sampling of renal tissue in patients with normal graft function at predetermined time points 7, typically between 1-12 months post-transplantation (post-TX). Protocol biopsies have been a useful diagnostic tool for the detection of subclinical rejection (SCR) and early chronic rejection, primarily interstitial fibrosis and tubular atrophy (IF/TA) [8-12]. However, being an invasive procedure, surveillance biopsy needs further assessment as regard efficacy on long and short term graft survival.
SCR has been increasingly recognized in adult kidney transplant recipients owing to the advent of surveillance biopsies. In children, however, surveillance biopsies are not routinely performed at most centers. That makes, the incidence, treatment options, and clinical outcomes of SCR remain unclear in children 8.
The aim of this study is to identify the subclinical histopathological graft data in pediatric kidney transplant recipients by doing early surveillance biopsy at 1 month and follow up at 4 month post-TX. Also we aimed to evaluate and compare the impact of different IS drugs on the graft by identifying the histological changes in surveillance biopsies.
Material and Methods
This is a case series (20 patients with 40 biopsies) that was conducted during the period between January 2015 and May 2017. The study included 20 subjects; all were transplanted at Kidney Transplantation Unit and are followed up at Kidney Transplantation Outpatient Clinic, Cairo University Children Hospital (Abo El Reech). The study protocol was approved by the Research Committee of Pediatric Department, Faculty of Medicine Cairo University and was performed in accordance with the Declaration of Helsinki. An informed consent was obtained from guardians of cases before their inclusion to the study.
Cases were included in the study according to the following criteria; a) Age between 6 and 18 years. b) Patients following up for at least four month post-TX. c) Cases either received antibody induction therapy [antithymocyte globuline (ATG) or Basiliximabe], or not according to immunological risk stratification. d) Transplant recipients received classic CNI based triple therapy protocol in the form of: CNI, MMF and steroids for at least 1 month post-transplantation. Cases whose weight less than 10 kg or those who were planned for referral (i.e. will be followed up for duration less than four months post-TX) were excluded from the study
Data of included cases
Full clinical assessment focusing on age of diagnosis, original renal disease, onset of renal replacement therapy (RRT), family history, weight, height, and body mass index. Laboratory investigations including: serum creatinine and trough level of immunotherapy. Perioperative circumstances including surgical complications, early graft function, and initial IS regimen. Course of graft function during follow up including Biopsy proven acute rejection (BPAR) episodes, and the need for modulating IS drugs.
Immunosuppression regimens
Eighteen patients received induction therapy with either ATG (5-9mg/kg) or basiliximab (20mg/dose) at day 0 and day 4 (2 cases received no antibody induction). Methylprednisolone started in the night before the operation at a dose of 150-250 mg/m2 then given in similar doses intraoperative at induction of anesthesia, at de clamping of graft vessels and 6 hours postoperative, then tapered gradually to oral steroids. MMF was given in a dose of 800-1200 mg/m2 three days before the operation. Cyclosporine (CsA) was given initially to all cases at the day of operation in a dose of 8-10mg/kg/day. Cases shifted to tacrolimus during their follow up received a dose of 0.12-0.15mg/kg/day.
All included cases received the classic triple CNI based immunotherapy for at least one month (steroids, CNI and MMF).The patients performed surveillance biopsy one month post transplantation, and then were subdivided into two groups based on their immunological risk stratification
Low immunological risk group not known to have original high risk disease {as congenital disorders of renal and lower urinary tract development, nephronophthisis and obstructive uropathy….ext}, first transplant, low panel reactive antibody (PRA) levels {<20%} and donor recipient mismatch not more than 3 out of 6 HLA alleles)
High immunological risk group including sensitized patients with high PRA levels {≥20%}, previous repeatedly positive cross match by lymphocytotoxcity, more than three out of six HLA alleles mismatch and history of a previous renal transplant)
The first group was (i.e. low immunological risk) switched to m-TORI (everolimus) (1.2-1.6 mg/m2) with low CNI (5mg/kg/day) and MMF was discontinued. While the second group continued on CNI and MMF. Results of the biopsy were compared between both groups three months later. Based on pathological findings detected in surveillance biopsy at 4 month post-TX, IS drugs were modified with shift of some cases from m-TORI / low CNI protocol back to the classic triple CNI based protocol and shift of some cases from CsA to tacrolimus.
