Manuscript accepted on :08-09-2021
Published online on: 20-09-2021
Plagiarism Check: Yes
Reviewed by: Dr. Yogesh Kumar

Second Review by: Dr. Ahmed Salah

Final Approval by: Dr. Ayush Dogra
Bhadekar N. S. and Zodape G.V*
and Zodape G.V*
Department of Zoology, S.S. and L.S. Patkar College of Arts and Science and V.P. Varde College of Commerce and Economics, S.V. Road, Goregaon West, Mumbai- 400 062, India
Corresponding Author E-mail: drgautamvz5@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2269
Abstract
The sponge Sigmadocia fibulata (Schmidt) was collected during low tides from West Coast of Mumbai. Crude extract was obtained by taking 10 gram of sponge samples in10 ml of methanol. The preparative TLC (Thin Layer Chromatography) was performed by using Toluene: Ethyl acetate: Diethylamine (7:2:1) (v/v). The isolated compounds were subjected to GC-MS and FTIR analysis. The structural properties of bio active compounds were determined.From the structural determination it was confirmed that S. fibulata contains bioactive compounds as Triacontanoic acid, methyl ester – (Skin irritant), Hexadecanoic acid, 2- hydroxyl- (hydroxymethyl) ethyl ester – (Fatty acid, Metabolite and Irritant) and 2-Nitro-1, 3-bis-oclyoxy-benzene, (A natural product found in Neolitsea daibuensis. It has a role as a plant metabolite and an algal metabolite). From their biological properties it was confirmed that S. fibulata contains bio active compound, which has biomedical and pharmaceutical properties.
Keywords
Analytical Study; Bioactive compounds; Sponge
Download this article as:| Copy the following to cite this article: Bhadekar N. S, Zodape G. V. Isolation and Partial Purification of Bioactive Compounds from Sponge Sigmadocia Fibulata (Schmidt) Collected from West Coast of Mumbai, India. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Bhadekar N. S, Zodape G. V. Isolation and Partial Purification of Bioactive Compounds from Sponge Sigmadocia Fibulata (Schmidt) Collected from West Coast of Mumbai, India. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3lyQtQk |
Introduction
Sponge contains a large number of unique and diverse natural products. For medicinal use many sponges have contributed the unique bioactive compounds. The arrays of secondary metabolites of sponges are ranging from amino acids and nucleotides to peroxides and sterols 1. Many bioactive compounds have immense pharmacological properties, which includes anti tumor, viral, fungal, anti-bacterial and many others. Some of these properties are now in clinical and preclinical trials2. Many natural products were also played important role in biological systems such as reproduction and play a crucial role as defense against predators, competitions for space, prevention of fouling and useful for the preparation of many antibiotics such as plakortin3 and manoalide from marine Sponges4. From living marine fauna, more than 60% of potentially bioactive compounds have been obtained till 1999 of which 70% of the bioactive compounds were isolated from sponges5. Throughout the globe, around 15000 different species of sponges were identified of which around 150 species were reported as freshwater sponges. Nearly 5000 different bioactive compounds were extracted from 500 different species of sponges of which 17% of sponges have commercial and traditional use including cosmetics6. Amongst the marine invertebrates, around 486 different species of sponges were reported in Indian waters7. In sponges, the class Demospongiae is well known for the production of secondary metabolites which have diverse function8. The interest of Marine natural products as one of the few de novo sources of drug discovery worldwide 9. However, in India, the bioactive potential from marine animals has been studied very scanty. In India the work carried on bioactive compounds by NIO Goa and others were from the coast of south and south- east India 10. The work on bioactive compounds from west coast of India has been carried out by 11 CIFE, Mumbai, and no efforts have gone into unraveling the details on bioactive compounds.12 studied the bioactive compounds from intertidal crab Leptodius exratus from Nariman point, west coast of Mumbai. Therefore an initial effort has been initiated for the isolation and partial purification of secondary metabolites from marine sponge S. fibulata (schmidt) from west coast of Mumbai.
Materials and Methods
Samples collection
The sponges S. fibulata was collected from Nariman Point, west coast of Mumbai during low tides. The sponges were brought to the laboratory in seawater and then washed with distilled water and sun dried.
Identification of sponges
Initial Preliminary identification was done by referring the literature. The shape and size of spicules were studied to confirm the specimen. The confirmation of identification was done by Dr. P.A. Thomas, Central Marine Fisheries Research Institute (CMFRI), Thiruvananthapuram, Kerala.
