Uma Sankar Gorla1,* , GSN Koteswara Rao1
, GSN Koteswara Rao1 , Umasankar Kulandaivelu1
, Umasankar Kulandaivelu1 , Rajasekhar Reddy Alavala1
, Rajasekhar Reddy Alavala1 , Siva Prasad Panda1
, Siva Prasad Panda1 and Rajkiran Kolakota2
and Rajkiran Kolakota2
1College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Andhra Pradesh, India
2Vignan Institute of Pharmaceutical Technology, Visakhapatnam, Andhra Pradesh, India.
Corresponding Author E-mail: umasankargorla@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2257
Abstract
Cocculus hirsutus, a tropical South Asian creeper,traditionally used as a diuretic, laxative, cardiotonic, anti-microbial, antidiabetic, anti-inflammatory and spermatogenic. However, the neuroprotective role was less explored; therefore, this researchwas conducted to investigate neuroprotective potentials of Cocculus hirsutus leaf hydroalcoholic extract in 6,7-Epoxytropine tropate (Scopolamine) induced cognitive impairment and oxidative lipid peroxidation in the brain of wistar albino rat. Scopolamine (1 mg/kg body weight, i.p.) was given in rats for 14 days to induce transient cognitive impairment. Donepezil (2 mg/kg body weight, orally) has been used for this research as a positive control. Behavioral studies were done using Morris water maze and elevated plus maze and neurobiochemical parameters such as acetylcholinesterase activity, reduced glutathione levels and activity of catalase were assessed in rats brain homogenate. Cocculus hirsutus leaf hydroalcoholic extract(200 mg/kg and 400 mg/kg) exhibited an improvement in spatial, exteroceptive learning and memory. The extract showed significant decline in the activity of acetylcholinesterase, enhancement of reduced glutathione levels and catalase activity (p<0.001). All the outcomes were assessed by Bonferroni post hoc tests with ANOVA for multiple comparison studies. This study reveals that hydroalcoholic extract of Cocculus hirsutusleaf acts as neuroprotective against scopolamine induced behavioral and neurobiochemical changes.
Keywords
Alzheimer’s Disease, Acetylcholinesterase, Behavioral Studies, Cocculus hirsutus, Scopolamine.
Download this article as:| Copy the following to cite this article: Gorla U. S, Rao G. K, Kulandaivelu U, Alavala R. R, Panda S. P, Kolakota R. Neuroprotective Potentials of Cocculus hirsutus Leaf Extract Against 6,7-Epoxytropine Tropate-Induced Memory Impairment in Rats. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Gorla U. S, Rao G. K, Kulandaivelu U, Alavala R. R, Panda S. P, Kolakota R. Neuroprotective Potentials of Cocculus hirsutus Leaf Extract Against 6,7-Epoxytropine Tropate-Induced Memory Impairment in Rats. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3lHLZIQ |
Introduction
6,7-Epoxytropine tropate (Scopolamine), also known as Hyoscine, is a natural belladonna alkaloid that competes with acetylcholine and non-selectively inhibits muscarinic receptors1. Its high affinity towards muscarinic receptors makes more suitable as preanesthetic medication to treat post-operative emesis and also effective to prevent motion sickness2. It readily permeates blood brain barrier and inhibits central muscarinic receptors that reduces post ganglionic cholinergic nerve stimulation results in transient cognitive impairment and neurophysiological changes3.Administration of scopolamine can be used as psychopharmacological experimental model of Alzheimer’s disease (AD)4.
