Ashraf S.A. El-Sayed1* , Hanaa Salah Maamoun1
, Hanaa Salah Maamoun1 , Gamal H. Rabie1
, Gamal H. Rabie1 , Ibrahim Shaker2
, Ibrahim Shaker2 , Bothaina A. Alaidaroos3
, Bothaina A. Alaidaroos3 , Mostafa G. Ali4
, Mostafa G. Ali4 and Amgad M. Rady5
and Amgad M. Rady5
1Enzymology and Fungal Biotechnology lab, Botany and Microbiology Department, Faculty of Science, Zagazig University, Zagazig, 44519, Egypt
2Limnology Department, Central Laboratory of Aquaculture Research
3Biology Department, Faculty of Science, King Abdulaziz University, Jeddah 21955, Saudi Arabia
4Botany and Microbiology Department, Faculty of Science, Benha University, Benha, 13518, Egypt
5Faculty of Biotechnology, October University for Modern Science and Arts, Cairo 12566, Egypt
Corresponding Author E-mail: ash.elsayed@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2229
Abstract
Tyrosinase is a copper-containing monooxygenase involved in thecatalysis of the hydroxylation and oxidation reaction of monophenols and diphenols, respectively, into O-quinones intermediates. Tyrosinase is mainly involved in melanogenesis via two reactions. Firstly, 3,4-dihydroxyphenylalanine is produced through tyrosine hydroxylation the nit oxidized into dopaquinone, and finally gives melanin. However, dopaquinones can results in neuronal damage and cell death through the excessive production, suggesting that tyrosinase may be implanted in the formation human brain’s neuromelanin and association with Parkinson’s diseases. Thus, down regulating the melanin pigments and its intermediates by inhibiting tyrosinase activity is the major pharmaceutical challenge to prevent hyperpigmentation, in addition to therapy of neuromelanin disorders. Thus, this review has been focused on exploring the biochemical and molecular properties of tyrosinase from different sources and its potential inhibition with different natural and synthetic compounds.
Keywords
Anticancer; Biochemical Properties; Inhibitors; Melanin Biosynthesis; Tyrosinase
Download this article as:| Copy the following to cite this article: El-Sayed A. S. A, Maamoun H. S, Rabie G. H, Shaker I, Alaidaroos B. A, Ali M. G, Rady A. M. Microbial Tyrosinase: Biochemical, Molecular Properties and Pharmaceutical Applications. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: El-Sayed A. S. A, Maamoun H. S, Rabie G. H, Shaker I, Alaidaroos B. A, Ali M. G, Rady A. M. Microbial Tyrosinase: Biochemical, Molecular Properties and Pharmaceutical Applications. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3jPZOm0 |
Introduction
Biological identity and types of tyrosinases
Tyrosinase (EC 1.14.18.1)is a copper-containing monooxygenase involved in production of O-quinones (diphenolase) through catalyzing the o-hydroxylation reaction of monophenols to produce O-diphenols (monophenolase) which upon oxidation gives O-quinonesas reported by Sanchez-Ferrer et al., (1995) (Fig. 1). Tyrosinase ismainly involved in the synthesis of melanin via two reactions. Firstly, upon O-hydroxylation reaction tyrosine transferred into 3,4-dihydroxyphenylalanine (L-DOPA) which undergoes further oxidation to produce dopaquinone, that finally by many enzymatic processes produces melanin. Practically, in microorganisms the melanin’s biosynthetic pathways are a little bit different from that of human cells. Funa et al., (1999) reported that in fungi, melanin can be synthesized from 1,8- Dihydroxynaphthalene
(DHN) precursors (DHN-melanin following polyketide pathway, using malonyl-CoA as a helper factor (Alspaugh et al., 1998). In human and few microbes, melanin pigments were synthesized by L-DOPA pathway using the precursors molecules of tyrosine that hydroxylated into L-DOPA then oxidized into dopaquinone and into further melanin pigment (Butler and Day, 1998). The melanin biosynthetic scheme in fungi has been illustrated in Fig. 1. Melanin is amorphous polymer, negatively charged and results from many enzymes catalyzed processes and autoxidative polycondensation of various hydrophobic groups in quinone (Jacobson, 2000, Langfelder et al., 2003, Ito and Fujita, 1985).Melanin pigments has the ability of deactivating the free radicals, peroxides, absorbing heavy metals, thus exhibits profound antioxidant activity (Olennikov et al., 2012;Różanowska et al., 1999;Cunha et al., 2010). Melanin is characterized by the presence of mobile π-electrons giving it extraordinary electronic properties and enabling it as a powerful protector against UV- and solar radiation (d’Ischia et al., 2009). Pigments of melanin (Olennikov et al., 2012; Różanowska et al., 1999;Cunha et al., 2010). Melanin is characterized by the presence of mobile π-electrons giving it extraordinary electronic properties and enabling it as a powerful protector against UV- and solar radiation (d’Ischia et al., 2009). Pigments of melanin can absorb light in a wide range, their absorption intensity ability increases gradually with decreasing wavelengths (Meredith and Sarna, 2006). Depending on the heat results from photon energy’s conversion, melanin canabsorb light (Rieszet al., 2006), which may result in protons transfer inside a monomer because duringthe photoexcitation of the pigmentenergy can be released (Olsen et al., 2007). Melanin’s photoionization and its subsequent partial destruction can be noticed after the interaction with the UV radiation in the range of (240–300 nm). Thus, during UV radiation cytotoxic byproducts can be formed despite the effective protecting roles of cellular melanin against both UV- and solar radiation. In melanin synthesis, a multi-copper oxygenase enzyme called tyrosinase is a rate limiting enzyme contains binuclear active sites for Copper (Garcia-Jimenez et al., 2017).
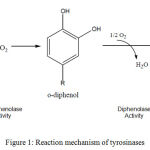 |
Figure 1: Reaction mechanism of tyrosinases. |
Tyrosinases have been well-characterized from many microorganisms as Streptomyces glausescens, Neurospora crassa and Agaricus bisporus (Kupper et al., 1989). It was reported that the melanin deficiency of oculocutaneous albinism was the main reason for human tyrosinase’smutations (Oetting and King, 1994). In human, the possible role of melanins is to prevent the pathogens to enter the wound which may results in healing of wound and the cuticle possibly can be sclerotized. While, in fungithe main role of tyrosinase is catalyzing the first step in the melanin pigment formation from tyrosine. The bacterialtyrosinases involved in melanin production was reported to be extracellular enzymes (Claus andDecker, 2006)
Melanogenesis
Melanins which produced by melanocytes (a membrane-bound granule), are the main pigment responsible for pigmentation of human’sskin, hair and eyes(Kim and Uyama, 2005) through melanogenesis. The number of melanosomes increases with continuous exposure to sunlight resulting in an increase their melanin content and final it transfers to keratinocytes. Skin pigmentation and melanogenesis are the main photo-protective factor responding to the bad effect of UV radiation from the skin and sun photo-carcinogenesis and removing reactive oxygen species (Mohania and Chandel et al., 2017). Melanogenes is process is a very complicated pathway consists of a mix of both chemically and reactions catalyzed by enzymes. Two different types of melanin can be produced by melanocytes through linkage with cysteine or glutathione: pheomelanin (red-yellow) and eumelanin (brown-black) (Fig. 2) (Slominski et al., 2004; Pillaiyar et al., 2015). The process of melanogenesis is started byL-tyrosine oxidation to prduce dopaquinone by tyrosinase enzyme and the eumelanin and pheomelanin will be synthesized from the resulted quinone which used as a precursor for the process of synthesis. In melanin synthesis,the formation of dopaquinoneis a rate-limiting step of because at a physiological pH value the rest of steps can occurs spontaneously (Halaban et al., 2002). Indoline, leukodopachrome (cyclodopa) can be produced after formation of dopaquinone, because during this intramolecular cyclization must be occured. Both of dopachrome and L-3, 4-dihydroxyphenylalanine (L-DOPA)can be increased because of redox exchange between leukodopachrome and dopaquinone respectively, which is another substrate for TYR and gives dopaquinone through oxidation by tyrosinase. TRP-2(dopachrome tautomerase (DCT)) catalyzes the decomposition of Dopachrome to produce dihydroxyindole (DHI) and dihydroxyindole-2-carboxylicacid (DHICA). Finally, eumelanin can be produced through the oxidation of the produced dihydroxyindoles (DHI and DHICA) by the action of TRP-1. In the same line, 5-S-cysteinyldopa or glutothionyldopa are produced from dopaquinone conversion in the presence of cysteine or glutathione. Further oxidation results in production of pheomelanin at end. Tyrosinase is very necessary for melanogenesis despitethe involvement of the three different enzymes, TYR, TRP-1 and TRP-2 in the melanogenesis pathway.