Surveillance biopsies
The patients had their blood pressure controlled (below the 95th centile for age, sex and height with medications). The blood coagulation profile, complete blood picture as well as bleeding time were normal at the time of biopsy. In addition, the allografts were evaluated by renal ultrasound. Post biopsy monitoring of urine output and blood pressure was performed with no documented biopsy related complications of the studied cases.
Core Tissue Biopsy needles (18 Gauge) were used. Pre-biopsy and during biopsy the distance between the lower kidney pole (the destined localization of biopsy) and the skin was determined. Afterwards the puncture site was labeled on the skin. After disinfection, sterile covering, anesthesia of the skin and a small puncture incision, puncture was performed with the ultrasound transducer (in sterile cover) in parallel with the puncture direction. The needle was continued to advance until it touches the lower pole of the kidney, depth of biopsy was aligned on the measured skin kidney pole distance. Afterwards kidney was punctured and the containing material was determined usually the needle was positioned in the convex lateral border in the superior pole. Post-biopsy hematuria was monitored by the visual inspection of urine samples on three separate occasions 13.
Biopsies were evaluated and scored according to the criteria of Banff classification of allograft rejection. Pathological data including glomerular, tubular, interstitial, and vascular findings were documented. All biopsies were stained for C4d by immunohistochemistry.
Statistical analysis
Data were tabulated and subjected to computer-assisted statistical analysis using Statistical Package for Social Science (SPSS) version 16.0. Nominal data were described as frequency and percentage and compared using Chi Square tests. Numerical data were described as mean and standard deviation and compared using t tests. Non-parametric data were described as median and interquartile range and were compared using Mann Whitney test. Numerical associations were tested using Pearson `s correlations. P values less than 0.05 were considered significant.
Results
Twenty renal transplant recipients were enrolled in the study. The mean age of included cases was 11.3 ± 3.5 years. Recipient male /female ratio was 15/5 while that of donors was 6/14. Eight cases (40%) were products of consanguineous marriage. Demographic, clinical and laboratory data of the studied cases are summarized in table 1.
Table 1: Demographic, clinical & laboratory data of the study group (n=20)
| Contant | Mean ±SD |
| Age at transplantation (years) | 11.3 ± 3.5 |
| Donor age at transplantation (years) | 36.9 ± 8.8 |
| Duration of F/U before RRT (years) | 2.7 ± 0.8 |
| Duration of RRT (years) | 2.9 ± 3.2 |
| Creatinine at discharge (mg/dl) | 0.61 ± 0.11 |
| Creatinine after 1 month (mg/dl) | 0.6 ± 0.12 |
| CsA trough level at discharge (ng/ml) | 240.1 ± 73.2 |
| CsA trough level at 1 month ( ng/ml) | 221.1 ± 45.2 |
| Initial Everolimus trough level ( ng/ml) | 7.1 ± 2.2 |
| FK trough level at 1 month ( ng/ml) | 9.5 ± 2.1 |
| Creatinine after 4 month (mg/dl) | 0.8 ± 0.14 |
| CsA trough level ( ng/ml) | 180 ± 73.2 |
| Everolimus trough level ( ng/ml) | 6.1 ± 2.9 |
| FK trough level ( ng/ml) | 7.2 ± 2.1 |
F/U (follow up), RRT (renal replacement therapy), D (donor), R (recipient), CsA (cyslosporine), FK (tacrolimus)
All included cases received living related renal graft except one case (living unrelated). Inherited nephropathy (IN) represented the etiology of ESRD in 40% of cases [6 cases with focal segmental glomerulosclerosis (FSGS), 1 case with Joubert syndrome and 1 case with caroli disease]. Three FSGS cases were sporadic while the other 3 had family history but were not subjected to genetic analysis due to its unavailability in current practice at time of diagnosis. Bilateral atrophic kidneys were the cause of ESRD in 25% of cases followed by neurogenic bladder as regard frequency of the study group [15%] (figure 1).