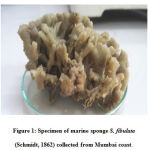 |
figure 1: Specimen of marine sponge S. fibulata (Schmidt, 1862) collected from Mumbai coast. |
Preparation of Sponge Extracts
The sponge extract was obtained by using the method as proposed by Braekman et al., 1992 with some modifications.
The extraction of sponge sample was done by following the method of13 with some modifications. 10 grams of sponge sample was added in 10 mL of methanol and kept standing for 24 hrs in conical flask by covering the mouth of the conical flask with stopper. After 24 hrs the sponges squeezes and made the suspension in methanol. The sample was then filtered through Whatman filter paper No.1. The sample so obtained was evaporated at low pressure by using Rotary Vacuum Evaporator at 45⁰C. The resultant compound so obtained was subjected to Millipore filter and finally dried in a vacuum desiccator and stored at 4⁰C in a refrigerator till further use.
 |
Figure 2: Crude extract of marine sponge S. fibulata (Schmidt, 1862) collected from Mumbai coast. |
Ethical approval
Ethical approval for the collection of S. fibulata was sought from the Maharashtra State Biodiversity Board, Nagpur and the voucher specimen of S. fibulata was sent to the repository center at NIO Goa, India, to deposit the sample. The Voucher numbers of the said specimen is 1-NIO1006/18 (S. fibulata).
TLC Analysis
Model Chem. Tech, HPTLC (High Performance Thin Layer Chromatography) available at Ancrom test lab. Mulund, Mumbai was used for the analysis of samples.
The aluminum TLC plates pre- coated with silica gel (60 F254) with 0.5 mm thickness were used for the experiment. The dried plates were charged and 10 mL of sponge crude extract was loaded on the plate and allowed the sample to dry at 100oC. The loaded plate then emerged in the saturated twin trough chamber containing Toluene: Ethyl acetate: Diethylamine (7:2:1) (v/v) as a mobile phase. The sample spots were run up to 9cm length in the mobile phase. The plate was removed from twin trough chamber and dried at110oC and dragendroff reagent was sprayed to develop the color spots. The spots were so obtained were observed under Deutorium lamp at 254 nm of the densitometric scanner. The spots scrapped off and dissolved in methanol. The pure samples were so obtained were filtered through Millipore filter and evaporated to dryness to get the pure compounds. The pure samples further processes for analytical studies.
GC-MS (Gas chromatography – Mass Spectrometry) Analysis
The dried powdered form samples were analyzed at IIT (SAIF) Powai, Mumbai. Analysis was performed with Jeol model Accu TOF GCV, GC MS (EI +ve or –ve) was used. The mass range of the spectrometer was ranges from 10 – 2000 amu at a mass resolution –of 6000.
FTIR (Fourier Transform Infrared Spectroscopy) Analysis
The dried isolated powdered sponge samples analyzed at IIT (SAIF) Powai, Mumbai. Analysis was performed with Bruker instrument corporation Germany make, 3000 Hyperion Microscope with Vertex 80 FTIR model was used. The scanning was done in the range of 4000-900cm-1. Analytical grade solvents and chemicals were used from M/S. S.D. fine chemicals, Thane, India.
Results and Discussion
Many marine sponges harbor a large number of secondary metabolites possessing a broad-spectrum of pharmacological applications have not yet been discovered. There are few sponges such as Hyrtios species, H.erectus, H.reticulatus, H gumminae , and H.tubulatus discovered bioactive compounds which have promising antifungal, antibacterial and antitumor properties14. The study carried out by 15 have obtained many compounds as Sterols (22ee)-24a-methyl-cholest-48(9), 22(23)-triene-3a,713-diol(1) and (22e)-24 amethylergost-6, 22(23)-diens-5a, 8a-epidioxy-3/3-o1(2) a fatty-acid nonadecanoic acid and aoily ester methyl nonadecanoate are the leab compounds where as complex compounds as (22e) –ergost-6, 22(23), 24(28)- triene -5a, 5a-epidioxy-3/3-o1 (3) and ergosterol (4) are the extra compounds present in sponge Suberites carnosus. 16 have identified a major parasite class of sponge called dеmospongiaе is well-known for generating the diverse and biggest number secondary metabolites amongst the marine invertebrates. Many sponges derived bioactive compounds as ara-a (anti-viral), ara-c (anti- cancer) and manoalide (phospholipids a 2 inhibitor), IPL512602 (anti-inflammatory), KRN 7000 (anticancer), laf 389(anticancer), discodemolide (anticancer), and HTI286 (anticancer) are now is being in clinical trials17,18. The study carried out by 19 found the antinocicеptivе and anti-inflammatory compounds from marine sponge Caissera. The structurally promising secondary metabolites of different genus of sponge Haliclona have been derived by20. The important pharmaceutical compounds have been identified by 21, 22 and they have identified the inhibition of HIV by two bis-quinolizidine alkaloids petrosins from Indian sponge Petrosia similis which inhibited HIV-1 replication. The study carried out by 23 , reported the gorgosterol type sterol lacking methyl substitution of the cyclopropane ring system, namely 22,23-methylene (22S, 23S) cholesterol 3 along with known compounds have been identified from marine sponge S. fibulata. The previous work have also been carried out on same sponge by 24,25 determined the mixture of steroids and peptides for the first time.