Transient and progressive cognitive impairment is due to deficiency of acetylcholine levels in the basal forebrain is one of the chief causes of AD5. Alzheimer’s disease etiopathogenesis may also include extracellular deposits of senile plaques of beta-amyloid, intracellular aggregation of neurofibrillary tau protein tangles and increased oxidative stress6. Cholinergic neurodegeneration in Alzheimer’s disease results in deficiency of acetylcholine at the areas of brain that causes impairment in cognitive functions like learning, thinking and memory7.Acetylcholinesterase enzyme inhibition is a significant target strategy for senile dementia by enhancing the levels of acetylcholine8. One of the major determinants for Alzheimer’s is oxidative stress that stimulates death of neuronal cells and plays a crucial role in neurobiochemical changes such as decreased levels of reduced glutathione and catalase activity9.Existing drugs have been approved to relieve some cognitive symptoms in Alzheimer’s disease10. On the other side, pharmacological effects of natural products seem to be advantageous in the treatment of neuropsychological and neurotoxicological disorders with no or fewer side-effects11.
Cocculus hirsutus (Menispermaceae) commonly referred to as “Patalgarudi (Sanskrit)” grows in India and many other countries in Asia. The leaves are ovate, obtuse, sub deltoid with soft hairs on both sides12. The traditional and research evidences support the use of various parts of Cocculus hirsutusas detoxifier, diuretic, antidiabetic, antipyretic, analgesic and anti-inflammatory agents13-15.Currently the neuroprotective potentials were less explored from the leaf of Cocculus hirsutus. Thus, the goal of our current pharmacological research was to demonstrate the neuroprotective potentials of Cocculus hirsutusleaf extract in rats against memory impairment induced by scopolamine.
Materials and Methods
Experimental animals
For the study, healthy wistar albino rats of both sexes weighing 180±20 g were used. The animals were housed in standard propylene cages and were kept in regulated environmental conditions at 25±2°C temperature, 30-60% Humidity and 12 hrcycles of light / dark. With typical pellet diet and water ad libitum, they were acclimatized to hygienic laboratory conditions for 2 weeks. The Institutional Animal Ethical Committee (IAEC) of Vignan Institute of Pharmaceutical Technology under registration number No. 2003/PO/Re/S/18/CPCSEA, dated 09/02/2018 approved the research protocol and all the studies were performedwith the IAEC and CPCSEA guidelines and regulations.
Collection of fruits and Preparation of the pulp extract
The fresh leaves of Cocculus hirsutuswere procured from Visakhapatnam, A.P., India and authenticated in Department of Botany, Andhra University, Visakhapatnam, A.P., India. The fresh leaves were collected, dried under shade conditions and powdered mechanically. Cocculus hirsutus leaf hydroalcoholic extract was prepared by using Soxhlet extraction for 48 hr. The extracted liquid was concentrated by placing on water bath, air dried overnight at room temperature and the final extract was stored in a refrigerator at 3±1°C.
Acute toxicity study of Cocculus hirsutus leaf extract
The extract was evaluated for acute toxicity in rats according to OECD guideline No. 42516. The rats were fasted overnight prior to dosing and the dosage was calculated based on the fasted body weight. Single doses of 2000 mg/kg of Cocculus hirsutus leaf hydroalcoholic extract was administered to the rats orally by gavage using a suitable intubation cannula. After administration, food was withheld for an additional 3-4 hr and monitored continuously for any toxic signs. The rats were kept under observation with a duration of 14 days for any mortality, weight, physiological and psychological changes. This study reported that hydroalcoholic leaf extract ofCocculus hirsutushas LD50>2000 mg/kg body weight.
Experimental protocol and dosage regimen
The experimental animals were segregated in to five groups of six rats each. According to acute toxicity data, 200 mg/kg and 400 mg/kg of test compound have been chosen for administration. In Group I, the rats were treated with normal saline (0.9%w/v, 1 ml/kg body weight, orally) to serve as normal control. In Group II, treatment of rats with scopolamine (1 mg/kg body weight, i.p.) dissolved in normal saline to serve as negative control. In Group III, rats were simultaneously treated with donepezil (2 mg/kg body weight, orally) and scopolamine (1 mg/kg body weight, i.p.) dissolved in normal saline to serve as a positive control. In Group IV, rats were simultaneously treated with Cocculus hirsutus leaf extract (200 mg/kg body weight, orally) and scopolamine (1 mg/kg body weight, i.p.) dissolved in normal saline. In Group V, rats were simultaneously treated with Cocculus hirsutusleafextract (400 mg/kg body weight, orally) and scopolamine (1 mg/kg body weight, i.p.) dissolved in normal saline. All the drugs were given for 14 consecutive days to all the representative groups with standard pellet diet and water ad libitum. The assessment of cognitive functions was carried out 30 min after scopolamine administration to the respective groups. For assessment of biochemical changes in the brain, the animals were sacrificed, brain was excised, washed with frozen saline, homogenized and frozen at -20°C for further use.
