Mohd Farhan Hanif Reduan1 , Mohammad Rasul Arif Mastika1
, Mohammad Rasul Arif Mastika1 , Fathin Faahimaah Abdul Hamid1
, Fathin Faahimaah Abdul Hamid1 , Noramalina Noralidin2
, Noramalina Noralidin2 , Nur Athirah Abd Manaf2
, Nur Athirah Abd Manaf2 , Rumaizi Shaari2
, Rumaizi Shaari2 , Intan Noor Aina Kamaruzaman1
, Intan Noor Aina Kamaruzaman1 and Muhammad Luqman Nordin2
and Muhammad Luqman Nordin2
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, PengkalanChepa, 16100 Kota Bharu, Kelantan, Malaysia.
2Department of Veterinary Paraclinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, PengkalanChepa, 16100 Kota Bharu, Kelantan, Malaysia
Corresponding Author E-mail: luqman.n@umk.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2260
Abstract
Oroxylum indicumalso known as ‘pucukbeko’ in Malaysia is often consumed as raw salad (ulam) due to the belief that the plant has numerous therapeutics activities that could improve health. Despite its medicinal potential,however, there has been very limited data on the plant’s safety and toxicity profile particularly for long term consumption. More depth insight and evidence-based studies are needed to verify its safety as a potential herb. Therefore, this study aims to investigate sub acute oral toxicity of ethanol extract of O. indicum in C57BL/6 male mice. Twenty-five mice (n=5) were orally administered at single dose of normal saline (control), vehicle (5% DMSO), extracts (100, 200 and 500 mg/kg bw), respectively in accordance with OECD Guideline 420 for 28 days. Liver, kidneys, heart, lungs, testes, spleen,and blood samples were collected to determine the effects of the extract on the relative organ weight, tissue changes, and blood profile alterations in the end of the study. The sub-acute toxicity results demonstrated no lethal effects and abnormal behavioural changes in mice treated with an expansion dose up to a maximum of 500 mg/kg. No significant (p>0.05) changes in body weights, relative organ weight and haematological evaluation. Nevertheless, there were significant differences (p<0.05) in the urea, mean corpuscular volume (MCV), and alanine transaminase (ALT) values but the levels were still within the acceptable range. Histopathological analysis of the liver and kidney tissues also revealed no striking lesions. This study displays that mice treated with an increasing dose ofO. indicum leaf ethanolic extract up to a maximum 500mg/kg bw did not cause any toxicological effects and considered safe to be consumed and used for therapeutic purposes
Keywords
C57BL/6 mice; Ethanol Extract; Haemato-Biochemistry; Oroxylum Indicum; Subacute oral toxicity study
Download this article as:| Copy the following to cite this article: Reduan M. F. H, Mastika M. R. A, Hamid F. F. A, Noralidin N, Manaf N. A. A, Shaari R, Kamaruzaman I. N. A, Nordin M. L. Sub-Acute Oral Toxicity Study of Ethanol Extract of Oroxylum Indicum Leafin C57BL/6 Mice. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Reduan M. F. H, Mastika M. R. A, Hamid F. F. A, Noralidin N, Manaf N. A. A, Shaari R, Kamaruzaman I. N. A, Nordin M. L. Sub-Acute Oral Toxicity Study of Ethanol Extract of Oroxylum Indicum Leafin C57BL/6 Mice. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3B8wxKa |
Introduction
The uses of herbal medicines are rapidly increasing across the world, and people are opting for these products as an alternative treatment of many health conditions and diseases1,2. Several factors that contribute to the demand ofpublic in using herbal remediesare due to various claims about the efficacy or effectiveness of the use of medicinal plant3andbecause high incidence of side effects derived from commercial drug4. The improvements in the quality, efficacy, and safety of herbal medicines with the development of science and technology towards the use of herbal remedies gives another option to people on the use of this product for many therapeutic purposes5. To the date, up to 70,000 plant species have been screened for biological activities6 and about 70% end up as a commercial drug7.With the increasing population and health conscious minded among people, the demands and pharmaceutical investment on herbs as a healthcare option is believed to have increased tremendously.