Characterization of isolated extracts of Sponge by HPTLC
The extracts isolated from the S. fibulata (Schmidt)collected from Nariman Point Mumbai coast are spotted on HPTLC plates and the plates were developed in a twin-trough chamber using I – butanol: methanol: water in the proportion of 3:1:1 (v/v), as a mobile phase. The plates were dried and sprayed with the dragendroff reagent .The photograph of the HPTLC plate of S. fibulata (Schmidt) and is shown in Photograph No.1, and 2. The spraying reagents gave positive tests for the presence of amides. The distinct and well-separated three spots were at Rf values of S. fibulata are G1- 0.029; G2- 0.137and G3-0.773. The densitometric scanning of these spots resulted in the quantification of these substances are found to be S. fibulata (67.69 %); (2.64 %); and (29.67 %) respectively. The pure compounds were isolated by scrapping the spots into methanol. Pure compounds were obtained by evaporating the solvent methanol by vacuum desiccators. These compounds are then characterized by FTIR analysis.
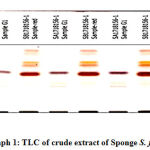 |
Photograph 1: TLC of crude extract of Sponge S. fibulata |
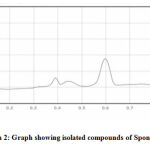 |
Photograph 2: Graph showing isolated compounds of Sponge S. fibulata |
Characterization of isolated extracts of sponge on GC-MS
GC-MS of the extracts isolated from the S. fibulata have been performed and the results are presented in Fig. No.3. the gas chromatograms of the extracts of the S. fibulata shown in Fig No. 3 G1 to G3, indicate that there are a large number of peaks. However the peaks at Rt value of S. fibulata are 28.18; 28.20 and 28.21 were found suitable for identification. The other peaks could not be identified due to the lack of database in the library as well as any references that are reported till now for the S. fibulata. The GC peaks obtained at Rt value of S. fibulata (Schmidt) were only employed for recording the mass spectra by irradiating the eluents at these Rt values through electron impact (EI+) source of the Mass spectrometer. Fig. No. 4 to 6, is the mass spectra of eluted compounds at Rt values 28.18; 28.20; 28.21; of S. fibulata (Schmidt). The M/Z mass peaks obtained for eluents at Rt values 28.18; 28.20; 28.21; corresponds to the substances of molecular mass of isolated compounds of S. fibulata (Schmidt) are466, 330, and 374respectively. It is observed that the mass spectra obtained for S. fibulata (Schmidt) the compounds are found to be different indicating that the different substances (compounds) are present in all isolated compounds of S. fibulata (Schmidt).The fragmentation peaks obtained for each of the mass spectra are as follows: For Rt value of compound 1 of S. fibulata at 28.18 ; M/Z at 466 (20%), M/Z at 365(10%), M/Z at 199 (27%), M/Z at 143 (38%), M/Z at 87 (82%), M/Z at 74 (100%), and M/Z at 57 (50%). For Rt value of compound 2 of S. fibulata at 28.20; M/Z at 330 (7%), M/Z at 299(12% ), M/Z at 239 (78%), M/Z at 134 (60%), M/Z at 98 (67%), M/Z at 74 (62%), and M/Z at 43 (100%) and for Rt value of compound 3 of S. fibulata at 28.21; M/Z at 374 (11%), M/Z at 267(8%), M/Z at 155 (37%), M/Z at 137 (14%), M/Z at 71 (78%),M/Z at 57 (96%), and M/Z at 43 (100%) The gas chromatograms shown in Fig No. 1,indicates that a large number of components are present in each of the isolated extracts S. fibulata (Schmidt). However, for the present study we have selected the components of GC peaks at Rt values of S.fibulata (Schmidt) are 28.18; 28.20 and 28.21 only, because they gave some conclusive evidence to identify the nature of the components on the database library. Therefore, the respective eluents at these Rt values were irradicated into the Electron impact (EI+) source of the mass spectrometer by which the molecules loose one electron and form positively charged molecules. Thus the molecules separate in ion trap mass spectrometer. The MS spectra shown in FigNo, 4 to 6, indicate that the mass peaks are due to the compounds having molecular weight of S. fibulata is 466, 330, and 374 respectively. These mass spectra are correlate to the Compounds as Triacontanoic acid, methyl ester – (Skin irritant), Hexadecanoic acid, 2- hydroxyl- (hydroxymethyl) ethyl ester – (Fatty acid, Metabolite and Irritant) and 2-Nitro-1, 3-bis-oclyoxy-benzene, (A natural product found in Neolitsea daibuensis. It has a role as a plant metabolite and an algal metabolite) of S. fibulata. The structures of these molecules are as shown in Table No. 4.