Behavioral parameters
Morris water maze test
Morris water navigation task was widely accepted for testing visual short-term memory and visual-spatial learning in rodents by observing and recording the escape latency time to reach the submerged invisible platform17. In this test, the maze consisted of black colored circular water pool (diameter 120 cm, height 50 cm) filled with water to 40 cm in depth. The maze was held at 25±2°C and was surrounded with various visual cues of different shapes. The pool was undisturbed and maintained the position of cues constant on all the days of experimentations. The maze was essentially divided into four equally spaced quadrants designed as North, South, East and West. In one of the quadrants, a black colored circular invisible platform with a diameter of 10 cm was fixed constantly 1 cm below the water surface so that the rat could escape from swimming. The rats were acclimatized to reach hidden platform in 120 s for a week with a minimum of five training sessions per day. The time taken to reach invisible platformby the treated rats was recorded as Escape Latency Time (ELT).
Elevated plus maze test
Elevated plus maze was widely used paradigm for evaluating the exteroceptive learning and memory in rodents by observing and recording the transfer latency time to reach any closed arm from the end of open arm 18-20.For rats, the maze consisted of a central dais (10 x 10 cm) with four arms radiating outwards i.e. two open arms (50 x 10 cm) alternative with two closed arms (50 x 10 x 20 cm), arranged at an angle of 90˚ degrees from each other. The height of plus maze was 50 cm from the ground level. On the 14th day of treatment period, after administration of drugs to the respective groups, each rat was positioned at the end of the open arm facing the central platform and the transfer latency time was recorded which reflects the acquisition of learning behavior of rats. The rats were acclimatized for 120 s to the maze and relocated to its home cage. After 24 hr of acquisition trail, the transfer latency time of each rat was documented that reflects the retention of information or memory. The time taken by the rats to reach any of the closed arms with all their four legs from the end of open arm is known as Transfer Latency Time (TLT). The maze was cleaned properly with wet tissue paper after each experiment to avoid the influence of residual stimuli if any.
Neurobiochemical parameters
After behavioral studies, rats were anesthetized under light ether and deliberately euthanized to evade any damage to the brain tissueby cervical dislocation. The entire brain tissue was immediately removed from the sacrificed rats, washed with ice-cold normal saline and the brains regions were separated. The tissue homogenate of different brain regions was used for analysis of neurobiochemical parameters such as acetylcholinesterase activity21, reduced glutathione levels22 and catalase activity23.
Brain acetylcholinesterase activity
Brain acetylcholinesterase activity was performed based on Ellaman’s photometric method in 1961 with minor modifications24. By using Teflon homogenizer, the hippocampal regions of the brains were homogenized with Tris HCl buffer (100 mM, pH 8) to prepare 10% homogenate. To 25 µL of supernatant, 50 µL of acetylthiocholine iodide (20 mM) and 925 µL of Ellman reagent (0.5 mM) prepared in Tris HCl buffer (100 mM, pH 8) were added. The degradation of acetylthiocholine iodide has been read at 412 nm and the outcomes were reported as µmols of acetylthiocholine hydrolyzed per milligram of protein (brain tissue) per minute.