Oroxylum indicum is widely distributed throughout India subcontinent and South East Asia countries, including Malaysia8. In Malaysia, it is commonly known as “pucukbeko, bonglai or bolaikayu” and eaten raw as a salad or cook with coconut milk. The leaves from the plant have previously been used among the older generation as herbal remedies and passed down to the new generation to be applied. Various scientific studies reported that O. indicum possesses anticancer9, antioxidant and hepatoprotective10, immunomodulaory properties11 and gastro-protective properties12. Despite the various claimed benefits of O. indicum leaf extract, the toxicity profiling of this plant is still lacking and have not been scientifically proven. Acute toxicity of ethanolic extract of Oroxylum Indicum are proven to be safe up to 500 mg/kg bw. This study was a continuation of a previous acute toxicity study of O. indicum leaf extract where the results suggests that the herbs considered safe in acute toxicity level13.Hence, this study aims to investigate the subacute oral toxicity of O. indicum leaf ethanolic extract in mice and to determine the prolonged toxic effect of the plant extract. Subacute toxicity study may support initial acute toxicity study of this plant where the duration of treatment for 28 days provides facts about the plausible adverse effects likely to arise for longer exposure.
Materials and Methods
Preparation of ethanolic extract
The plant leaves were purchased from a local market in Pengkalan Chepa, Kelantan in January 2021. The leaflets have 2-4 pairs, 7.0 x 3.5-8.0 cm, ovate-elliptic, acuminate, base obliquely rounded-obtuse. The leaves of O. indicum were then washed with distilled water and oven-dried at 40°C for 4 days. The dried leaves of O. indicum were ground into fine particles form and 150g of the pulverized leaves were soaked in 1.5 L of ethanol for 72 hours at room temperature. Then, the extract was filtered using Whatman filter paper (No. 1001-240, Grade 1) and was dried and evaporated by using a rotary evaporator controlled in a water bath at 60°C. The final yield products were stored under -20°C. The extract was tested for its sterility by inoculating 0.1g of the extract in 1ml of sterile nutrient broth. The extract was considered sterile with the absence of turbidity after incubated at 37˚C for 24 hours. The plant part was identified by the botanist from Herbarium, Universiti Kebangsaan Malaysia, Selangor, Malaysia. A voucher specimen (ID025/2020) was deposited at the herbarium for reference.
Experimental animals and husbandry
A total of 25 males C57BL/6 mice aged between 6 to 8 weeks with a body weight of 25 to 30 g were obtained from the Animal Research and Service Centre, Universiti Sains Malaysia, Health Campus, Kubang Kerian, Kelantan. The mice were placed in polycarbonate cages with optimum environmental conditions of temperature (24 ± 2°C), relative humidity (45 ± 5%), and lightning duration (12 hours daylight and 12 hours dark hours) were provided. Filtered tap water and commercial mice pellets were provided ad libitum. Wood shaving was provided as the bedding which was cleaned and changed daily. All mice underwent an acclimatization period for 5 days prior to the toxicity experiment. The research was conducted according to the guidelines and approval by the Institutional Animal Care and Use Committee (IACUC), Universiti Malaysia Kelantan (IACUC Ref No: UMK/FPV/ACUC/FYP/16/2020).
Subacute oral toxicity study
The study was designed according to Organisation for Economic Co-operation and Development Guideline Test No. 420 (OECD, 2001)14. The mice were randomly assigned into 5 experimental groups consisted of 5 mice per group. All the mice were fasted overnight from the feed but had free access to water before experimentation. The experimental groups were orally gavage with a single dose of 100 mg/kg bw, 200 mg/kg bw, and 500 mg/kg bwof extracts accordingly, whereas the control and vehicle groups were treated with normal saline and 5% dimethyl sulfoxide (DMSO) respectively. No food was provided for the first 2 hours after O. indicum leaf extract administration. The extract was administered once daily, in the morning for 28 days.The conditions of all experimental mice were closely observed for the first 30 minutes to 6 hours, intermittently at 7 to 24 hours, and twice daily for 28 days for any abnormalities or signs of toxicity and mortality. The conditions which were accessed include changes in body weight, food and water intake, coat colour, mucous membrane, respiratory rate, neurological signs, and behavioural changes.