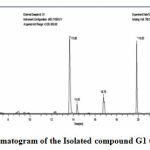 |
Figure 3: Gas Chromatogram of the Isolated compound G1 to G3, of S. fibulata. |
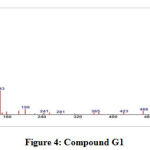 |
Figure 4: Compound G1 |
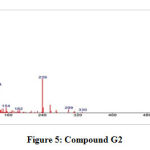 |
Figure 5: Compound G2 |
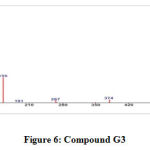 |
Figure 6: Compound G3. |
FTIR (Fourier Transform Infrared Spectroscopy) analysis
The FTIR studies are carried out in KBr pallets. Each of the compounds isolated at Rf values at G1- 0.029; G2- 0.137and G3-0.773 on TLC for each of the isolated compounds of S. fibulata extracts gave the IR spectras as shown in Fig No 7 to 9.All the IR spectra’s of the compounds at the respective Rf values mentioned above are found to be dissimilar and different of the extracts isolated from S. fibulata. The wave numbers of some of the important IR peaks along with their intensity, type of vibration and probable groups present in the respective compounds isolated at Rf values G1- 0.029; G2- 0.137and G3-0.773 are as shown in table.1 to 3.
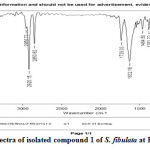 |
Figure 7: FTIR spectra of isolated compound 1 of S. fibulata at Rf values G1- 0.029. |
 |
Figure 8: FTIR spectra of isolated compound 2 of Sigmadocia fibulata at Rf values G2- 0.137. |
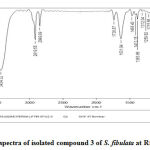 |
Figure 9: FTIR spectra of isolated compound 3 of S. fibulata at Rf values G3-0.773. |
Table 1: Correlation of IR spectra of the compound isolated from S. fibulata at Rf value 0. 029 on TLC
| Sr.No. | Wave number: cm-1 intensity | Type of Ir vibrations | Probable group Assignment |
| 1 | 3826.10 | N-H- streatching vibration | O-H, N-H group |
| 2 | 3750.62 | N-H- streatching vibration. | O-H, N-H group |
| 3 | 3445.73 | N-H -streatching vibration. | O-H, N-H group |
| 4 | 2956.73 | N-H -streatching vibration. | O-H, N-H group |
| 5 | 2920.15 | N-H -streatching vibration. | O-H, N-H group |
| 6 | 2850.82 | N-H -streatching vibration. | O-H, N-H group |
| 7 | 1739.03 | C=N -streatching | C=0,C=C, C=N group |
| 8 | 1632.18 | C=N- streatching | C=0,C=C, C=N group |
| 9 | 1464.50 | C=N- streatching | C=0,C=C, C=N group |
| 10 | 1383.22 | C=N -streatching | C=0,C=C, C=N group |
| 11 | 1217.97 | C-O – stretching | C-O group |
| 12 | 1119.42 | C-N stretching | C-N group |
| 13 | 1022.33 | – | C-C group |
| 14 | 924.02 | – | C-C group |
| 15 | 861.17 | C-H bending | Rock |
| 16 | 418.68 | – | Rock |
Table 2: Correlation of IR spectra of the compound isolated from S. fibulata at Rf value 0.137on TLC
| Sr.No. | Wave number: cm-1 intensity | Type of Ir vibrations | Probable group Assignment |
| 1 | 3434.32 | N-H stretching | O-H aliphatic primary amine |
| 2 | 2919.55 | C-H stretching | C-H Alkyl stretch |
| 3 | 2849.99 | C-H stretching | C-H group |
| 4 | 1736.87 | C=O stretching | C=O group |
| 5 | 1631.94 | C=C stretching | C=C alkene group |
| 6 | 1466.40 | C-H bending | C=N group |
| 7 | 1413.15 | C-H bending | C=N group |
| 8 | 1388.86 | C-O | C-O group |
| 9 | 1312.04 | C-O | C-O group |
| 10 | 1259.82 | C-O stretching | C-O alkyl aryl ether group |
| 11 | 1119.07 | C-O stretching | C-C group |
| 12 | 1023.97 | C-C | C-C group |