Brain reduced glutathione levels
Brain levels of reduced glutathione were assessed according to the standard protocol described by Leopold Flohe and Wolfgand A. Gunzler (1984)25.Briefly, the brain hemispheres were homogenized with phosphate buffer (0.1 M, pH 7.4) to prepare 5% homogenate and centrifuged for 10 min at 4°C at 1500 rpm. After centrifugation, the supernatant collected was used to analyze the levels of glutathione peroxidase. The activity of Glutathione peroxidase was stated as µmol of GSH utilized per milligram of protein per minute at 37°C.
Brain catalase activity
The activity of brain catalase was assayed according to colorimetric technique of Sinha (1972)26. 1 mL of brain tissue homogenate in 5 mL phosphate buffer (pH 7.4) was combinedin 4 mL of H2O2 (0.2 M) in phosphate buffer. After 3 min of adding H2O2, dichromate acetic acid (2 mL) was added to 1 mL of above-mentionedreaction mixture. The final reaction mixture was placed in hot water bath for 10 min, cooled under running tap water and record the absorbance against blank at 540 nm. The activity of brain catalase was expressed as µmol of H2O2 consumed per milligram of protein per minute at 37℃27.
Statistical Analysis
All findings are expressed as Mean ± Standard Error of the Mean (n=6) and assessed by Analysis of Variance (ANOVA) accompanied by Bonferroni post tests for multiple comparative studies using Graph Pad Prism application, version 5.0. The “P” value p<0.001was considered as statistically significant.
Results
Effect of Cocculus hirsutus leaf extract on escape latency in scopolamine treated rats using Morris water navigation task
Escape Latency Time was considered as parameter to test the impact of Cocculus hirsutusleafextract on spatial learning and memory in rats with dementia caused by scopolamine. The rats treated with scopolamine showed a significant increase in ELT than the saline treated controls (*p<0.001) indicating the impairment of cognition (Figure 1). Administration of Cocculus hirsutusleafextract (200 mg/kg and 400 mg/kg) was found that the ELT was significantly reduced ($p<0.001) and decreased the effects of scopolamine as compared with ($p<0.001) group alone treated with scopolamine (Table 1).
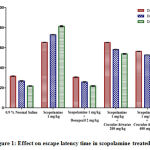 |
Figure 1: Effect on escape latency time in scopolamine treated rats. |
Table 1: Effect of Cocculus hirsutus leaf extract and standard drug donepezil on escape latency in scopolamine treated rats.
| Groups | Treatment | Day 7 | Day 10 | Day 14 |
| Group I | Normal saline
(0.9 %W/V, 1 ml/kg) |
31.63±0.38 | 26.81±0.50 | 21.76±0.31 |
| Group II | Scopolamine
(1 mg/kg) |
65.40±0.18* | 72.98±0.28* | 81.35±0.51* |
| Group III | Donepezil+ Scopolamine
(2 mg/kg + 1 mg/kg) |
30.57±0.38$ | 25.82±0.48$ | 21.87±0.31$ |
| Group IV | Extract + Scopolamine
(200 mg/kg + 1 mg/kg) |
65.21±0.23$# | 58.23±0.28$# | 53.9±0.31$# |
| Group V | Extract + Scopolamine
(400 mg/kg + 1 mg/kg) |
56.37±0.21$# | 52.76±0.13$# | 46.33±0.27$# |
Results are expressed as Mean±SEM (n=6). SEM = Standard error mean. The statistically significant difference has been determined by ANNOVA accompanied by Bonferroni post tests for multiple comparison and are statistically significant with ‘*’p<0.001 compared with the control group, ‘$’p<0.001 compared with the negative control, ‘#’p<0.001 compared with the positive control. ANNOVA = Analysis of variance.
Effect of Cocculus hirsutus leaf extract on transfer latency in scopolamine treated rats using elevated plus maze test.