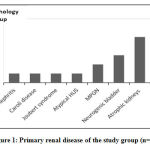 |
Figure 1: Primary renal disease of the study group (n=20). |
Two (10%) cases didn’t receive antibody induction therapy [one of them had low immunological risk (HLA typing revealed zero donor/recipient mismatch) while the other was planned to receive ATG but developed severe bronchospasm with induction of anesthesia]. All cases started maintenance immunosuppression postoperatively with the classic triple therapy protocol for at least one month post-TX (steroids, CNI and mycofenolate). All cases received cyclosporine [with mean initial cyclosporine trough level 240.1 ± 73.2 ng/ml], 65% of cases received MMF, while 35% received enteric coated mycofenolic acid. All cases had immediate excellent graft function (adequate urine output and gradual decline of serum creatinine) with normal graft ultrasound and Doppler imaging. Serum creatinine at hospital discharge
postoperatively was 0.61 ± 0.11mg/dl. During the four months follow up duration of the studied cases, no major infections or immunotherapy associated side effects were noted.
Surveillance biopsies and immunosuppression modification
Surveillance graft biopsies taken at 1 month post-TX were completely normal in 9 (45%) cases. Acute and chronic scoring system based on Banff classification is illustrated by table 2. Subclinical changes were found in 4 (20%) cases (2 cases with SCR and 2 cases with borderline changes), five (25%) cases had CNI associated adverse effects (table 3). Only one biopsy showed +ve C4d staining. Based on these pathological findings; 4 cases received antirejection therapy, 2 cases were planned to be shifted to m-TOR based regimen (low immunological risk) continued on same triple therapy protocol, 5 cases were shifted from CsA to tacrolimus, and 1 case was shifted from CsA to tacrloimus after antirejection therapy (figure 2,3)
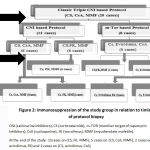 |
Figure 2: immunosuppression of the study group in relation to timing of protocol biopsy |
Table 2: Acute & chronic score of biopsies at 1 and 4 months post-transplantation
| Acute score at 1 month no (%) | Chronic score at 1 month no (%) | ||||||||
| T | 0 | 13 (65%) | Ct | 0 | 17 (85) | ||||
| 1 | 1 (5%) | 1 | 0 (0%) | ||||||
| 2 | 3 (15%) | 2 | 0 (0%) | ||||||
| I | 0 | 16 (80%) | Ci | 0 | 17 (85%) | ||||
| 1 | 1 (5%) | 1 | 0 (0%) | ||||||
| 2 | 0 (0%) | 2 | 0 (0%) | ||||||
| V | 0 | 17 (85%) | Cv | 0 | 17 (85%) | ||||
| 1 | 0 (0%) | 1 | 0 (0%) | ||||||
| 2 | 0 (0%) | 2 | 0 (0%) | ||||||
| G | 0 | 17 (85%) | Cg | 0 | 17 (85%) | ||||
| 1 | 0 (0%) | 1 | 0 (0%) | ||||||
| 2 | 0 (0%) | 2 | 0 (0%) | ||||||
| Acute score at 4 months no (%) | Chronic score at 4 months no (%) | ||||||||
| T | 0 | 13 (65%) | Ct | 0 | 15 (75%) | ||||
| 1 | 1 (5%) | 1 | 1 (5%) | ||||||
| 2 | 2 (10%) | 2 | 0 (0%) | ||||||
| I | 0 | 14 (70%) | Ci | 0 | 15 (75%) | ||||
| 1 | 2 (10%) | 1 | 1 (5%) | ||||||
| 2 | 0 (0%) | 2 | 0 (0%) | ||||||
| V | 0 | 16 (80%) | Cv | 0 | 15 (75%) | ||||
| 1 | 0 (0%) | 1 | 1 (5%) | ||||||
| 2 | 0 (0%) | 2 | 0 (0%) | ||||||
| G | 0 | 13 (65%) | G | 0 | 16 (80%) | ||||
| 1 | 2 (10%) | 1 | 0 (0%) | ||||||
| 2 | 1 (5%) | 2 | 0 (0%) | ||||||
Three biopsies at 1 month & four biopsies at 4 months could not be scored
Acute changes:
g: (Glomelular changes) 0, 1, 2, 3 No, mild, moderate, severe glomerulitis.