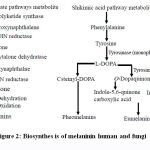 |
Figure 2: Biosynthes is of melaninin human and fungi. |
Melanocyte is the only cells that can produce tyrosinase in the Golgi and endoplasmic reticulum, following its production it transferred to melanosomes, for synthesis of the pigment melanin. Tryosinase enzyme contains three histidine residues surrounding two ions of copper giving tyrosinase its catalytic activity of. During the formation of pigments the active sites are characterized by three states named; oxy, met and deoxy forms (Fig. 3). The specificity of tyrosinase enzyme backs to the fact that at the enzyme active site, two copper ions interacts with dioxygen to produce anintermediate which is highly reactive participatingin the hydroxylation reactions of monophenols to diphenols (monophenolase activity) and in o-diphenols oxidation to o-quinones (diphenolase activity) (Decker and Tuczek, 2000).
Many studies has widely focused on monophenolase mechanism for tyrosinase activity (sanjust et al., 2003) depending on the three different forms of the enzyme. In the cycle of monophenolase, firstly, the monophenol reacts with oxy form only at the axial position of one of its coppers. O-hydroxylation reaction of monophenol takes place as a result for the trigonal bipyramidal rearrangement using the bound peroxide. The result of this is the coordinated o-diphenol, which upon oxidation gives o-quinone, giving a ready deoxy form for further binding with dioxygen. The met form can react with o-diphenol giving the coordinated o-diphenol in the cycle of monophenolase. Both the oxy and met forms can oxidize theo-diphenol giving o-quinone in the diphenolase cycle. In contrast, monophenol can inhibitsthe reduction o-diphenol by competing with it for the met form site binding. From the kinetic point of view the monophenolic substrates reduce monophenolase activity in compareo-diphenolic substrates which increases diphenolase activity (Wilcox et al., 1985). It is conceivable that for o-hydroxylation the monophenolic substrates require a rearrangement from the axial to equatorial arrangement for simple electron transfer, while, the o-diphenolic substrates doesn’t need like this rearrangement at the copper site. Catalytic activity of tyrosinase on monophenols is lower than on o-diphenols as the kinetic studies of the steady state showed(Cabanes et al., 1987). The activity of monophenolase is characterized by a lag time (Fenoll et al., 2001) in which the concentration of both substrate and enzyme with the presence of hydrogen donor are a limiting factors (Cooksey etal., 1997). In the kinetic studies, lag time is the time needed for the resting met form to be transferred into the active deoxy form in presence of a reducing agent, results through the action of the little amounts of the oxy form that found with the met form. In the presence of cofactors which is a reducing agents, especially the derivatives of o-diphenol such as L-DOPA and (+)-catechin, tyrosinase can be activated and the lag time was shortened or totally abolished (Fig.3). At a very low concentration,L-DOPA is the reducing agent which effectively can eliminates lag time.The process of neuromelanin production can be catalyzed by tyrosinase which catalyzes the oxidation of dopamine producing dopaquinones. This findings suggests that a significant role might be play by tyrosinase in the formation of neuromelanin in the human brain cells and responsible for the neurodegeneration associated with Huntington’s diseases and Parkinson’s disease (Chen et al., 2014). Thus, melanin synthesis regulation through tyrosinase inhibition can be a good motivation for researchers concerned with hyperpigmentation prevention.
Crystal structure of Tyrosinases
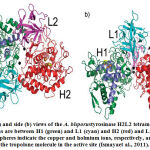
The structural properties of tyrosinase are different in nature with the distribution in different sources (Jaenicke and Decker, 2003; Mayer, 2006). The structural variations of tyrosinases are mainly in amino acid sequences, molecular configuration, size, post-translational modification mechanisms,and active site structure. Tyrosinases are composed of two ions of copper (Cu A and Cu B) each attached by six histidine residues in their active site. The crystal structure of a tyrosinase was identified for the first time inplant in its active form was purified from the leaves of walnut and showed activity for both monophenolase and a bulky residue at the blocker position (Zekiri and Molitor et al., 2014). Walnut tyrosinase (jrTYR) was purified from leaves of walnut and the X-ray crystallography was used to determine structure to a 1.8 Å resolution, showing identical structure with polyphenol oxidase (PPO) (Sendovski et al. 2011; Mauracher et al. 2014). It shares a higher similarity in the structure with Ipomoea batatas catechol oxidases (Klabunde and Eicken, 1998) and Vitis vinifera (Virador et al., 2010). The region of active-site which contains in its center abinuclear copper is formed of a bundle of four helices (α4, α5, α12, and α14). Each copper ion in the active-site is fixed by three histidine residues (His)(Fig. 4). Copper A (CuA) is coordinated by His87, His108, and His117, where His87 and His117 are located on α-helices (α4 and α5) and His108 on a loop. A thioether bond is formed from the CƐ atom of His108in with the sulfur atom of accompanied cysteine (Cys91), giving a limited flexibility of His108. Copper B (CuB) is arranged by His239, His243, and His273, located on α-helices. A hydroxide anion serves as A Bridge between the two copper centers which are 2.1 Å from each copper ion. The N terminal loops can be stabilized by two disulfide bonds (Cys11-Cys26 and Cys25-Cys88), anchoring them to the main core, and copper incorporation(Fujieda et al., 2013). In jrTYR the two disulfide bonds are located at a distance ofabout 8 Å next to each other. A phenylalanine residue (Phe260) in jrTYR is located at the position of blocker residue above CuA. It has been suggested that in catechol oxidases the bulky phenylalanine prevents the substrates from binding to CuA, leadingto the lack of monophenolase activity (Olivares et al., 2002; Sendovski et al., 2011).The absence in activity of monophenolase in catechol was suggested to be due to the limited flexibility of the CuA site in combination with a bulky residue of oxidases, since these restrictions will prevent the rotation of substrate, which is necessary for the hydroxylation of monophenols (Goldfeder et al., 2014).
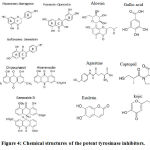 |
Figure 4: Chemical structures of the potent tyrosinase inhibitors. |
Molecular, Biochemical properties and sources of Tyrosinase
The molecular subunit structure of tyrosinase was slightly varied with the biological identity of the producing microbes. Tyrosinase had a homodimer subunit structure of 50 kDa from Aspergillus nidulans(Birse and Clutterbuck, 1990), Agaricus bisporus (Lopez-Tejedor and Palomo, 2018) and Trichoderma reesei(Selinheimo et al., 2007). The structure of molecular subunit of tyrosinase from Streptomyces spp was 30kDa (Wu et al., 2010). The enzyme Mycothermus thermophiles had an approximate native molecular mass 320 kDa, with subunit structure 80 kDa on the SDS-PAGE, revealing their composition of four identical subunits (Sutay Kocabas et al., 2008). Also, the enzyme from Thermomicrobium roseumhas two identical subunits with 43 kDa (Kong and Hong et al., 2000).The molecular mass of tyrosinase from Streptomyces glaucescens, Neurospora crassa and Trametes sanguinea was 30.9,46 and 45 kDa, respectively (Kim and Uyama, 2005; Selinheimo et al., 2007).