Transfer Latency Time was used to test the impact of Cocculus hirsutusleafextracton acquisition of learning and retention of memory in rats with dementia caused by scopolamine. The rats treated with scopolamine showed remarkable increase in TLT than the saline treated controls (p<0.001) indicating the impairment of memory and learning (Figure 2). The animals treated with Cocculus hirsutusleafextract(200 mg/kg and 400 mg/kg) showed significant decrease (p<0.001) in TLT indicating substantial improvement in learning and memory and decreases the effects of scopolamine as compared with that of reference standard donepezil (1mg/kg) (Table 2).
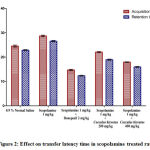 |
Figure 2: Effect on transfer latency time in scopolamine treated rats. |
Table 2: Effect of Cocculus hirsutus leaf extract and standard drug donepezil on transfer latency in scopolamine treated rats.
| Groups | Treatment | Acquisition trial | Retention trial |
| Group I | Normal saline
(0.9 %W/V, 1 ml/kg) |
24.53±0.40 | 22.87±0.23 |
| Group II | Scopolamine
(1 mg/kg) |
28.70±0.36* | 26.57±0.28* |
| Group III | Donepezil+ Scopolamine
(2 mg/kg + 1 mg/kg) |
14.69±0.27$ | 12.36±0.17$ |
| Group IV | Extract + Scopolamine
(200 mg/kg + 1 mg/kg) |
22.08±0.20$# | 18.94±0.26$# |
| Group V | Extract + Scopolamine
(400 mg/kg + 1 mg/kg) |
17.95±0.12$# | 15.96±0.23$# |
Results are expressed as Mean±SEM (n=6). SEM = Standard error mean. The statistically significant difference has been determined by ANNOVA accompanied by Bonferroni post tests for multiple comparison and are statistically significant with ‘*’p<0.001 compared with the control group, ‘$’p<0.001 compared with the negative control, ‘#’p<0.001 compared with the positive control. ANNOVA = Analysis of variance.
Neurobiochemical Assessment
Effect on brain acetylcholinesterase activity
Administration of scopolamine markedly increase the degradation of acetylcholine as indicated by significant enhancement in the activity of acetylcholinesterase (*p<0.001) in the hippocampal region of rat’s brain as compared to the control group (Figure 3A). Concurrent administration of donepezil and scopolamine showed significant reduction in acetylcholinesterase activity ($p<0.001) in hippocampus compared with the negative control. Furthermore, Cocculus hirsutus leaf extract(200 mg/kg and 400 mg/kg) treated groups exhibited significant decrease in acetylcholinesterase activity in the hippocampal region of rat’s brain when compared with ($p<0.001) group alone treated with scopolamineand (#p<0.001) positive control (Table 3).
Effect on brain reduced glutathione levels
Chronic scopolamine administration to rats resulted in oxidative stress as shown by significant decline in the levels of reduced glutathione (*p<0.001) when compared to saline treated rats (Figure 3B). Donepezil significantly inhibited the effects of scopolamine and marked increase in reduced glutathione levels ($p<0.001) when compared to scopolamine-alone treated group (negative control). The co-administration of Cocculus hirsutus leaf extract(200 mg/kg and 400 mg/kg) and scopolamine significantly reverses the decline the levels of reduced glutathione induced by scopolamine ($p<0.001). The Cocculus hirsutus leaf extract treated groups showed significant difference when measured with (#p<0.001) positive control (Table 3).
Effect on brain catalase activity
Administration of scopolamine to the rats prominently increases the oxidative damage by significant decrease in the activity of catalase (*p<0.001) when compared to the normal control (Figure 3C). Concurrent administration of donepezil and scopolamine to the rats showed significant rise in the catalase activity by inhibiting the effects of scopolamine when compared to those administered with scopolamine-alone ($p<0.001). Combined administration of Cocculus hirsutusleafextract (200 mg/kg and 400 mg/kg) and scopolamine in rats resulted in significant increase of catalase activity when compared with negative control ($p<0.001). The extract treated groups also exhibited significant difference when measured with (#p<0.001) positive control (Table 3).