i: (interstitial changes) 0, 1, 2, 3 No, mild, moderate, severe interstitial mononuclear cell infiltration .
t: (tubular changes) 0, 1, 2, 3 No, mild, moderate, severe tubulitis .
v: (vascular changes) 0, 1, 2, 3 No, mild, moderate, severe intimal arteritis
Acute score is the sum of acute changes
Chronic changes
cg: (chronic glomerular changes) 0, 1, 2, 3 No, mild, moderate, severe chronic transplant glomerulopathy.
ci: (chronic interstitial changes) 0, 1, 2, 3 No, mild, moderate, severe interstitial fibrosis.
ct: (chronic tubular changes) 0, 1, 2, 3 No, mild, moderate, severe tubular atrophy and loss .
cv: (chronic vascular) 0, 1, 2, 3 No, mild, moderate, severe fibrous intimal thickening.
Chronic score is the sum of chronic changes
Surveillance graft biopsies were done four month post transplantation as follow up pathological data after modification of their immunosuppression protocol (table 2). C4d staining results after 4 month was negative in18 biopsies while sample was inadequate for staining in 2 biopsies. Based on these pathological findings; 6 cases received antirejection therapy, 4 cases shifted from m-TOR based regimen to classic triple therapy, 2 cases shifted from evirolimus, CsA to evirolimus, tacrolimus (figure 2,3). Clinico-pathological interpretation of graft biopsies at 1 & 4 months post-transplantation is illustrated by table 3.
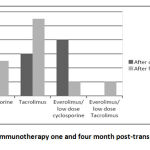 |
Figure 3: Immunotherapy one and four month post-transplantation. |
Table 3: Clinico-pathological interpretation of graft biopsies at 1 and 4 months
| One month biopsies | N (%) |
| Normal | 9 (45%) |
| Acute SCR
Borderline SCR |
2 ( 10%)
2 ( 10%) |
| Acute CNI toxicity | 5 (25%) |
| Sclerosing arteriopathy
Denovo FSGS |
1 (5%)
1 (5%) |
| Four month biopsies | N (%) |
| Inadequate (medulla) | 1(5%) |
| Normal | 7 (35%) |
| Acute AMR | 3 (15%) |
| Chronic active subclinical TCMR | 1 (5%) |
| Chronic active AMR with subclinical TCMR | 1 (5%) |
| IFTA (I), borderline SCR | 1 (5%) |
| Acute tubular injury | 2 (10%) |
| Membranous Pattern of glomerular injury | 1 (5%) |
| IFTA (I), ,tubular injury | 3 (15%) |
SCR (subclinical rejection), CNI (calcinurine inhibitors), FSGS (focal segmental glomerulosclerosis), AMR (antibody mediated rejection), TCMR (T cell mediated rejection), IFTA (interstial fibrosis/tubular atrophy).