Various factors affecting TYR properties such as pH value, reaction temperature, substrates and kinetics of enzymatic properties. The maximum activity of tyrosinase from Agaricus bisporus, S. polyantibioticus and Trichoderma reesei was reported at 30-35°C(Zaidi et al. 2014, Selinheimo et al., 2006). Le Roes-Hill et al. (2015) recognized that, the highest catalytic activity of tyrosinase from Streptomyces pharetrae was reported at 40ºC. Tyrosinase from Trametes sanguineus showed an optimum temperature at 60 to 65˚C (Halaouli and Asther et al., 2005). The maximum activity of tyrosinase from Bacillus thuringiensis was detected at reaction temperature 75˚C (Liu et al., 2004). The pH 9.5 was the optimum for tyrosinase of Thermomicrobium roseum,the enzyme loss about 75% its of activity at pH 6 but retains fullactivity upon standing 20 h in the pH-range 8.5-10.0 (Kong and Hong, 2000). Thepurified enzyme optimal pH of T. reesei was 9 (Selinheimo et al., 2006).The highest activity of tyrosinase from A. bisporus was at pH 7.0(Zaidi et al. 2014), while, the enzymeoptimal pH from Streptomyces sp was 6.8 (Yoshimoto et al., 1985). Themaximal activity of tyrosinase from Streptomyces pharetrae and Streptomyces polyantibioticus was reported at pH 6.5 (Le Roes-Hill et al., 2015). The activity of enzyme from A. nidulans was optimal at pH 7.0 (Birse and Clutterbuck, 1990). Similarly, the kinetics of methionine γ-lyase (El-Sayed et al., 2014, 2015, 2016, 2019), cystathionine γ-lyase (El-Sayed et al., 2015a,b,c), arginine deiminase (El-Sayed et al., 2017, 2018, 2019) have been reported.
The affinity of tyrosinase from different microorganisms to their substrates was reported. The km(maximum affinity) value of tyrosinase of A. nidulans was 2 mM using hydroquinone monomethylether as substrate (Birse and Clutterbuck, 1990). Tyrosinase from B. thuringiensis and P. putidahadkm0.563 mMand 0.23 mM (Liu et al. 2004; McMahon et al., 2007). The kinetic constants Km, kcat and kcat/Km for tyrosinase from A. bisporus of L-tyrosine were 0.21 mM, 7.9 s-l, and 37.6 M-1’s-l, respectively (Garcia-Jimenez et al., 2016). The Km, kcatvalue for tyrosinase from S. antibioticus to L-tyrosine were 5.1 mM and 2.6 s-l at pH 7.2, respectively (Fogal et al., 2015). The km value and kcat/Km value of enzyme from Ralstonia solanacearum for L-tyrosine were 0. 9 mM and 1.42 M-1’s-l, respectively (Molloy et al., 2013). Tyrosinases have been produced from different bacterial, fungal and plant sources as listed on Table 1.
Table 1: Different sources of tyrosinase
| Sources | Species | References |
|
Bacteria |
Pseudomonas maltophilia, | Claus and Decker, 2006; |
| Marinomonas mediterranea | Liu et al., 2005; | |
| Sinorhizbium meliloti | Liu et al., 2005; | |
| Symbiobacterium thermophilum | Claus and Decker, 2006; | |
| Thermomicrobium reseum | McMahon et al., 2007 | |
| Bacillus thuringiensis | Matoba et al., 2006 | |
| Bacillus megaterium | Shuster and Fishman, 2009 | |
| Mycobacterium avium-intracellulare | Harris et al., 1990 | |
| Streptomyces castaneoglobisporus | Olivares and Solano, 2009 | |
| Pseudomonas putida | Matoba et al., 2006 | |
| Ralstoniasolana cearum | Matoba et al., 2006 | |
| Verrucomicrobium spinosum | Matoba et al., 2006 | |
| Streptomyces castaneoglobisporus | Matoba et al., 2006 | |
| Streptomyces cyaneofuscatu | Harir M.; Bellahcene M. et al., 2017. | |
| Fungi | Lentinula edodes | Kanda et al., 1996 |
| Aspergillus nidulans | Birse and Clutterbuck, 1990 | |
| Aspergillus oryzae | Nakamura et al., 2000 | |
| Amanita muscaria | Mueller et al., 1996 | |
| Portabella mushrooms | Halaouli et al., 2005 | |
| Lentinula boryana | deFaria et al., 2007 | |
| Aspergillus niger PA2 | Agarwal P. et al., 2017 | |
| Agaricus hortensis | Madhosingh and Sundberg, 1974 | |
| Trichoderma reesei | Selinheimo, E. et al., 2009 | |
| Mycothermus thermophilus | Sutay Kocabas D. et al., 2008. | |
| Neurospora crassa | Parvez, S. et al., 2007 | |
| Agaricus bisporus | Jin-Jie Hu and Xiao-Lin Bai et al., 2017 | |
| Lentinula boryana | Otavio de Faria et al., 2007 | |
| Plants | Solanum melongena | Janovitz-Klapp et al., 1989 |
| Monastrell grape | Janovitz-Klapp et al., 1989 | |
| Apple | Janovitz-Klapp et al., 1989 | |
| Sunflower seed | Janovitz-Klapp et al., 1989 | |
| Tea leaf (Camellia sinensis) | Teng J. et al., 2017 | |
| Bromus (Poaceae) and other grass genera | Holzapfel C. et al., 2010 | |
| portulaca grandiflora | Lee et al., 1997 |
Biotechnological applications of tyrosinases
Pharmaceutical applications
Tyrosinase can be found in macro organisms as animals, plants and microorganisms (Chen and Shi, 2014). Tyrosinase in human’s cell, is a very important step which regulates the melanin production in the melanosomes of melanocyte cells resulting in pigmentation of skin, hair and eyes giving the protection against Ultra Violetlight (Videira et al., 2013).In addition, tyrosinase playing a pivotal role inimmune response and healing of wounds (Marino et al., 2011). Tyrosinase has an important medical application including L-DOPA production, a drug used in the treatment of the Parkinson’s disease, and other neurological diseases (Valipourand and Burhan, 2016). Tyrosinase is used for production of hydroxytyrosol as an estrogenic intermediate (Zhang et al., 2007), treatment of vitiligo (Seo et al., 2003).
Food Industry
Tyrosinase has been implemented in various food processing by production of various food additives (Valipour and Burhan, 2016). Tyrosinase has been frequently used as emulsifiers in manufacturing natural polymers can be cross linked for the production of new polymers (Faria et al., 2007), based on the accessibility and abundance of target protein(Heck et al., 2012). Tyrosinase can be used to yield a novel bioproducts through grafting of specific compounds to biopolymers. The byproducts of food processes can be converted to environmentally valuable items by tyrosinase (Aberg et al., 2004). Tyrosinase as cross-linking enzymes are exploited in tailoring the gelation properties of foods, that has been characterized as a highly specific agent in the reaction they work on, in addition to utilizing food matrix components like proteins (Selinheimo, 2008).
Enzymatic browning of plant-derived foods
The fruit and vegetables browning have gained a high attention in the field of food processing, as it lower its economic price. Browning by enzymes is a main factor in fruits damaging during the process of post-harvest and handling, because of mixing tyrosinase enzyme and their polyphenolic substrates after crushing operations leading to rupture of cell structure (Yi et al., 2010).Tyrosinase oxidize the phenolic compounds in fruits leading to bad changes in flavor, color and texture, thereby reducing its price in markt (Xu andZhang et al., 2017). The browning process depends upon many factors, such as enzyme concentration and substrate, availability of oxygen, temperature and pH (Zheng et al. 2008).
The process of enzymatic browning was reported to start with O-diphenol such as L-DOPA transformation to O-quinone, which produces brown melanin pigment, upon further oxidation (Busch, 1999).Several chemical and/or physical agents have been used to control the browning by enzymes such as blanching,chemicals application,high temperature and microwave (Ioannou and Ghoul, 2013, Zhang et al., 2018b). Unfortunately,these processes are characterized by many disadvantages as changing of nutritional quality and organoleptic of the end products,
representing a potential risk for human health (Tinelloand Lante, 2018). Therefore, several enzyme inhibitors, namely ascorbic acid (Kubglomsong et al., 2018), for browning prevention citric acid and kojic acid, have been used(Friedman, 1996).