Necropsy, gross organ examination and sample collection
All surviving mice were sacrificed with inhalation of carbon dioxide and decapitation using a guillotine. The internal organs mainly liver, kidneys, lungs, heart, brain, and spleen were isolated, cleaned, and weighed. The organs were examined for the abnormalities and changes in the sizes, colour, consistency, and texture. After that, all organs were preserved in 10% formalin for further histopathology evaluation. The relative organ weights were determined as follows:
Relative organ weight = weight of organ/bodyweight of the mice on the sacrifice day x 100%13..
Haematology and serum biochemistry analyses
Blood samples were also collected in heparin tubes using 25G needles via cardiac puncture. The serum samples were centrifuged at 5000 rpm for 30 seconds prior to analysis using microcentrifuge. The complete blood count tested were total white blood cell count and differentials count, total red blood cell count, haemoglobin concentration, packed cell volume, mean corpuscular volume, mean corpuscular haemoglobin concentration, and platelet count. The serum samples were submitted for biochemical analysis for creatinine and blood urea nitrogen (BUN), alanine aminotransferase (ALT), and total protein, including albumin and globulin. All haematological procedures were conducted using IDEXX VetTest Chemistry analyser at Faculty of Veterinary Medicine, Universiti Malaysia Kelantan.
Histopathological evaluation
After 48 hours of formalin preservation, a small block of tissue was placed on the cassette and processed by using an automated tissue processor. Then, the tissues were sectioned into a thickness of 5 μM using a rotary microtome and dried overnight in an oven at 37°C. Haematoxylin and Eosin (H&E) were used to stain the sectioned tissues for the examination of abnormalities and lesions. All the tissue sections were scored according to the established scoring method by15.
Statistical analysis
Data were analysed statistically using Statistical Package for Social Science (SPSS) software version 23. Test of analysis of variance (ANOVA) was conducted to compare the data variations between and within groups. Post hoc analysis using Duncan test was further carried out. The results were considered statistical significance when p<0.05.
Results
Behaviour, physical observations, body weight changes, relative organs weight and mortality rate if mice upon treatment.
All the toxicity signs including pain, stress, abnormal behavioural, physical changesand mortality were absent in all groups of mice.Body weights of the mice taken at initial of experimentation (day 0), day 7, and day 14, day 28 of post extract administration did not show any significant (p>0.05) changes compared to control groups (Table 1). There were no significant differences (p>0.05) observed in the organ’s weights of liver, kidneys, heart, lungs, testes, and spleen (Table 2).Administration of the extract at different doses (100, 200, and 500 mg/kg bw) resulted in no death of the mice within 28 days period of the study and observation (Table 2). This finding indicates that the inoculation of O. indicum leaf ethanolic extract up to 500 mg/kg bwfor 28 days is safe.
Table 1: The body weight (mean ± SEM) in subacute oral toxicity study of Oroxylum indicum leaf ethanolic extract in mice.
| Day/Week | Control | Vehicle | Low dose (100 mg) | Medium dose (200 mg) |
High dose (500 mg) |
| Day 0-7 (Week 1) | 25.4 ± 0.20a | 26.10 ± 0.10a | 25.53 ± 0.32a | 25.78 ± 0.37a | 25.73 ± 0.33a |
| Day 14 (Week 2) | 25.43 ± 0.07a | 26.17 ± 0.33a | 25.68 ± 0.28a | 25.72 ± 0.29a | 26.02 ± 0.35a |
| Day 21 (Week 3) | 25.90 ± 0.20a | 26.77 ± 0.32a | 26.02 ± 0.22a | 26.08 ± 0.36a | 26.20 ± 0.27a |
| Day 28 (Week 4) | 26.30 ± 0.06a | 26.80 ± 0.41a | 26.40 ± 0.24a | 26.28 ± 0.42a | 26.68 ± 0.25a |
*Values in the same row with similar superscript letter were not significantly different (p > 0.05).