By comparing the immunotherapy status of the studied group one and four months post-transplantation, we found that the number of the patients receiving tacrolimus had significantly increased (p = 0.02) while the number of the patients on everloimus/low dose CsA had significantly decreased (p = 0.014) (table 4). Comparison effect of different immunosuppression on acute at 1 & 4 months post-transplantation is illustrated by (table 5)
Table 4 : comparing the immunotherapy status of the studied group one and four months post-transplantation
| Immunotherapy | One Month | Four Month | P value |
| N of cases on Cyclosporine | 3 | 2 | 0.605 |
| N of cases on Tacrolimus | 1 | 7 | 0.006* |
| N of cases on Everolimus | 6 | 1 | 0.019* |
Table 5: Effect of different immunosuppression on acute changes of graft biopsy
| Cyclosporine
(n=6)
|
P value | Tacrolimus
(n=6) |
P value |
Evirolimus
(n=8) |
P value |
|||||
| 1 month
N (%) |
4 month
N (%) |
1 month
N (%) |
4 month
N (%) |
1 month
N (%) |
4 month
N (%) |
|||||
| No score | 2 (33) | 1(17) | 0.5 | 1(17) | 1(17) | 1 | 0 (0) | 2 (25) | 0.4 | |
| G | 0 | 4 (67) | 4 (66) | 1 | 5(83) | 5(83) | 1 | 8 (100%) | 3 (37.5%) | 0.1 |
| 1 | 0(0) | 1(17) | – | 0(0) | 0(0) | – | 0 (0%) | 3 (37.5%) | 0.18 | |
| 2 | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0 (0%) | 0 (0%) | – | |
| T | 0 | 3(50) | 4(66) | 0.7 | 2(33) | 5(83) | 0.25 | 8 (100%) | 3 (37.5%) | 0.1 |
| 1 | 1(17) | 1(17) | 1 | 0(0) | 0(0) | – | 0 (0%) | 1 (12.5%) | 0.7 | |
| 2 | 0(0) | 0(0) | – | 3(50) | 0(0) | 0.18 | 0 (0%) | 2 (25%) | 0.4 | |
| I | 0 | 4(67) | 4(66) | 1 | 3(50) | 5(83) | 0.4 | 8(100%) | 5 (62.5%) | 0.4 |
| 1 | 0(0) | 1(17) | 0.7 | 1(17) | 0(0) | 0.7 | 0 (0%) | 1 (12.5%) | 0.7 | |
| 2 | 0(0) | 0(0) | – | 1(16) | 0(0) | 0.7 | 0 (0%) | 0 (0%) | – | |
| V | 0 | 4(67) | 5(83) | 0.7 | 5(83) | 5(83) | 1 | 8 (100%) | 6 (75%) | 0.5 |
| 1 | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0 (0%) | 0 (0%) | – | |
| 2 | 0(0) | 0(0) | – | 0(0) | 0(0) | – | 0 (0%) | 0 (0%) | – | |
There were no significant differences on comparing acute and chronic scores for cases on different immunosuppression protocols after 1 and 4 months. By comparing vascular changes of studied biopsies of cases on different immunotherapy protocols after four month, we found significant decrease in number of cases with normal biopsy among evirolimus group (p=0.03). As we have 5 cases of evirolimus group with inadequate biopsy sample for assessment of vascular changes in comparison to only 1 case on each of cyclosporine & tacrolimus groups, this significance could not seriously be taken in consideration (Figure 4).
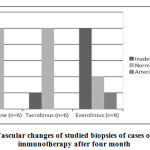 |
Figure 4: Vascular changes of studied biopsies of cases on different immunotherapy after four month |
Discussion
This prospective interventional cohort study aimed to study the baseline pathological graft data one month post transplantation and to evaluate the histopathological impact of different immunosuppression protocols on the graft four months post transplantation. The mean age of our study group was 11.3 ± 3.5 years. Few studies, with similar objectives, were conducted on pediatric renal transplant recipients 14-17. Similar studies also conducted on adult population 18, 19. Still, adult studies are used as references in many renal transplantation issues in which pediatric studies are lacking.
Rush et al. previously noted that treatment of SCR episodes occurring in the first six months post-transplantation leads to improvement of graft function 20. In addition, it was demonstrated that chronic lesions detected by surveillance biopsies conducted as early as 3 or 6 or 12 months are each associated with long term graft loss 21.
In our study subclinical changes rate was 20 % (10% acute SCR, and 10% borderline subclinical changes) at 1 month and 30 % (25% acute SCR and 5% borderline subclinical changes) at 4 month protocol biopsies. In a similar study; subclinical borderline changes were present in 20–30% of biopsies performed between three and six months in pediatric and adult renal transplantation [22]. Bruel et al, also performed protocol biopsies at three and six months for 28 pediatric renal transplant recipients; they found subclinical borderline rejection in 3.5%, SCR in 3.5% and IF/TA grade 1 in 25% 14. The difference of results may be partially related to the different timing of biopsy. Both results, however, showed lower SCR rates than previously reported results that concluded that SCR (Banff grade ≥ IA) detected by protocol biopsies is more common in the first months after transplantation with a frequency of 7–17% between three and six months post-transplant 23-25.