Table 3: Effect of Cocculus hirsutus leaf extract and standard drug donepezil on neurobiochemical levels in scopolamine treated rats.
| Groups | Treatment | AChE activity (µmol/mg protein/min) | Reduced Glutathione levels (µmol/mg protein/min) | Catalase activity (µmol/mg protein/min) |
| Group I | Normal saline
(0.9 %W/V, 1 ml/kg) |
10.70±0.19 | 26.27±0.15 | 96.10±0.09 |
| Group II | Scopolamine
(1 mg/kg) |
24.60±0.24* | 11.61±0.15* | 30.80±0.16* |
| Group III | Donepezil+ Scopolamine
(2 mg/kg + 1 mg/kg) |
11.83±0.26$ | 25.09±0.17$ | 89.09±0.11$ |
| Group IV | Extract + Scopolamine
(200 mg/kg + 1 mg/kg) |
19.75±0.34$# | 18.55±0.17$# | 65.21±0.25$# |
| Group V | Extract + Scopolamine
(400 mg/kg + 1 mg/kg) |
16.41±0.17$# | 23.47±0.16$# | 85.61±0.19$# |
Results are expressed as Mean±SEM (n=6). SEM = Standard error mean. The statistically significant difference has been determined by ANNOVA accompanied by Bonferroni post tests for multiple comparison and are statistically significant with ‘*’p<0.001 compared with the control group, ‘$’p<0.001 compared with the negative control, ‘#’p<0.001 compared with the positive control. ANNOVA = Analysis of variance.
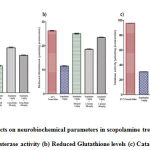 |
Figure 3: Effects on neurobiochemical parameters in scopolamine treated rats (a) Acetylcholinesterase activity (b) Reduced Glutathione levels (c) Catalase activity. |
Discussion
Alzheimer’s is a transient, heterogenous and progressive neurodegenerative disease characterized by agnosia, aphasia and apraxia with the loss of memory and cognitive dysfunction28. Collective evidences suggested AD’s behavioral and cognitive symptoms of contributed to impaired neurogenesis in the hippocampus29. Due to increased lifetime expectancy, now-a-days Alzheimer’s has become a public health load30. Many studies reported that the pathogenesis of Alzheimer’s disease includes elevated acetylcholinesterase levels and increased oxidative stress that are found to be associated with enhanced levels of protein carbonyls, lipid peroxidation and decreased superoxide dismutase activity, levels of reduced glutathione and the activity of catalase in the hippocampus of rodents. The prevalence and severity of the disease motivated us to reconnoiter the abilities of medicinal plants to manage this illness.Our results showed that chronic administration of scopolamine influences different regions of brain that affects the spatial learning and cognition by increasing acetylcholinesterase activity and oxidative stress. In the current study, 14 days pre-treatment of animals with Cocculus hirsutus leaf extractat different doses proved to have neuroprotective potentials that significantly reduces the effects induced by scopolamine as an indication from improvement in behavior and neurobiochemical scores of acetylcholinesterase, reduced glutathione and catalase.
Morris water navigation test was used as behavioral task for assessing visual short-term memory and visual-spatial learning in rats. The results on 7th day, 10th day and 14th day shows that donepezil and Cocculus hirsutus leaf extract significantly reduces the time taken to reach submerged hidden flatform (ELT) from a fixed quadrant in scopolamine induced rats.Our results with Morris water maze test confirmed that leaf extract counteracted the scopolamine induced cognitive deficits thus Cocculus hirsutusis a neuroprotective.
The elevate plus maze test for exteroceptive learning and memory usually based on natural rejection of rodents to high and open spaces. Transfer latency time was considered as the parameter to evaluate acquisition and memory retention in rodents. Generally, the animals exhibited shortened transfer latency time in the retention trial as compared to acquisition trial for entering into the open arms of elevated plus maze. In this test, 14 days pretreatment with Cocculus hirsutus leaf extract significantly reduced the transfer latency time in the rats treated with scopolamine as compared with the standard drug donepezil. Our results clearly suggested that Cocculus hirsutushas neuroprotective effect because it enhances learning and retention of memory.