Tyrosinase inhibitors
Tyrosinase can be over expressed by the inner factors as hormones which stimulate melanocyte- and other environmental factors such as irradiation by UV, which are the main reason of disorders of hyperpigmentation, including lentigo, nevus, age spots, solar lentigo, melasma, ephelides and post-inflammatory states (Slominskiet al., 2004). Also, it was reported that tyrosinase may be the causative agent of Parkinson’s disease and other neurodegenerative diseases (D’Mello et al., 2016). Therefore, tyrosinase inhibitors attract the attention of various researchers, especially for medical and cosmetic applications. Several inhibitors for tyrosinase as kojic acid and hydroquinone may be effective in clinical applications and formulations in topical use (Fujimoto et al., 1999). Generally, in the presence of a monophenolic or a diphenolic substrates these inhibitors are examined and the enzyme activity was measured based on dopachrome produced(Lin et al., 2008). The inhibitors mainly contain copper-binding agents and competes for binding with the activesites on tyrosinase (Robb, 1984). Analogues of the main substrate include numerous aromatic acids, phenols and their derivatives can be used as a competitive inhibitor (Nicolas et al., 1994) to tyrosinase. Because the metal loproteinic identity of tyrosinase, metal chelators such as thiourea derivatives, kojic acid, carbon monoxide,cyanide, tropolone,could be used as enzyme inhibitor. Several natural compounds with anti-tyrosinase activityhave been recently recommended because of their lower toxicity and good bioavailability, mainly for food, medicinal and cosmetic applications.
Plants secondary metabolites
Flavonoids are polyphenol derivatives containing pyrane rings and phenols frequently found in the seeds, leaves, bark and plant’s flowers. Over the identified 4000 flavonoids, these compounds mainly participate in the protection of plant against its pathogens and UVradiation (Harborne andWilliams, 2000). Flavonoids are the largest groupsof natural inhibitors of tyrosinase (Wanget al., 2014) that are classifiedinto six groups: flavanols, flavones, flavonols, flavanones, isoflavones and anthocyanidins. Most of flavonoids are competitively inhibit tyrosinase by binding with tyrosine. Some of flavonoids, such as catechin, rhamnetin,kaempferol,quercetin and morin are analogue of tyrosine that competitively inhibits tyrosinase activity (Kim 2013, Kubo et al., 2000, Xieet al., 2003).Steppogenin, is a flavanone derivative,isolated from the Cudrania tricuspidatatwigs, showed a potential inhibition to tyrosinase activity by about10-timesgreater than kojic acid(Zehng et al., 2013).
Aloe sin, a hydroxychromone glucoside purified from Aloe vera. It has been shown to be a potent and selective tyrosinase’s inhibitor which has direct inhibitory effects on melanogenesis (Jin and Lee, 1999).After UV radiation aloesin may be able to inhibit the hyperpigmentation in a dose-dependent manner (Choiet al., 2002).
Coumarins are heterocyclic compounds naturally occurs, consists of an aromatic ring connected to a condensed six-member lactone ring. Both coumarins and their derivatives are characterized to be biologically and pharmacologically active especially as inhibitors for tyrosinase (Bubols et al., 2013; Liu and Wuet al., 2012 and Hassan et al. 2018). Esuletin, dihydroxylcoumarin isolated from Euphorbia lathyris, displaying a potential tyrosinase inhibition. Other coumarin analogs, 9-hydroxy-4-methoxypsoralen, isolated from Angelica dahurica (Ahmad et al., 2004). From the molecular modeling studies, the position of hydroxyl group substituent of coumarin has an important role in tyrosinase inhibition. Hydroxycoumarin, trans-N-coumaroyltyramine and cis-N-coumaroyltyramine from Humulus japonicas showed a powerful tyrosinase inhibition (Yang and Oh, 2018).
Gallic acid (3,4,5-trihydroxy benzoic acid) is a polyphenol compound displaying a potential inhibitory effect to tyrosinase activity (Kubo et al., 2000, Lu and Nie et al., 2006) by reducing dopaquinone back to L-DOPA through a redox reaction. Gallic acid and its dervitives esters are used in the field of food industry as additives. Gallic acid prevents the oxidation reaction of L-DOPA enhanced by tyrosinase (Masudaet al., 2008).Most of gallic acid derivatives have been isolated from green tea and Galla rhois (Khan et al., 2006).
Anthraquinones have various pharmacological applications especially anti-tyrosinase activity that beingmore powerful than kojic acid (Leu etal., 2008).Among the natural anthraquinones, benzaldehyde, benzoic acid, anisaldehyde, cinnamic acid, anisic acid and vanillic acid (Maghsoudi et al., 2013, Nihei and Kubo, 2017).These compounds displaying noticeable inhibitory activities towards tyrosinase. For example, 3,4-dihydroxybenzaldehyde-Oethyloxime (IC500.3 µM) had a potent tyrosinase inhibitory effect that being consistent to the authentic inhibitor, tropolone (IC500.13 µM) (Ley and Bertram, 2001). The aldehyde group and terminal methoxy group in derivatives of benzaldehyde playing an important role on its effect as inhibitor by chelating the copper ion present in tyrosinase’s active site (Conrad et al., 1994).
Fungal secondary metabolites
Fungi produce a plethora of bioactive compounds especially with potential inhibitory effect to tyrosinase (Critonet al., 2008).
Among these metabolites, kojic acid has been reported as the most powerful “well-known” inhibitors to tyrosinase activity. Kojic acid (5-hydroxy- 2-hydroxymethyl-γ-pyrone) has been used commonly as skin-whitening cosmetic (Da Costaet al., 2018) and as anadditive in food for inhibition of browning by enzymes(Bentley, 2006). Kojic acid works as as a bidentate chelator for the reaction of metal ions transition such as Cu+2 and Fe+3, with an inhibitory effect to the monophenolase and diphenolase enzyme activity of tyrosinase from mushroom (Kim and Uyama, 2005).Nevertheless, the usage of kojic acid in cosmetics is limited because under the light it’s unstable and also, in aerobic condition, thereby;many trails for creating synthetic kojic acid derivatives were reported aiming to improving the ability metal chelating and structural stability (Saghaie et al., 2013). Kojic acid was frequently produced by A. albus, A. candidus, A. niger and Penicillium sp(Wei et al. 1991).
Azelaic acid (1, 7-heptanedicarboxylic acid),which is a saturated dicarboxylic acid in a straight-chain, that mainly obtained from yeast, Pityrosporum ovale through lipo peroxidation of free and esterified cis-polyunsaturated fatty acids. This acid is a cytotoxic agent on malignant melanocytes of primary cutaneous melanoma, whilehas no effect on normal melanocytes (Schallreuter and Wood, 1990).
Yeast metallothioneins are ubiquitous cytosolic proteins witha selective binding to heavy metal ions (Zn2+, Cu2+ and Cd2+) and cysteine(Hamer, 1986). It was reported that Neurospora crassa copper-metallothionein is a metal donor for apo-tyrosinase. Metallothionein from Aspergillus niger was reported as an inhibitor for various mushroom tyrosinases.
Agaritine, β-N-(γ-L(+)-glutamyl)-4-hydroxymethyl phenylhydrazine, giving a pigment reduction activity by preventing the formation of melanin by acting as uncompetitive and partially competitive inhibitors to tyrosinase (Valverde et al., 2015). Agaritine is mainly produced by Agaricus bisporus mushrooms, suggesting that agaritine canacts in vivo a role as regulator of mushroom tyrosinase activity endogenously (Valverde et al., 2015).