Our four month SCR rate is near to that of Hymes et al. They showed that 29% of their pediatric renal transplant recipients had SCR at 3 months on classic triple therapy [26]. Higher rate however was reported by Seikku et al. as they had 39% prevalence of SCR on 3 month surveillance biopsies in a small group of pediatric patients [27]. At 6 months, the prevalence of SCR was reported by Kanzelmeyer et al to be 25% of their studied cases 28.
Aoun et al outlined the importance of protocol biopsy in different immunosuppressive drugs by comparing protocol biopsies findings in pediatric renal transplant recipients on cyclosporine versus tacrolimus-based immunosuppression where results of 3 months protocol biopsies of 18 patients on cyclosporine showed 13 normal, two borderline and three Banff II rejections, while the ten patients on tacrolimus showed no rejection [16]. Some of the hesitation in performing surveillance biopsies in children is related to the perceived risks associated with biopsies of stable allografts. However, adverse outcomes related to the use of surveillance biopsies are rare. In the current study, no biopsy associated complications occurred. Relatively large single-center retrospective study of ultrasound guided percutaneous biopsies of renal allografts in children did not report any serious adverse events such as the need for blood transfusion or surgical intervention secondary to the biopsy procedure 29.
Our results showed that everolimus, low dose cyclosporine immunosuppression protocol had negative impact on the graft pathological findings. That is similar to what was found by a 24-month, multicenter, open-label, randomized trial which showed that incidence of biopsy proven acute rejection (BPAR) and antibody mediated rejection (AMR) was higher on everolimus group than CNI based group, but this trial was conducted on adult recipients 18.
In Another single-center analysis, Liefeldt et al reported an increased risk for donor specific antibody (DSA) post-transplantaion after conversion to CNI-free therapy with introduction of everolimus [30]. Studies of very early switch at seven weeks, or halving of CNI dose from two weeks onwards with full withdrawal at month 2 have shown a markedly high rate of BPAR (27.5% and 31%, respectively), even when higher concentrations of everolimus were used (trough levels of 6–10ng/mL). There may also be an increased risk of AMR in patients switched early from CNI based therapy to everolimus in a steroid-free regimen30-32.
On the other hand, our finding regarding impact of evirolimus on graft pathology was not consistent with results of other previously conducted studies on everolimus and low CNI immunosuppression protocol usage in pediatric population 17, 33-35.
Brunkhorst et al. results showed that 24 % of the everolimus/ low dose CNI group developed BPAR and there was no CNI induced nephrotoxicity, while in the CNI group 45 % developed BPAR and 4%CNI induced nephrotoxicity 33 Ettenger et al also reported promising results of evirolimus with full-dose cyclosporine and steroids. This regimen was used in a multicenter trial involving 17 de novo pediatric renal transplant patients, 40% of whom were living related donors graft recipients 34. However, this regimen is lacking the main privilege of evirolimus usage which is avoidance of CNI associated nephrotoxicity.
Our results supported that tacrolimus had good impact on graft pathological findings. That was on the same line with Aoun `s results who concluded that patients on tacrolimus had less acute rejection episodes detected on protocol biopsies 3 months after transplant 16. The above finding was previously supported by the Symphony trial that found a better graft function at one year and lower rate of BPAR using a tacrolimus-based protocol targeting trough levels of 3–7 ng/mL as compared to protocols utilizing standard dose CsA, low dose CsA, or sirolimus 36.
Limitations of this study include the small number of included cases and the short duration of their follow up. That was partially due to the lack of justification of protocol biopsy usage in pediatric recipients as routine clinical practice. Long term graft outcome of cases underwent protocol biopsies versus those who did not need to be studied.
Conclusion
surveillance biopsy appears to assist in management of pediatric renal allograft recipients. Still it`s benefit as an invasive tool to improve long term graft outcome after subclinical injury in pediatric patients to be evaluated. Individualization of immunosuppression post transplantation is a must particularly in children who need to achieve linear growth and have longer life expectancy. Although promising as regard avoidance of CNI induced nephrotoxicity, early use of evirolimus based regimen may be guarded by higher rejection rates. Tacrolimus usage in CNI based classic protocol may have better outcomes than cyclosporine as regard graft function.