Neurobiochemical results shows that administration of scopolamine for 14 consecutive days markedly increase the degradation of acetylcholine by increasing the acetylcholinesterase activity and diminishes reduced glutathione levels and catalase which gives a measure of lipid peroxidation (oxidative stress) in rats brain. Pretreatment of rats with 200 mg/kg and 400 mg/kg of Cocculus hirsutus leaf extract significantly increases the levels of acetylcholine due to decreased acetylcholinesterase activity and enhances reduced glutathione levels and catalase activity as a sign of reduced oxidative stress in the brains induced by scopolamine. This Cocculus hirsutus leaf extract proved to have neuroprotective activity in accordance with the results obtained.
Conclusion
The possible outcomes of this study indicate that Cocculus hirsutus leaf extract counteracted the impairment of memory and oxidative stress induced by scopolamine. Therefore, it could be inferred that Cocculus hirsutus can be an appreciated plant resource for age-related dementia management. More molecular studies are required to investigate the mechanisms underlying the neuroprotective effects of Cocculus hirsutus by targeting the other hypothesis of Alzheimer’s.
Acknowledgement
The authors are grateful to the management K L College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, A.P. and Vignan Institute of Pharmaceutical Technology, Visakhapatnam, A.P. for the facilities granted for the research work.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Renner U. D,Oertel R andKirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit.,27(5):655-665 (2005).
CrossRef - Martin L and Andrew J. T. The muscarinic antagonist’s scopolamine and atropine are competitive antagonists at 5-HT3 Neuropharmacology,108:220-228 (2016).
CrossRef - Sahreh S and Mohammad A. M. Diosmin is neuroprotective in a rat model of scopolamine-induced cognitive impairment. Biomed Pharmacother.,108:1376-1383 (2018).
CrossRef - Bajo R, Pusil S, Lopez M. E, Canuet L, Pereda E, Osipova D, et al. Scopolamine effects on functional brain connectivity: a pharmacological model of Alzheimer’s disease. Sci rep.,5:9748 (2015).
CrossRef - Atul P, Pranay S, Preeti P, Rajesh S. Y and Prakash C. B. Scopolamine induced behavioral and biochemical modifications and protective effect of Celastruspaniculatous and Angelica glauca in rats. Int J NutrPharmacolNeurol Dis.,4(3):158-169 (2014).
CrossRef - Samaila M. C, Che Norma M. T, Mohamad Aris M. M, Mohamad Taufik H. B, Zulkhairi A andSaravanan J. The use of nootropics in Alzheimer’s disease: is there light as the end of the tunner?.Biomed Res Ther.,6(1):2937-2944 (2019).
CrossRef - Amira M, Shuntaro Y, Yoshinori K andKuniyoshi S. In vitro Neuroprotective Activities of Compounds from Angelica Shikokiana Molecules,20:4813-4832 (2015).
CrossRef - Bindhu K. H and Vijayalakshmi A. Neuroprotective effect of Carica papaya leaf extract against aluminium toxicity: An experimental study on cognitive dysfunction and biochemical alteration in rats. Indian J Pharm Educ.,53(3 Suppl 2):s392-s398 (2019).
CrossRef - Smith M. A, Perry G, Richey P. L, Sayre L. M, Anderson V. E, Beal M. F, et al. Oxidative damage in Alzheimer’s. Nature,382(6587):120-121 (1996).
CrossRef - Jeffrey L. C, Travis M and Kate Z. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther.,6:37 (2014).
CrossRef - Kumar H, More S. V, Han S. D, Choi J. Y and Choi D. K. Promising therapeutics with natural bioactive compounds for improving learning and memory – A review of randomized trials. Molecules,17:10503-10539 (2012).