| 13 | 921.70 | C-C | C-C group |
| 14 | 862.66 | C-C | C-C group |
| 15 | 15 | Rock |
Table 3: Correlation of IR spectra of the compound isolated from S. fibulata at Rf value 0.773on TLC
| Sr.No. | Wave number: cm-1 intensity | Type of Ir vibrations | Probable group Assignment |
| 1 | 1500-1600 | C-C | O-H, N-H group |
| 2 | 1050-1150 | N=O | O-H,N-H group |
| 3 | 2190-2140 | C-O | C-H group |
| 4 | 1000-1350 | C=C | C-H group |
| 5 | 2880-2900 | C-N | |
| 6 | 3300 | C-H |
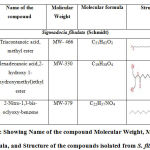 |
Table 4: Showing Name of the compound Molecular Weight, Molecular formula, and Structure of the compounds isolated from S. fibulata. |
Conclusion
From the above results it is concluded that, the compounds extracted from sponge S. fibulata showed antibacterial, pesticidal, and biomedical properties as we have studied it well. Our findings are significant for the development of multi-drug therapy for both pharmaceuticals and biomedical applications. Therefore we have screen the crude extracts of sponges for its structural elucidation to find the new drugs for pharmaceutical industry. The study further suggests that, Sigmadocia fibulatafurther screening is required for molecular level to understand the physiology and mode of action of the compound. The clinical study is also required which may be useful for pharmaceutical industry to manufacture the new drugs for safe performance and safety indexes to be studied to eradicate the diseases from mankind in future.
Acknowledgement
Authors would like to thank to, Dr. P.A.Thomas, Principal Scientist, Central Marine Fisheries Research Institute (CMFRI), Thiruvananthapuram, Kerala for identification of sponge species. Authors are also thanks to, The Director, APT Testing & Research Pvt. Ltd. (ATR) Pune, for experimentation and providing their assistance (IAEC NO. APTRF/ RP 57/1819 dated 01 February 2019).Thanks is also due to The Director, Ancrom test lab. Mulund, Mumbai, for providing HPTLC (High Performance Thin Layer Chromatography) facilities and also to the Director, (SAIF) IIT, Powai, Mumbai for providing the facilities of analytical study.
Conflict of Interest
There is no conflict of interest
Funding Sources
None
References
- Masry -El Fahmy HA, Abdelwahed HH, ASH. Synthesis and Antimicrobial activity of some new benzimidazole derivatives. Molecules. 2000; 5:1429-1438.
CrossRef - Newman GM, Cragg J. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004; 67: 1216-1238.
CrossRef - Higgs DJ, Faulkner. Plakortin,an antibiotic from Plakortis halichondrioides. J Org Chem., 1978; 43: 3454-3457.
CrossRef - De Silva PJ, Scheuer. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (Polejaeff). Tetrahedron Letters. 1980; 21: 1611-1614.
CrossRef - Abas HH, Zulfigar Y, Chan KL. Cytotoxicity and drug metabolism screening of several marine sponges from Pulau Pasir, Kedah and Pulau Aur, Johor. Asean Review of Biodiversity and Environmental Conservation (ARBEC) 1999.
- Rifai S, Fassouane A, El-Abbouyi A, Wardani A, Kijjoa A, Van Soest R. Screening of antimicrobial activity of marine sponge extracts. Journal de Mycologie Médicale, 2005; 15: 33-38.