Chemically synthesized tyrosinase inhibitors
Captopril ((2S)-N-(3-mercapto-2-methylpropionyl)-L-proline) is a drug has a wide application in the hypertension treatment and heart failure due to its effect in inhibition of the angiotensin-converting enzyme (Cleland, 1994). This drug is characterized by irreversibly inhibition to monophenolase of tyrosinase from mushroom, although,it exhibits irreversible competitive inhibition to diphenolase of tyrosinase (Kuo and Ho, 2013). Inhibition of monophenolase and diphenolase activities of tyrosinase by captopril showing a positive kinetic cooperativity. Also, Captopril is known as a copper chelator,so, its inhibitory mechanism could be rationalized bychelating copper ions at the active site of tyrosinase. As well as, Tropolone (2-hydroxy- 2,4,6-cycloheptatriene) is one of the most potenttyrosinase inhibitors (Ismaya and Rozeboom, 2011). It is analogous in its structure to tyrosinase’so-diphenolic substrates as well as an effective copper chelator.
Resorcinols such as m-coumaric acid, L-mimosine and 4-substituted resorcinols has also been reported with slow-binding inhibition of tyrosinases from different sources(Garcia-Jimenez et al., 2016). However, resorcinol is a poor tyrosinase inhibitor, substitution in the 4-position yields increased inhibitory activity. The highest inhibition was obtained with hydrophobic substituents in the 4-position such as 4-hexyl- and 4-dodecylresorcinol (McEvily et al.,1992). Hexylresorcinol was reported as an effective inhibitor to be used in the food industry due to its characters which includes, water solubility, stability, nontoxicity, non-mutagenicity and non-carcinogenicity, and it has been used in the prevention of shrimp melanosis because it recognized as safe and also,in controlling of browning in fresh and hot-air-dried apple pecies as well as potatoes and avocados.
Hydrogen peroxide is reported as inhibitors of diverseenzymes which contain copper- such as dopamine b-monooxygenase and tyrosinase produced by mushroom (Andrawis and Kahn, 1985; Connor et al., 2011). Activities of both monophenolase and diphenolase of mushroom tyrosinase can be lost on exposure to high concentration ofH2O2. In the presence of relatively high concentrations of H2O2, tyrosinase is converted to an oxy-oxytyrosinase (inactivated form of tyrosinase), which is a hypothetical intermediate analogous to the ternary complex formed between oxytyrosinase and o-dihydroxyphenol.
In conclusion, the biochemical, molecular and structural properties of tyrosinases from different microbial sources has been extensively documented. The potential chemical inhibitors of different biological identities for tyrosinases have been addressed that could be used as anti-melanin hyperpigmentation as well as in treatment of neuromelanin disorders.
Acknowledgement
None
Conflict of Interest
None
References
- Aberg C. M., Chen T., Ayotunde O., Srinivasa R. R., Gregory F. P. (2004). Enzymatic Grafting of Peptides from Casein Hydrolysate to Chitosan. Potential for Value-Added Byproducts from Food- Processing Wastes. Journal of Agriculture and Food Chemistry 52(4):788–793.
- Ahmad V.U., Ullah F., Hussain J., et al. (2004). Tyrosinase inhibitors from Rhododendron collettianum and their structure-activity relationship (SAR) studies. Chemical and Pharmaceutical Bulletin 52:1458–61.
- Andrawis A. and Kahn V. (1985). Inactivation of mushroom tyrosinase by hydrogen peroxide. Phytochemistry 24: 397– 405.
- Arafa A.M, Abd-Elghany A.E, El-Dahmy S.I, Abdelaziz S, El-Ayouty Y, El-Sayed A.S.A (2020): Partial purification and characterization of algal phenylalanine ammonia-lyase as a novel approach for myristicin biotransformation. Journal of Microbiology and Biotechnology. 30(4): 622-632.
- Badria F. A., El Gayyar M. A. (2001) A new type of tyrosinase inhibitors from natural products as potential treatments for hyperpigmentation. Bollettino chimico farmaceutico 140: 267–271.
- Bentley R. (2006). From miso, sake and shoyu to cosmetics: a century of science for kojic acid. Natural Product Research 23:1046–62.
- Birse C.E. and Clutterbuck A.J. (1990). N-acetyl-6-hydroxytryptophan oxidase, a developmentally controlled phenol oxidase from Aspergillus nidulans. J. Gen. Microbiol. 136, 1725-1730.
CrossRef - Bubols G.B., Vianna D.R., Medina-Remon A., et al. (2013). The antioxidant activity of coumarins and flavonoids. Mini Reviews in Medicinal Chemistry13: 318–34.
- Busch J.M. (1999). Enzymic browning in potatoes: a simple assay for a polyphenol oxidase catalysed reaction. Biochemistry and Molecular Biology Education 27:171–
- Cabanes J., García-Cánovas F., Lozano J. A., García-Carmona F. (1987). A kinetic study of the melanization pathway between L-tyrosine and dopachrome. Biochimica et Biophysica Acta 923: 187–195.
- Chen X., Shi Y., Chai W., Feng H., Zhuang J., Chen Q. (2014). Condensed tannins from Ficus virens as tyrosinase inhibitors: structure, inhibitory activity and molecular mechanism, PloS One, 9, e91809.
- Chen X., Shi Y., Chai W., Feng H., Zhuang J., Chen Q. (2014). Condensed tannins from Ficus virens as tyrosinase inhibitors: structure, inhibitory activity and molecular mechanism, PloS One, 9, e91809.
- Choi S., Lee S.K., Kim J.E., et al. (2002). Aloesin inhibits hyperpigmentation induced by UV radiation. Clinical And Experimental Dermatology 27:513–15.
- Claus H. and Decker H. (2006). Bacterial tyrosinases. Systematic and Applied Microbiology 29: 3–14.
- Cleland J. G. (1994). The clinical course of heart failure and its modification by ACE inhibitors: insights from recent clinical trials. European Heart Journal 15: 125–130.
- Connor K.L., Colabroy K.L., Gerratana B. (2011). A heme peroxidase with a functional role as an L-tyrosine hydroxylase in the biosynthesis of anthramycin. Biochemistry 50(41):8926-8936.
- Conrad J. S., Dawso S. R., Hubbard E. R., Meyer T. E., Strothkamp K. G. (1994). Inhibitor binding to the binuclear active site of tyrosinase: temperature, pH, and solvent deuterium isotope effects. Biochemistry 33: 5739–5744.
- Cooksey C. J., Garratt P. J., Land E. J., Pavel S., Ramsden C. A., Riley P. A. et al. (1997). Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. Journal of Biological Chemistry 272: 26226–26235.
- Criton M. and Le Mellay-Hamon V. (2008). Analogues of N-hydroxy-N0-phenylthiourea and N-hydroxy-N0-phenylurea as inhibitors of tyrosinase and melanin formation. Bioorganic&Medicinal Chemistry Letters 18:3607–10.
- Cunha MM, Franzen AJ, Seabra SH, Herbst MH, Vugman NV, Borba LP, de Souza W, Rozental S (2010) Melanin in Fonsecaea pedrosoi: a trap for oxidative radicals. BMC Microbiol 10:80–85.
CrossRef - d’Ischia M, Pezzella A, Meredith P, Sarna T (2009) Chemical and structural diversity in eumelaninsunexplored bio-optoelectronic materials. Angew Chem In Ed Engl 48:3914–3921.
CrossRef - D’Mello S. A. N., Finlay G. J., Baguley B. C., Askarian-Amiri M. E. (2016). Signaling pathways in melanogenesis, Int. J. Mol. Sci., 17, 1144.
- Da Costa J.P. et al. (2018). Biological effects of kojic acid on human monocytes in vitro, Biomed. Pharmacother. 101;100–106.
CrossRef - Decker H. and Tuczek F. (2000). Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends in Biochemical Sciences 25:392–7.
- El-Sayed, A.S.A, Shindia, A.A. Diab, A.A. Rady A.M. (2015): Purification, Immobilization, Biochemical characterization of thermostable L-Arginase from thermotolerant Penicillium chrysogenum. Archives of Pharmacal Research (DOI: 10.1007/s12272-014-0498-y).
- El-Sayed, A.S.A. Ibrahim, H., Sitohy, M.Z. (2014): Co-Immobilization of PEGylated Aspergillus flavipes L-methioninase with glutamate dehydrogenase: A catalytically stable consortium. Enzyme and Microbial Technology 54: 59-69.