Acknowledgment
Sincere and heartfelt thanks are expressed to transplanted children and their families, who were the backbone of this study. Furthermore, colleagues from Pediatric Nephrology Unit (PNU), urology and radiology departments, nurses, officers and anyone who ever showed support to this work are deeply appreciated.
Conflict of interest
No conflicts of interest declared.
Funding Source
Thare are no funding source
References
- Goldstein SL, Rosburg NM, Warady BA, Seikaly M, McDonald R, Limbers C, et al. Pediatric end stage renal disease health-related quality of life differs by modality: a PedsQL ESRD analysis. Pediatr Nephrol. 2009; 24(8):1553–60.
CrossRef - Kazi JI, & Mubarak M. Biopsy findings in renal allograft dysfunction in a live related renal transplant program. J Transplant Technol Res. 2012; 2:108.
CrossRef - Cochat P & Harambat J. Maximizing growth in children after renal transplantation. Transplantation. 2009; 88: 1321–1322.
CrossRef - Kahan BD, Kaplan B, Lorber MI, Winkler M, Cambon N, Boger RS. RAD in de novo renal transplantation: Comparison of three doses on the incidence and severity of acute rejection. Transplantation. 2001;71: 1400–1406.
CrossRef - Gaumann A, Schlitt HJ, Geissler EK. Immunosuppression and tumor development in organ transplant recipients: The emerging dualistic role of rapamycin. Transpl Int . 2008; 21: 207–217.
CrossRef - Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366: 520–529.
CrossRef - Nankivell BJ, & Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006; 6: 2006–12.
CrossRef - HymesLC, GreenbaumL, AmeralSG, Warshaw BL.Surveillance renal transplant biopsies and subclinical rejection at three months post-transplant in pediatric recipients.Pediatr Transplant 2007;11(5):536-9
CrossRef - Buchmann TN, Wolff T, Bachmann A et al.Repeat true surveillance biopsies in kidney transplantation. Transplantation. 2012;93:908-913.
CrossRef - Buchmann TN, Wolff T, Bachmann A, Guerke L, Steiger J, Mihatsch MJ et al. The utility of 6-month protocol renal biopsy under modern immunosuppression. Clin Nephrol. 2008;70:490-495.
CrossRef - Pascual J, Perez-saez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management.Transplant Rev(Orlando).2012; doi:10.1016/j.trre.2012.07.002.
CrossRef - Moreso F, Carrera M, Goma M. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation. 2012; 93:41-46.
CrossRef - Rush D. Can protocol biopsy better inform our choices in renal transplantation? Transplant Proc. 2009; 41:s6-s8.
CrossRef - Gülcü A, Göktay Y, Soylu A et al. Doppler US evaluation of renal biopsy complications in children. Diagn Interv Radiol. 2013;19: 15–19.
CrossRef - Bruel A, Allain-Launay E, Humbert J, Ryckewaert A, Champion G, Moreau A et al. Early protocol biopsies in pediatric renal transplantation: interest for the adaptation of immunosuppression. Pediatr Transplant . 2014;2:142-9.
CrossRef - Brunkhorst LC, Fichtner A, Höcker B, Burmeister G, Ahlenstiel-Grunow T, Krupka K, et al. Efficacy and Safety of an Everolimus- vs. a Mycophenolate Mofetil-Based Regimen in Pediatric Renal Transplant Recipients. PLoS One. 2015;10(9):e0135439.
CrossRef - Aoun B, Decramer S, Vitkevic R, Wannous H, Bandin F, Azema C, et al. Protocol biopsies in pediatric renal transplant recipients on cyclosporine versus tacrolimus-based immunosuppression. Pediatr Nephrol. 2013;28(3):493-8.
CrossRef - Ferraresso M, Belingheri M, Ginevri F, Murer L, Dello Strologo L et al. Three-yr safety and efficacy of everolimus and low-dose cyclosporine in de novo pediatric kidney transplant patients. Pediatr Transplantation. 2014;18: 350–356.
CrossRef - De Fijter JW, Holdaas H, Øyen O, Sanders JS4 Sundar S, Bemelman FJ, et al.Early conversion from calcineurin inhibitor- to everolimus-based therapy following kidney transplantation: Results of the randomized ELEVATE trial. Am J Transplant. 2016; doi: 10.1111/ajt.14186.