CrossRef - Thomas PA. Porifera. In: Alfred JRB, Das AK, Sanyal AK, editors. Faunal diversity in India. Calcutta: ENVIS Central Zoological Survey of India. 1998; p. 28-36.
- Newbold RW, Jensen PR, Fenical W, Pawlik JR. Antimicrobial activity of Caribbean sponge extracts. Aquat Microb Ecol. 1999; 19:279-84.
CrossRef - McConnell OJ, Longley RE, Koehn FE. The discovery of marine natural products with therapeutic potential. Biotechnology. 1994; 26:109-74.
CrossRef - Tilvi, S.; Naik, C.G. Tandem mass spectrometry of kahalalides : Identification of two new cyclic Depsipeptides, Kahalalide R and S from Elysia grandifolia. J. Mass. Spectrom. 2007; 42 (1); 70-80.
CrossRef - Vankateshwaran K.; Pani Prasad K. Microchemolytic assay P-41-42 in Laboratory manual on advanced Techniques in marine Biotoxinology (Venka K. & Pani Prasad K. Ed) CAS in Fishery science. CIFE Mumbai India., 1997; P-76.
- Zodape; G.V.; Kulkarni B.G. Occurrence of Anatoxin -A & Homoanatoxin -A in Intertidal crab Leptodius exratus in Mumbai Nariman Point Coast. Bionano Frontier. 2010; Vol.3 (2); 259-264
- Braekman JC, Daloze D, Stoller C, Van Soest RWM. Chemotaxonomy of Agelas (Porifera: Demospongiae). Biochem Syst Ecol., 1992; 20(5):417-31.
CrossRef - Shady Nourhan Hisham, El-Hossary Ebaa M., Fouad Mostafa A., Gulder Tobias A. M., Mohamed Salah Kamel and Abdelmohsen Usama Ramadan. Bioactive Natural Products of Marine Sponges from the Genus Hyrtios; Molecules 2017; 22: 781.
CrossRef - Prahhu D Mishra, Solitimbi VVahidulla, L D’Souza & S Y Ramat (1996); Lipid constituents of marine sponge Suberites carnosus, Indian Journal of Chemistry Vol. 3513, pp. 806-809
- Newbold RW, Jensen PR, Fenical W, Pawlik JR. Antimicrobial activity of Caribbean sponge extracts. Aquat Microb Ecol. 1999; 19:279-84.
CrossRef - Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, Soest RWV et al. CSF shunts infections in pediatrics. A seven-year experience. Am J Dis Child 1984; 138:1103-1108.
CrossRef - Rodríguez-Nieto, S., González-Iriarte, M., Carmona, R., et al., 2002. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 16, 261–263.
CrossRef - Azevedo LG, Peraza GG, Lerner C, Soares A, Murcia N, Muccillo BA et al. Investigation of the anti-inflammatory and analgesic effects from an extract of Aplysina caissara, a marine sponge. Fundament Clinical Pharmaco 2008; 22(5):549-556.
CrossRef - Oliveira JHD, Grube A, Kock M, Berlinck RG, Macedo ML, Ferreira AG et al. Ingenamine G and cyclostellettamines G-I, K, and L from the new Brazilian species of marine sponge Pachychalina sp. J Nat Prod 2004; 67:1685-1689
CrossRef - Blunt JW, Copp BR, Munro MHG, Northcote PT, Prunes MR. Marine natural products Nat Prod Rep 2006; 23:26- 78.
CrossRef - Groud TV, Reddy NS, Swamy NR, Ram TS, Venkateswarlu Y. Anti-HIV active petrosins from the marine sponge Petrosia similis. Biol Pharm Bull 2003; 26:1498-1501.
CrossRef - Thota S.P.R., Sarma N.S., Murthy Y.L.N. Occurrence of a rare gorgosterol type steroid in marine sponge Sigmadocia fibulata; Journal of Natural Products 2015;8 : 05-08
CrossRef - Lo, J.M., Wang, W.L., Chiang, Y.M., Chen, C.M. Ceramides from the Taiwan red alga C. spongiosum & symbiotic sponge S. symbiotica, J. Chinese Chem. Soc 2001; 8:821.
CrossRef - Tan, L.T., Williamson, R.T., Gerwick, W.H., Watts, K.S., McGough, K., Jacob, R. cis, cis- and trans, trans- Ceratospongamide, New bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica, J. Org. Chem 2000; 65: 419.
CrossRef