CrossRef - El-Sayed, A.S.A., Abdel-Azim, S., Ibrahim H., Yassin, M.A., Abdel-Ghany S., Esener, S. Ali, GS (2015): Biochemical stability and molecular dynamic characterization of Aspergillus fumigatus cystathionine -Lyase in response to various reaction effectors. Enzyme and Microbial Technology 81: 31–46.
CrossRef - El-Sayed, A.S.A., El Sayed M,T, Rady, A., Zein N, Enan G., Shindia, A.,A, El-Hefnawy, S.A, Sitohy, M. Sitohy, B. (2020): Exploiting the biosynthetic potency of Taxol from fungal endophytes of Conifers plants; Genome mining, and metabolic manipulation. Molecule, 25, 3000; doi:10.3390/molecules25133000.
CrossRef - El-Sayed, A.S.A., George, N.M., Bolbol, A.A., Mohamed, M.S. (2019): Purification and biochemical characterization of Aspergillus terreus ornithine decarboxylase: Curcumin is a potent enzyme inhibitor. Molecule, 24: 1-16.
CrossRef - El-Sayed, A.S.A., Hassan, A.E.A., Shindia, A.A., Mohamed, S.G., Sitohy, M.Z. (2016) Aspergillus flavipes L-methionine γ-lyase dextran conjugates with enhanced structural proteolytic stability and anticancer efficiency. Journal of Molecular Catalysis: B-enzymatic, 133: S15-S24.
CrossRef - El-Sayed, A.S.A., Khalaf, S., El-Batrik, M.I. Ali, GS, Esener, S. (2015): Cystathionine γ-Lyase from Aspergillus carneus; purification, PEGylation, chitosan immobilization, characterization and pharmacokinetic properties. Journal of Molecular Microbiology and Biotechnology 25: 301-310.
CrossRef - El-Sayed, A.S.A., Khalaf, S.A. and Ahmed, H.A. (2013): Characterization of homocysteine -lyase from submerged and solid fermented cultures of Aspergillus fumigatus JX006238. Journal of Microbiology and Biotechnology, 23: 499-510.
CrossRef - El-Sayed, A.S.A., Khalaf, S.A., Abdel Hamid, G., El-Batrik, M.I. (2015): Screening, morphological and molecular identification of cystathionine γ-lyase producing fungi. Acta Biologica Hungarica, 66 (1) 119–132.
CrossRef - El-Sayed, A.S.A., Laura E. Luff, Salah E. Abdel-Ghany, Gul Shad Ali, Esener, S. (2017) Molecular and Spectroscopic Characterization of Aspergillus flavipes and Pseudomonas putida L-Methionine γ-lyase in vitro. Applied Biochemistry and Biotechnology, 181:1513–1532.
CrossRef - El-Sayed, A.S.A., Nada, H.M, Hassan M.N. (2015): Purification, immobilization, and biochemical characterization of L-arginine deiminase from thermophilic Aspergillus fumigatus KJ434941: Anticancer Activity In vitro. Biotechnology Progress, 31 (2): 396-405.
CrossRef - El-Sayed, A.S.A., Shindia, A.A., Abou-Zaid, A.A. Yassin, A.M. (2019): Aspergillus nidulans arginine deiminase- Dextran conjugates with enhanced molecular stability, proteolytic resistance, pharmacokinetic properties and anticancer activity. Enzyme and Microbial Technology 131: 12: 109432.
CrossRef - El-Sayed, A.S.A., Shindia, A.A., AbouZaid, A.A. Yassin, A.M., Ali, G.S., Sitohy M (2019): Biochemical characterization of peptidylarginine deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzyme and Microbial Technology. 124: 41-53.
CrossRef - El-Sayed, A.S.A., Shindia, A.A., Ammar, H., Yassin, M.A., Hussein, H.A., Awad, S.A, Ali, G.S. (2021): Production and bioprocess optimization of microtubule-stabilizing antitumor Epothilone B analogue from Aspergillus fumigatus, an endophyte of Catharanthus roseus, with the response surface methodology. Enzyme and Microbial Technology, 143: 109718.
CrossRef - El-Sayed, A.S.A., Shindia, A.A., Zaher, Y. (2013): Purification and characterization of L-amino acid oxidase from the solid cultures of Aspergillus oryzae ASH. Microbiology 82: 750-759.
CrossRef - El-Sayed, A.S.A., Yassin, M.A., Ali, G.S. (2015): Transcriptional and proteomic profiling of Aspergillus flavipes in response to sulfur starvation. PLOS One 3;10(12).
- El-Sayed, M.T, El-Sayed, A.S.A. (2020): Bioremediation and tolerance of zinc ions using Fusarium solani. Heliyon, 6 (2020) e05048.
- Faria R.O., Vivian R. M., Maria A. L. A. A., Nadia K., David A. M. (2007). The Biotechnological Potential of Mushroom Tyrosinaes. Food technology and Biotechnology 45(3): 287-294.
- Fenoll L. G., Rodríguez-López J. N., García-Sevilla F., García- Ruiz P. A., Varón R., García-Cánovas F. et al. (2001.) Analysis and interpretation of the action mechanism of mushroom tyrosinase on monophenols and diphenols generating highly unstable o-quinones. Biochimica et Biophysica Acta 1548: 1–22.
- Fogal, S., Carotti, M., Giaretta, L., Lanciai, F., Nogara, L., Bubacco, L., Bergantino, E. (2015). Human tyrosinase produced in insect cells a landmark for the screening of new drugs addressing its activity. Mol. Biotechnol. 57, 45-57.
CrossRef - Friedman M. (1996). Food browning and its prevention: an overview. Journal of Agriculture and Food Chemistry. 44: 631–653.
- Fujieda N.. Yabuta S.. Ikeda T.. Oyama T., Muraki N., Kurisu G., Itoh S. (2013). Crystal Structures of Copper-depleted and Copper-bound Fungal Pro-tyrosinase INSIGHTS INTO ENDOGENOUS CYSTEINE DEPENDENT COPPER INCORPORATION. J. Biol. Chem. 288, 22128–22140.
- Fujimoto N., Onodera H., Mitsumori K., Tamura T., Maruyama S., Ito A. (1999). Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats, Carcinogenesis, 20, 1567e1571.
- Garcia-Jimenez A., Teruel-Puche J.A., Berna J., Rodriguez-Lopez J.N., Tudela J., Garcia-Ruiz P.A., Garcia-Canovas F. (2016). Characterization of the action of tyrosinase on resorcinols. Bioorg. Med. Chem. 24, 4434-4443 .
CrossRef - Garcia-Jimenez A., Teruel-Puche J.A., Garcia-Ruiz P.A. et al. (2017). Action of 2,2’,4,4’-tetrahydroxybenzophenone in the biosynthesis pathway of melanin. Int J Biol Macromol 98:622–9.
- Goldfeder M., Kanteev M., Isaschar-Ovdat S., Adir N., Fishman A. (2014). Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun., 5, 4505.
CrossRef - Halaban R., Patton R.S., Cheng E. et al. (2002). Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. Journal of Biological Chemistry 277:14821–8.
- Halaouli S., Asther M., Kruus K., Guo L., Hamdi M.; Sigoillot, J.C.; Lomascolo, A. (2005). Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J. Appl. Microbiol. 98, 332-343.
CrossRef - Hamer D. H. (1986). Metallothionein 1,2. Annual Review of Biochemistry. 55: 913–951.
- Harborne J.B. and Williams C.A. (2000). Advances in flavonoid research since1992. Phytochemistry 55:481–504.
- Hassan M., Ashraf Z., Abbas Q. et al. (2018). Exploration of novel human tyrosinase inhibitors by molecular modeling, docking and simulation studies. Interdiscip Sci ;10:68–80.
- Heck T., Greta F., Michael R., Linda T. (2013). Enzyme catalyzed protein crosslinking. Applied Microbiology and Biotechnology, 97(2): 461-475.
- Ioannou I. and Ghoul M. (2013). Prevention of enzymatic browning in fruit and vegetables. European Scientific Journal 9(30):310–
- Ismaya W.T., Rozeboom H.J., Weijn A., Mes J.J., Fusetti F., Wichers H.J., Dijkstra B.W. (2011). Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry, 50, 5477–5486.