Table 2: The relative organs weights (mean ± SEM) in subacute oral toxicity study of Oroxylum indicum leaf ethanolic extract in mice.
| Organs | Control | Vehicle | Low dose (100 mg) | Medium dose (200 mg) |
High dose (500 mg) |
| Liver | 1.23 ± 0.12a | 1.36 ± 0.09a | 1.43 ± 0.13a | 1.50 ± 0.09a | 1.40 ± 0.15a |
| Kidneys | 0.57 ± 0.20a | 0.20 ± 0.01a | 0.38 ± 0.10a | 0.38 ± 0.08a | 0.40 ± 0.12a |
| Lungs | 0.17 ± 0.03a | 0.16 ± 0.06a | 0.28 ± 0.02a | 0.15 ± 0.05a | 0.23 ± 0.13a |
| Heart | 0.30 ± 0.10a | 0.10 ± 0.01a | 0.18 ± 0.05a | 0.25 ± 0.09a | 0.20 ± 0.07a |
| Spleen | 0.26 ± 0.03a | 0.25 ± 0.02a | 0.30 ± 0.05a | 0.24 ± 0.01a | 0.27 ± 0.04a |
*Values in the same row with similar superscript letter were not significantly different (p > 0.05).
Haematological and serum biochemical analyses
There were no significant differences (p>0.05) in the hemogram parameters in all groups in both treatment and control groups (Table 3). For the serum biochemistry analysis of kidney, liver, and protein, there were no significant differences (p>0.05) for both treatment and control groups (Table 4).
Organ gross examination and histopathological evaluation of the organs
No gross lesions were observed related to toxicity in both treatment and control groups. The histopathological scoring revealed no abnormalities and lesions were noticed from the tissue sections of the liver and kidney of mice in all treatments and control groups as shown in Figures 1-10.Figure 1: Liver section of the control group mice; Figure 2: Kidney section of the control group mice; Figure 3: Liver section of the vehicle group mice; Figure 4: Kidney section of the vehicle group mice; Figure 5: Liver section of the low dose group mice; Figure 6: Kidney section of the low dose group mice; Figure 7: Liver section of the medium dose group mice; Figure 8: Kidney section of the medium dose group mice; Figure 9: Liver section of the high dose group mice; Figure 10: Kidney section of the high dose group mice. (H&E, 40x magnification)
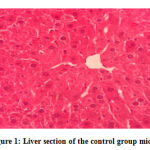 |
Figure 1: Liver section of the control group mice. |
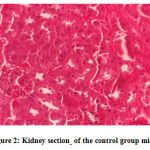 |
Figure 2: Kidney section of the control group mice. |
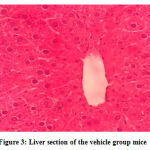 |
Figure 3: Liver section of the vehicle group mice. |
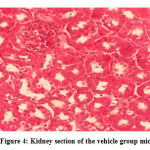 |
Figure 4: Kidney section of the vehicle group mice. |
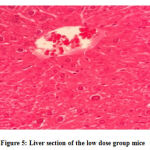 |
Figure 5: Liver section of the low dose group mice. |
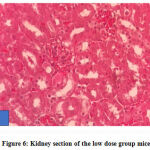 |
Figure 6: Kidney section of the low dose group mice. |
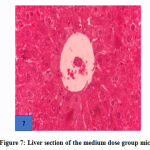 |
Figure 7: Liver section of the medium dose group mice. |
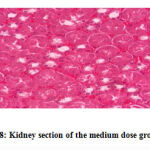 |
Figure 8: Kidney section of the medium dose group mice. |
 |
Figure 9: Liver section of the high dose group mice. |
 |
Figure 10: Kidney section of the high dose group mice. |
Table 3: The haematological evaluation (mean ± SEM) in subacute oral toxicity study of Oroxylum indicum leaf ethanolic extract in mice.