CrossRef - Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Nashan B, et al. Design and rationale of the ATHENA study–A 12-month, multicentre, prospective study evaluating the outcomes of a de novo everolimus-based regimen in combination with reduced cyclosporine or tacrolimus versus a standard regimen in kidney transplant patients: study protocol for a randomised controlled trial. 2016;17:92. doi: 10.1186/s13063-016-1220-9.
CrossRef - Rush DN, Nickerson P, Jeffery JR, McKenna RM, Grimm PC JR et al.Protocol biopsies in renal transplantation: research tool or clinically useful? Curr Opin Nephrol Hypertens. 1998; 7: 691-694.
CrossRef - Yilmaz S, Tomlanovich S, Mathew T, Taskinen E, Paavonen T, Navarro M, et al. Protocol core needle biopsy and histologic Chronic Allograft Damage Index (CADI) as surrogate end point for long-term graft survival in multicenter studies. J Am Soc Nephrol. 2003;14(3):773-9
CrossRef - Heilman RL, Devarapalli Y, Chakkera HA, et al. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant . 2010; 10: 563–570.
CrossRef - Dart AB, Schall A, Gibson IW, Blydt-Hansen TD, Birk PE. Patterns of chronic injury in pediatric renal allografts. Transplantation. 2011; 89: 334–340.
CrossRef - Nankivell BJ, Borrows RJ, Fungcl-S, O’connell PJ, Allen RDM, Chapman The natural history of chronic allograft nephropathy. N Engl J Med. 2003; 349: 2326–2333.
CrossRef - EL-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009; 9: 527–535.
CrossRef - Hymes LC, Warshaw BL, Hennigar RA, Amaral SG, Greenbaum LA. Prevalence of clinical rejection after surveillance biopsies in pediatric renal transplants: does early subclinical rejection predispose to subsequent rejection episodes? Pediatr Transplant. 2009; 13: 823-826.
CrossRef - Seikku P, Krogerus L, Jalanko H, Holmberg Better renal function with enhanced immunosuppression and protocol biopsies after kidney transplantation in children. Pediatr Transplant: 2005; 9: 754–762.
CrossRef - Kanzelmeyer NK, Ahlenstiel T, Drube J, Froede K, Kreuzer M, et al. Protocol biopsy-driven interventions after pediatric renal transplantation . Pediatr Transplant. 2010; 14: 1012-1018.
CrossRef - Vidhun J, Masciandro J, Varich L, Salvatierra O Jr, Sarwal M. Safety and risk stratification of percutaneous biopsies of adult-sized renal allografts in infant and older pediatric recipients. Transplantation. 2003; 76: 552-557.
CrossRef - Liefeldt L, Brakemeier S, Glander P, et al. Donor – specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation.Am J Transplant. 2012; 12:1192–8.
CrossRef - Mjörnstedt L, Sørensen SS, von Zur Mühlen B, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial inkidney transplantation. Am J Transplant. 2012;12:2744–53.
CrossRef - Montagnino G, Sandrini S, Iorio B, et al. A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low-dose cyclosporine. Nephrol Dial Transplant. 2008;23:707–14.
CrossRef - Brunkhorst LC, Fichtner A, Höcker B, Burmeister G, Ahlenstiel-Grunow T, Krupka K, et al. Efficacy and Safety of an Everolimus- vs. a Mycophenolate Mofetil-Based Regimen in Pediatric Renal Transplant Recipients. PLoS One. 2015;10(9):e0135439.
CrossRef - Ettenger R, Hoyer PF, Grimm P, Webb N, Loirat C, Mahan JD, et al. Multicenter trial of everolimus in pediatric renal transplant recipients: Results at three year. Pediatr Transplant . 2008;12:456–463.
CrossRef - Grushkin C, Mahan JD, Mange KC, Hexham JM, Ettenger R. De novo therapy with everolimus and reduced-exposure cyclosporine following pediatric kidney transplantation: A prospective, multicenter, 12-month study. Pediatr Transplant. 2013; 17: 237–243.
CrossRef - Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357: 2562–2575.
CrossRef