CrossRef - Satish V, Ravichandrian V. D, Usha G andPadmaa M. P. Antimicrobial studies on the extracts of Cocculus hirsutus and HyptissuaveolensPoit. Indian J Nat Prod Resour., 1:49-52 (2010).
- Logesh R, Das N, Adhikari-Devkota A andDevkota H. P. Cocculus hirsutus (L.) W. Theob. (Menispermaceae): A review on traditional uses, phytochemistry and pharmacological activities. Medicines, 7(11):69 (2020).
CrossRef - Nayak S. K andSinghai A. K. Anti-inflammatory and analgesic activity of roots of Cocculus hirsutus.Indian J Nat Prod., 9:12-14 (1993).
- Ganapaty S and Vijay K. Hypoglycemic activity of aerial parts of Cocculus hirsutus on alloxan-induced diabetes. Indian J Nat Prod., 22:17-20 (2006).
- Singh S. K, Sinha S. K andShirsat M. K. Design, synthesis and evaluation of 4-Aminopyridine analogues as Cholinesterase inhibitors for management of Alzheimer’s diseases. Indian J Pharm Educ.,52(4):644-654 (2018).
CrossRef - Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods.,11(1):47-60 (1984).
CrossRef - Raghavendra M, Rituparna M, Kumar S and Acharya S. B. Role of Ocimum sanctum in the experimental model of Alzheimer’s disease in rats. Int J Green Pharm.,3(1):6-15 (2009).
CrossRef - Kulkarni P. D, Ghaisas M. M, Chivate N andSankpal P. S. Memory enhancing activity of Cissampelospariera in mice. Int J Pharm Pharma Sci.,3(2):206-211 (2011).
- Yamini N, AfsarSh, Devi K, Deepak K, Premalatha S and Pavan K. B. Effect of ethanolic seed extract of Bauhinia purpurea on cognition in scopolamine induced Alzheimer’s disease rat’s model. J Compr Pharm.,2(4):145-150 (2015).
CrossRef - Gauresh S. S, Mruniya S. N, Aakash D. P, Mandar B. M, Priya J. G, Kirti S. L and Sadhana S. Neuroprotective effect of Cubebin: A dibenzylbutyrolactonelignan on scopolamine-induced amnesia in mice. Indian J Med Res.,146(2):255-259 (2017).
CrossRef - Flohe L and Gunzler W. A. Assays of glutathione peroxidase.,105:114-121 (1984).
CrossRef - Aebi H. Catalase in vitro.Methods Enzymol.,105:121-126 (1984).
CrossRef - Ellman G. L, Courtney K. D, Valentino A. J and Robert M. F. A new and rapid colorimetric determination of acetylcholinesterase activity. ,7(2):88-90 (1961).
CrossRef - Leopold F andWolfgand A. G. Assays of glutathione peroxidase. Methods Enzymol.,105:114-120 (1984).
CrossRef - Asru K. S. Colorimetric assay of catalase. Anal Biochem.,47(2):389-394 (1972).
CrossRef - Mundugaru R, Sivanesan S, Udaykumar P, Vidyadhara D, Prabhu S. N and Ravishankar B. Neuroprotective functions of Alpinia galanga in forebrain ischemia induced neuronal damage and oxidative insults in rat hippocampus. Indian J Pharm Educ.,52(4S):s77-s85 (2018).
CrossRef - Jewart R. D, Green J, Lu C. J, Cellar J and Tune L. E. Cognitive, behavioral and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry.,13(4):324-328 (2005).
CrossRef - Demars M, Hu Y. S, Gadadhar A and Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res.,88(10):2103-2117 (2010).
CrossRef - Kalaria R. N, Maestre G. E, Arizaga R, Friedland R. P, Galasko D, Hall K, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management and risk factors. Lancet Neurol.,7(9):812-826 (2008).
CrossRef