| Parameters | Control | Vehicle | Low dose(100 mg) | Medium dose (200 mg) | High dose(500 mg) | |
| RBC (x1012/L) | 6.56 ± 1.00a | 6.13 ± 0.23a | 4.69 ± 1.14a | 5.31 ± 0.96a | 5.04 ± 0.69a | |
| Hb (g/L) | 12.23 ± 1.37a | 10.37 ± 0.12a | 12.42 ± 2.37a | 10.03 ± 1.79a | 9.08 ± 1.06a | |
| HCT(L/L) | 23.60 ± 1.02a | 27.75 ± 1.95a | 24.43 ± 5.46a | 9.98 ± 5.76a | 6.70 ± 3.86a | |
| MCV (Fl) | 44.40 ± 0.20a | 44.9 ± 0.30a | 45.23 ± 0.74a | 46.17 ± 0.22a | 45.30 ± 0.66a | |
| MCHC(g/L) | 38.70 ± 3.0a | 37.90 ± 3.20a | 50.73 ± 9.60a | 42.73 ± 2.67a | 40.90 ± 1.67a | |
| Platelets (109/L) | 185.67 ± 25.43a | 208.00 ± 54.15a | 170.75 ± 29.05a | 175.75 ± 30.04a | 233.20 ± 56.18a | |
| WBC(x109/L) | 1.87 ± 0.28a | 3.13 ± 0.81a | 4.10 ± 0.92a | 2.00 ± 0.35a | 2.00 ± 0.21a | |
| Neutrophils (x109/L) | 0.26 ± 0.08a | 0.17 ± 0.10a | 0.12 ± 0.05a | 0.11 ± 0.03a | 0.16 ± 0.01a | |
| Lymphocytes (x109/L) | 1.63 ± 0.46a | 2.53 ± 0.83a | 3.35 ± 0.84a | 1.73 ± 0.33a | 1.60 ± 0.26a | |
| Monocytes (x109/L) | 0.02 ± 0.002a | 0.02 ± 0.01a | 0.01 ± 0.05a | 0.03 ± 0.02a | 0.01 ± 0.05a | |
*Values in the same row with similar superscript letter were not significantly different (p > 0.05)
Table 4: The serum biochemistry evaluation (mean ± SEM) in subacute oral toxicity study of Oroxylum indicum leaf ethanolic extract in mice.
| Parameters | Control | Vehicle | Low dose (100 mg) | Medium dose(200 mg) | High dose(500 mg) |
| Urea (mmol/L) | 26.40 ± 1.00a | 26.00 ± 3.30a | 30.20 ± 2.01a | 28.50 ± 1.50a | 25.00 ± 4.16a |
| Creatinine (µmol/L) | 0.12 ± 0.04a | 0.26 ± 0.12a | 0.15 ± 0.01a | 0.22 ± 0.06a | 0.28 ± 0.05a |
| ALT (U/L) | 104.20 ± 1.02a | 90.70 ± 0.95a | 115.80 ± 1.80a | 122.50 ± 2.01a | 102.30 ± 2.16a |
| Total protein (g/L) | 6.56 ± 0.50a | 6.20 ± 0.23a | 6.88 ± 0.60a | 7.07 ± 0.74a | 6.01 ± 0.22a |
| Albumin (g/L) | 3.40 ± 0.12a | 3.01 ± 0.10a | 2.60 ± 0.28a | 2.69 ± 0.50a | 2.10 ± 0.16a |
| Globulin (g/L) | 4.60 ± 0.30a | 3.80 ± 0.29a | 3.58 ± 0.60a | 3.20 ± 0.43a | 4.20 ± 0.15a |
*Values in the same row with similar superscript letter were not significantly different (p > 0.05).
Discussion
To the best of our knowledge, to date, there is no subacute toxicity study of O. indicum to support its medicinal potential even though the plant is extensively used by local people as raw salad. In this 28-day oral subacute toxicity study revealed that no death was found in mice indicating that the LD50 of O. indicum ethanolic lead extract could be more than 500 mg/kg. Therefore, the long-term consumption of the plant extract below 500 mg/kg may be considered relatively safe.
Several parameters were assessed in this study. Administration of O. indicum extract up to maximum dose of 500 mg/kg did not cause any mortality among mice and did not showed any abnormal behavioural changes that indicates signs of toxicity such as lethargy, inactive, isolated itself and no fur-grooming. Mortality rates and behaviour changes are important parameters in toxicity study16,17. In addition, the LD50 of the O. indicum extract was up to 500 mg/kg bw which suggests a wide safety margin for therapeutic doses.