CrossRef - Jaenicke E. and Decker H. (2003). Tyrosinases from crustaceans form hexamers. Biochemical Journal 371(2): 515–523.
CrossRef - Jin Y.H., Lee S. J., Chung M. H. et al. (1999). “Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism,” Archives of Pharmacal Research, vol. 22, pp. 232–236.
- Jones K., Hughes J., Hong M., et al. (2002). Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Research 15:335–40.
- Kebeish, M.R, E-Sayed, A.S.A., Fahmy, H. and Abdel-Ghany, A. (2016): Molecular cloning, biochemical characterization and antitumor properties of a novel L-asparaginase from Synechococcus elongates. Biochemistry, 81, 1173–1181.
- Khan M.T., Khan S.B., Ather A. (2006). Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosaJafri and their structure-activity relationship. Bioorganic and Medicinal Chemistry 14:938–43.
- Kim Y.J. (2013). Rhamnetin attenuates melanogenesis by suppressing oxidative stress and pro-inflammatory mediators. Biological and Pharmaceutical Bulletin 36:1341–7.
- Kim, Y. and Uyama, H. (2005). Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cellular and Molecular Life Scien., 62(15): 1707-1723.
- Klabunde T., Eicken C., Sacchettini J. C., Krebs B. (1998). Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol, 5, 1084–1090.
CrossRef - Kong K.H., Hong M.P., Choi S.S., Kim Y.T., Cho S.H. (2000). Purification and characterization of a highly stable tyrosinase from Thermomicrobium roseum. Appl. Biochem. 31, 113-118.
CrossRef - Kong K.H., Hong M.P., Choi S.S., Kim Y.T., Cho S.H. (2000). Purification and characterization of a highly stable tyrosinase from Thermomicrobium roseum. Appl. Biochem. 31, 113-118.
CrossRef - Kubglomsong S., Theerakulkait C., Reed R.L., Yang L., Maier C.S., Stevens J.F. (2018).. Isolation and identification of tyrosinase-inhibitory and copper-chelating peptides from hydrolyzed rice-bran-Derived albumin. J. Agric. Food Chem. 66 (31), 8346–8354.
CrossRef - Kubo I. and Kinst-Hori I. (1999). Tyrosinase inhibitory activity of the olive oil flavor compounds. Journal of Agricultural and Food Chemistry 47: 4574–4578.
- Kubo I., Kinst-Hori I., Kubo Y. et al. (2000). Molecular design of antibrowning agents. Journal of Agricultural and Food Chemistry 48:1393–9.
- Kuo T. C. and Ho F. M. (2013). Competitive Inhibition of Mushroom Tyrosinase by Captopril’, Res. J. BioTechnol, 8, 26–29.
- Kupper U., Niedermann D. M., Travaglini G., Lerch K. (1989). Isolation and characterization of the tyrosinase gene from Neurospora crassa. Journal of Biological Chemistry. 264: 17250– 17258.
- Le Roes-Hill M., Palmer Z., Rohland J., Kirby B., Burton S. (2015). Partial purification andcharacterization of two actinomycete tyrosinases and their application in cross-linking reactions. Mol. Catal. B 122, 353-364.
CrossRef - Lee S. E., Kim M. K., Lee S. G., Ahn Y. J., Lee H. S. (2000). Inhibitory effects of Cinnamomum cassia bark-derived materials on mushroom tyrosinase. Food Science and Biotechnology 9: 330– 33.
- Lee S.G., Karadeniz F., Seo Y., Kong C.S. (2017). Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (thunb.) bullock via inhibition of tyrosinase and tyrosinaserelated proteins. Molecules ;22:1480–90.
- Leu Y.L., Hwang T.L., Hu J.W., Fang J.Y. (2008). Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytotherapy Research 22:552–6.
- Ley J.P., Bertram H.J. (2001). Hydroxy- or methoxy-substituted benzaldoximes and benzaldehyde-o-alkyloximes as tyrosinase inhibitors. Bioorg Med Chem 9:1879–85.
- Lin J.W., Chiang H.M., Lin Y.C., Wen K.C. (2008). Natural products with skin-whitening effects. Journal of Food and Drug Analysis 16(2):1–
- Liu J., Wu F., Chen L. et al. (2012). Biological evaluation of coumarin derivatives as mushroom tyrosinase inhibitors. Food Chem ;135:2872–8.
- Liu N., Zhang T., Wang Y.J., Huang Y.P., Ou J.H., Shen P. (2004). A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Appl. Microbiol. 39, 407-412.
CrossRef - Lopez-Tejedor D. and Palomo J.M. (2018). Efficient purification of a highly active H-subunit of tyrosinase from Agaricus bisporus. Protein Expr Purif 145:64–70.
- Lu Z., Nie G., Belton P. et al. (2006). Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int., 48, 263—274.
CrossRef
- Maghsoudi S., Adibi H., Hanzeh M, et al. (2013). Kinetic of mushroom tyrosinase inhibition by benzaldehyde derivarives. Journal of Reports in Pharmaceutical Sciences 2:156–64.
- Marino S.M., Fogal S., Bisaglia M. et al. (2011). Investigation of Streptomyces antibioticus tyrosinase reactivity toward chlorophenols, Arch. Biochem. Biophys. 505: 67–74. DOI: 10.1016/j.abb.2010.09.019.
CrossRef - Masuda T., Odaka Y., Ogawa N., et al. (2008). Identification of geranic acid, a tyrosinase inhibitor in lemongrass (Cymbopogon citratus). Journal of Agricultural and Food Chemistry 56:597–601.
- Mauracher S. G., Molitor C., Al-Oweini R., Kortz U., Rompel A. (2014). Latent and active abPPO4 mushroom tyrosinase cocrystallized with hexatungstotellurate(VI) in a single crystal. Acta Crystallogr. Sect. D, 70, 2301–2315.
CrossRef - Mayer A.M. (2006). Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 67(21): 2318-2331.
- McEvily J. A., Iyengar R., Otwell W. S. (1992). Inhibition of enzymatic browning in foods and beverages. Critical Reviews in Food Science and Nutrition 32: 253–273.
- McMahon, A.M., Doyle E.M., Brooks S., OConnor K.E. (2007). Biochemical characterization of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida Enzyme Microb. Technol. 40, 1435-1441.
CrossRef - Meredith P, Sarna T (2006) The physical and chemical properties of eumelanin. Pigment Cell Res 19:572–594.
CrossRef - Mohania D., Chandel S., Kumar P., Verma V., Digvijay K., Tripathi D., Choudhury K., Mitten S.K., Shah D. (2017). Ultraviolet radiations: skin defense-damage mechanism, Adv. Exp. Med. Biol. 996:71–87.
CrossRef - Molloy S., Nikodinovic-Runic J., Martin L.B., Hartmann H., Solano F., Decker H., OConnor K.E. (2013). Engineering of a bacterial tyrosinase for improved catalytic efficiency towards D-tyrosine using random and site directed mutagenesis approaches. Biotechnol. Bioeng. 110, 1849-1857.
CrossRef - Nicolas J.J., Richard-Forget F.C., Goupy P.M., Amiot M.J., Aubert S.Y. (1994). Enzymatic browning reactions in apple and apple products. Critical Reviews in Food Science and Nutrition 34:109–
- Nihei K.I. and Kubo I. (2017).Substituent effect of benzaldehydes on tyrosinase inhibition. Plant Physiol Biochem;112:278–82.
- Oetting W. S. and King R. A. (1994). Molecular basis of oculocutaneous albinism. Journal of Investigative Dermatology. 103: 131S–136S.
- Olennikov DN, Tankhaeva LM, Rokhin AV, Agafonova SV (2012) Physicochemical properties and antioxidant activity of melanin fractions from Inonotus obliquus Chem Natural Compounds 48:396–403.
CrossRef - Olivares C., García-Borrón J. C., Solano F. (2002). Identification of Active Site Residues Involved in Metal Cofactor Binding and Stereospecific Substrate Recognition in Mammalian Tyrosinase. Implications to the Catalytic Cycle†. Biochemistry. 41, 679–686.