Body weight change is a good indicator of animal health. Body weight changes not only influenced by weight gain after food intake, but also will be affected by the internal organ weight. Body weight changes can be caused by adverse effects of chemicals or drugs consumption. Changes in body weight due to exposure to potentially toxic substances could indicate an effect of toxicity13. If more than 10% of body weight loss from initial body weight after consuming crude extract, it may indicate to the toxic effects of the administration. The increasing body weight of mice for all group with no significant different (P>0.05) in the end suggests the herbs is safe to be consumed. This result agrees with17,18, who also recorded a significant increase in the body weight of mice and rats respectively when administered with ethanolic plant extracts. It can be stated that leaves did not interfere energy metabolism of animal. Similarly, no significant different of relative organ weight of control and treated group (P> 0.05), indicating no cellular changes or injury happened such as hypertrophy and hyperplasia due to the direct exposure of toxicants on cells.
The blood can be used as parameters analyses to the systemic toxicity of any chemical or compound as the blood cells are first exposed to toxicants and most susceptible target. The toxicants may directly harm the cells in the bloodstream or have an effect on the hematopoietic tissue in the bone marrow. This can lead to a decrease of blood cells count and osmotic fragility and affects the health status of the animal19,20. Furthermore, the severity of toxicity can be predicted from the significant alteration of the blood parameters15. In this study, there were no significant differences among all groups for haemogram analyses except in mean corpuscular volume. The readings, however, remained within the acceptable value.
Substances such as compound, medication and xenobiotics entering the body must undergo metabolism and excretion processes majorly in the liver and kidneys. Any severe organ dysfunction may result in compound accumulation or toxic-concentrated metabolites21. The serum level of creatinine and BUN were used to estimate kidney status in this study. Creatinine and BUN are by-products of protein metabolism in the kidney glomerulus and circulated in the blood22. Moreover, Alanine aminotransferase (ALT) and nitrogenous wastes marker of the kidney such as blood urea nitrogen (BUN) and creatinine are typically used as markers for the liver and kidney toxicities as they directly involve in detoxification of metabolites23,24. In this study, there were no significant differences (p>0.05) for both treatment and control groups indicating that the extracts were not toxic at the dosage up to 500 mg/kg. The histopathological results supported evidence related to the body weight, haematology and biochemical findings as histopathology is an essential parameter for assessing pathological changes in the organs due to systemic toxicity.
Conclusion
In conclusion, the oral sub-acute toxicity of Oroxylum indicum leaf extract has shown to be more than 500 mg/kg and this study suggest the plant is generally safe for long-term consumption. However, for clearer and more comprehensive picture of the toxicity effect of O. indicum, a well-designed of chronic toxicity studyis encouraging to be carry out.
Acknowledgement
The authors would like to thank Universiti Malaysia Kelantan for providing UMK Fundamental Research Fund (R/Fund/A0600/ 01391A /002/2020/00735). The authors also would like to acknowledge Madam Nur Eizzati Badrul Hisham, Mr Ahmad Fadli Ahmad Sanusi, Mr Muhamad FaizJuha and Miss Nani Izreen Mohd Sani from Histopathology and Bacteriology Laboratory, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan for their technical assistance while conducting the research.
Conflict of Interest
There are authors no conflict of Interest
Funding Source
None
References
- Ekor M, 2014. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontier in Pharmacology. 4 (2014): 177.
CrossRef - Nordin, S. F., Nordin, M. L., Osman, A. Y., Hamdan, R. H., Shaari, R., & Arshad, M. M. (2017). The effect of Matricaria Chamomilla L on the growth performance of red hybrid tilapia. Biomedical and Pharmacology Journal, 10(4), 1905-1915.
CrossRef - Helmstädter, A., &Staiger, C. (2014). Traditional use of medicinal agents: a valid source of evidence. Drug Discovery Today, 19(1), 4-7.
CrossRef - Harirforoosh, S., Asghar, W., & Jamali, F. (2013). Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Journal of Pharmacy & Pharmaceutical Sciences, 16(5), 821-847.
CrossRef - Bandaranayake, W. M. (2006). Quality control, screening, toxicity, and regulation of herbal drugs. Modern Phytomedicine, 25–57.
CrossRef - Veeresham, C. (2012). Natural products derived from plants as a source of drugs. Journal of advanced pharmaceutical technology & research, 3(4), 200.
CrossRef - Steenhuysen, J. 2007. “Mother Nature Still A Rich Source Of New Drugs.” Reuters Limited. March 19, 2007.
- Deka DC, Kumar V, Prasad C et al.,2013. Oroxylum indicum-a medicinal plant of North East India: An overview of its nutritional, remedial and prophylactic properties. Journal of Applied Pharmaceutical Science. 3: 104-112.
- Kamkaen N, Wilkinson JM and Cavanagh HM, 2006. Cytotoxic effect of four Thai edible plants on mammalian cell proliferation. Thai Pharmaceutical and Health Science Journal. 1(3): 189-95.
- Tenpe CR, Upaganlawar A, Burle S et al., 2009. In vitro antioxidant and preliminary hepatoprotective activity of Oroxylum indicum Vent leaf extracts. Pharmacology online. 1 (2009): 35-43.
- Zaveri M, Gohil P and Jain S, 2006. Immunostimulant activity of n-butanol fraction of root bark of Oroxylum indicum, vent. Journal of Immunotoxicology. 3(2): 83-99.
CrossRef - Zaveri M and Jain S, 2007. Gastroprotective effects of root bark of Oroxylum indicum, vent. Journal of Natural Remedies. 7(2): 269.
- Reduan, M. F. H., Hamid, F. F. A., Nordin, M. L., Shaari, R., Hamdan, R. H., Chung, L. T., … &Noralidin, N. (2020). Acute oral toxicity study of ethanol extract of Oroxylum indicum leaf in mice. The Thai Journal of Veterinary Medicine, 50(4), 573-581.
- OECD 2001. “Test No. 420: Acute Oral Toxicity – Fixed Dose Procedure, OECD guidelines for the testing of chemicals, section 4. Paris: OECD Publishing.” [Online]: Available: https://dx.doi.org/10.1787/9789264070943-en. Accessed May 21, 2020.
CrossRef - Nurul SAS, Hazilawati H, Mohd RS et al., 2018. Subacute oral toxicity assessment of ethanol extract of Mariposa christiavespertilionis leaves in male Sprague Dawley rats. Toxicological Research. 34 (2):85-95.
CrossRef - Abid, R., & Mahmood, R. (2019). Acute and sub-acute oral toxicity of ethanol extract of Cassia fistula fruit in male rats. Avicenna journal of phytomedicine, 9(2), 117.
- Aliyu, A., Shaari, M. R., Ahmad Sayuti, N. S., Reduan, M. F. H., Sithambaram, S., Noordin, M. M., … & Hamzah, H. (2020). Subacute Oral Administration of Clinacanthus nutans Ethanolic Leaf Extract Induced Liver and Kidney Toxicities in ICR Mice. Molecules, 25(11), 2631.
CrossRef - Porwal, M., Khan, N. A., & Maheshwari, K. K. (2017). Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdeniatenacissima leaves in experimental rats. Scientia Pharmaceutica, 85(3), 29.
CrossRef - Reduan FH, Shaari RM, Nurul SAY et al., 2020. Acute and subacute dermal toxicity of ethanolic extract of Melastomamalabathricum leaves in Sprague-Dawley rats. Toxicological Research. 2020: 1-8. https://doi.org/10.1007/s43188-019-00013-5.
CrossRef - Odeyemi, O. O., Yakubu, M. T., Masika, P. J., &Afolayan, A. J. (2009). Toxicological evaluation of the essential oil from Mentha longifolia L. subsp. capensis leaves in rats. Journal of medicinal food, 12(3), 669-674.
- Garza, A. Z., Park, S. B., &Kocz, R. (2020). Drug Elimination. StatPearls [Internet].
- Salazar, J. H. (2014). Overview of urea and creatinine. Laboratory Medicine, 45(1), e19-e20.
CrossRef - Dina PF, Hazilawati H, Rosly SM et al., 2011. Expression of circulating CD146 associated with endovascular dysfunction in adenine-induced chronic renal damage in rats using an evagreen real-time RT-PCR assay. Pertanika Journal of Tropical Agricultural Sciences.34: 381-391.
- Sajjaratul NNA, Hazilawati H, Rosly SM et al., 2016. Blood profiles and histopathological changes of liver and kidney tissues from male Sprague Dawley rats treated with ethanol extracts of Clinacanthus nutans leaf. Journal of Clinical Toxicology. 6:1-10.