CrossRef - Olsen S, Riesz J, Mahadevan I, Coutts A, Bothma JP, Powell BJ, McKenzie RH, Smith SC, Meredith P (2007) Convergent proton-transfer photocycles violate mirror-image symmetry in a key melanin monomer. J Am Chem Soc 129:6672–6673.
CrossRef - Pillaiyar T., Manickam M., Jung S.H. (2015). Inhibitors of melanogenesis. Expert Opinion on Therapeutic Patents 7:775–88.
CrossRef - Riesz J, Gilmore J, Meredith P (2006) Quantitative scattering of melanin solutions. Biophys J 90(11):4137–4144.
CrossRef - Robb D.A. (1984). Tyrosinase. In: Lontie R (ed) Copper proteins and copper enzymes, vol 2. CRC Press, Boca Raton, pp 207–
- Różanowska M, Sarna T, Land E, Truscott T (1999) Free radical scavenging properties of melanin: interaction of eu- and pheomelanin models with reducing and oxidizing radicals. Free Rad Biol Med 26:518–525.
CrossRef - Saghaie L., Pourfarzam M., Fassihi A., Sartippour B. (2013).Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Journal of Pharmaceutical Sciences and Research 8:233–42.
- Sánchez-Ferrer A., Rodríguez-López J.N., García-Cánovas F., García- Carmona F. (1995). Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta.1247 (1):1-11.
- Sanjust E., Cecchini G., Sollai F., Curreli N., Rescigno A. (2003). 3-Hydroxy kynurenine as a substrate/activator for mushroom tyrosinase. Archives of Biochemistry and Biophysics. 412: 272– 278.
- Schallreuter K. U.; Wood J. W. (1990) A possible mechanism of action for azelaic acid in the human epidermis. Archives of Dermatological Research 282: 168–171.
CrossRef - Selinheimo E., Saloheimo M., Ahola E., Westerholm-Parvinen A., Kalkkinen N., Buchert J., Kruus K. (2006). Production and characterization of a secreted, C-terminally processed tyrosinase from the filamentous fungus Trichoderma reesei. FEBS J. 273, 4322-4335.
CrossRef - Selinheimo, E., NiEidhin, D., Steffensen, C., Nielsen, J., Lomascolo, A., Halaouli, S, Record, E., OBeirne, D., Buchert, J., Kruus, K. (2007). Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol. 130, 471-480.
CrossRef - Sendovski M., Kanteev M., Ben-Yosef V. S., Adir N., Fishman A. (2011). First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol., 405, 227–237.
CrossRef - Sendovski M., Kanteev M., Ben-Yosef V. S., Adir N., Fishman A. (2011). First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol., 405, 227–237.
CrossRef - Seo-Yum S., Vinay K. S., Niti S. (2003). Mushroom Tyrosinase: Recent Prospects. Journal of Agricultural and Food Chemistry, 51: 2837-2853.
- Shiino M., Watanabe Y., Umezawa K. (2001). Synthesis of N-substituted N-nitrosohydroxylamines as inhibitors of mushroom tyrosianse. Bioorganic and Medicinal Chemistry 9: 1233–1240.
- Slominski A., Tobin D. J., Shibahara S., Wortsman J. (2004). Melanin pigmentation in mammalian skin and its hormonal regulation, Physiol. Rev., 84, 1155e1228.
- Slominski A., Tobin D.J., Shibahara S. et al. (2004). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological Reviews 84:1155–228.
- Sutay Kocabas D., Bakir U., Phillips S.E., McPherson M.J. (2008). Purification, characterization, and identification of a novel bifunctional catalase-phenol oxidase from Scytalidium thermophilum; Ogel, Z.B.; Appl. Microbiol. Biotechnol. 79, 407-415.
CrossRef - Tinello F. and Lante A. (2018). Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 50, 73–83. doi: 10.1016/j.ifset.2018.10.008.
CrossRef - Valipour E., Burhan A. (2016). Increased Production of Tyrosinase from Bacillus Megaterium strain M36 by the Response Surface Method. Archives of biological sciences 68(3): 659-668.
- Valverde M., Hernández‑Pérez T., Paredes‑López O. (2015). Edible mushrooms: Improving human health and promoting quality life. Int J Microbiol; 2015:376387.
- Vasantha K.Y., Murugesh C.S., Sattur A.P. (2014). A tyrosinase inhibitor from Aspergillus niger. Journal of Food Science and Technology51(10): 2877–
- Videira I. F., Moura D. F., Magina S. (2013). Mechanisms regulating melanogenesis, An. Bras. Dermatol., 88, 76e83.
CrossRef - Virador V. M., Grajeda J. P. R., Blanco-Labra A., E. Mendiola- Olaya E., Smith G. M., Moreno A., Whitaker J. R. (2010). Cloning, sequencing, purification, and crystal structure of Grenache (vitis vinifera) polyphenol oxidase. J. Agric. Food Chem. 58, 1189–1201
CrossRef - Wang Y., Curtis-Long M.J., Lee B.W. et al. (2014). Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorganic and Medicinal Chemistry 22:1115–20.
- Wei C.I., Huang T.S., Chen J.S., Marshall M.R., Chung K.T. (1991). Production of kojic acid by Aspergillus candidus in three culture media. Journal of Food Protection 54(7):546–
- Wilcox D. E, Porras A. G, Hwang Y. T, Lerch K., Winkler M. E, Solomon I (1985). Substrate analogue binding to the coupled binuclear copper active site in tyrosinase. Journal of American Chemistry Society 107: 4015–4027.
- Wu J., Chen J., Gao J., Liu X., Cheng W., Ma X. (2010). Cloning, Characterization and expression of two new polyphenoloxidase cDNAs from Agaricus bisporus. Biotechnology Letters 32(10): 1439–1447.
- Xie L.P., Chen Q.X., Huang H., et al. (2003). Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry 68:487–91.
- Xu H., Zhang X., Karangwa E. and Xia S. (2017). Correlating enzymatic browning inhibition and antioxidant ability of Maillard reaction products derived from different amino acids. Journal of the Science of Food and Agriculture. 97, 4210–4218.
- Yang H.H., Oh K.E., Jo Y.H., et al. (2018). Characterization of tyrosinase inhibitory constituents from the aerial parts of Humulus japonicus using LC MS/MS coupled online assay. Bioorg Med Chem; 26:509–15.
- Yi W., Cao R., Peng W. et al. (2010). Synthesis and biological evaluationof novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors. European Journal of Medicinal Chemistry 45:639–46.
- Yoshimoto T., Yamamoto K., Tsuru D. (1985). Extracellular tyrosinase from Streptomyces KY-453: purification and some enzymatic properties. J. Biochem. 97, 1747-1754.
CrossRef - Zaidi K.U., Ali A.S., Ali S.A (2014). Purification and characterization of melanogenic enzyme tyrosinase from button mushroom. Enzyme Res, 120739.
CrossRef - Zehng Z.P., Tan H.Y., Chen J., Wang M. (2013). Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structureactivity relationship study. Fitoterapia 84:242–7.
- Zekiri F, Molitor C, Mauracher SG, Michael C, Mayer RL, Gerner C, Rompel A. (2014). Purification and characterization of tyrosinase from walnut leaves (Juglans regia).Phytochemistry. 101:5–15.
- Zhang T., Wen S., Tan T. (2007). Optimization of the medium for glutathione production in Saccharomyces cerevisiae. Process Biochem,; 42:454-458.
- Zhang Z., Wang J., Zhang X., Shi Q., Xin L., Fu H. et al. (2018b). Effects of radio frequency assisted blanching on polyphenol oxidase, weight loss, texture, color and microstructure of potato. Food Chem. 248, 173–182. doi: 10.1016/ j.foodchem.2017.12.065.
- Zheng Z.P., Cheng K.W., Chao J., Wu J., Wang M. (2008). Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera). Food Chemistry 106:529–535
CrossRef - Zolghadri S., Bahrami A., Khan M.T.H., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., Saboury A.A. (2019). A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 34, 279–309.
CrossRef