Salma Osman Noorelhuda Mohammed1 , Nadir Musa Khalil Abuzeid1
, Nadir Musa Khalil Abuzeid1 , Sara Abdelghani2
, Sara Abdelghani2 , Lienda Bashier Eltayeb3*
, Lienda Bashier Eltayeb3*
1Department of Medical microbiology, Faculty of Medical Laboratory Sciences., Omdurman Islamic University.
2Department of Parasitology, Faculty of Medical Laboratory Sciences, Al Neelain University, Khartoum, Sudan.
3Department of Medical Laboratory Sciences, Collage of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudia Arabia.
Corresponding Author E-mail: lindarose009@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/2247
Abstract
Background:Methicillin-resistant Staphylococcus aureus (MRSA) has gained significant health solicitude globally due to its resistance to nearly almost antimicrobial agents, and garlic is one of nature's most powerful antibiotics that must be used as a pharmaceutical regimen. The current study aimed to determine the In-Vitro antibacterial efficacy of crude garlic extract against MRSA. Methods: The aqueous and 70% ethanol crude garlic (Alllium sativum)extract was prepared. Disc diffusion method was performed to assess the antimicrobial activity for100 clinical isolates of MRSA collected, The reference standard strain was Staphylococcus aureus (ATCC 25923). Results: All MRSA strains assessed were significantly sensitive to 70% ethanolic extract at various concentrations range from 200 to 25%, exhibited inhibitory effects against clinical isolates and Staphylococcus aureus (ATCC 25923) with the means of inhibition zones ranging from 17.76- 14.35 mm and 15-13 mm in length, while the aqueous extracts were less in both clinical isolates and Staphylococcus aureus (ATCC 25923) ranging from 11.93-8.62 mm and 11-8 mm respectively, methanol and distilled water were not affected on growth. Conclusion: The findings demonstrate that 70%ethanol extract of crude Allium sativum has significantly inhibitory effect on methicillin-resistant Staphylococcus aureus is better than aqueous extract. This study does not undermine the value of antibiotic use, but instead the probability of using them in low dosage to minimize their negative consequences.
Keywords
Allium sativum; Aqueous Extract; Alcoholic Extract; MRSA
Download this article as:| Copy the following to cite this article: Mohammed S. O. N, Abuzeid N. M. K, Abdelghani S, Eltayeb L. B. In-Vitro Antibacterial Activity of crude Garlic (Allium Sativum) Extract Against Clinical Isolates of Methicillin Resistant Staphylococcus aureus. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Mohammed S. O. N, Abuzeid N. M. K, Abdelghani S, Eltayeb L. B. In-Vitro Antibacterial Activity of crude Garlic (Allium Sativum) Extract Against Clinical Isolates of Methicillin Resistant Staphylococcus aureus. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3AXf6fp |
Introduction
Plant-derived antimicrobials have extremely large therapeutic effects, and they are efficacious in treating infectious diseases even while reducing many of the problems associated with synthetic antimicrobials 1.The different pharmacological effects of plant products are generally the result of secondary product combinations derived from plants. Such plant compounds are often secondary metabolites such as alkaloids, steroids, and fatty acid gums that have a certain physiological impact on the body 2, 3, 4.There are many natural elements and chemicals in garlic, including sulfur compounds, numerous enzymes,different minerals, vitamins, and amino acids. Allicin (diallyl thiosulfinate ordiallyl disulfide)is among garlic’s greatest crucial biologically vigorous substances 5. Even though allicin is deemed the prime antioxidant compound, other compounds may represent stronger roles; such as polar compounds of phenolic and steroidal origin, that display diverse pharmacological countenance without odor and are furthermore heat-stable 6.
The efficacy and broad-spectrum antimicrobial activity of garlic versus huge species of bacteria (Staphylococcus aureus, Streptococcus pneumonia, Streptococcus fecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumonia, Pseudomona saeruginosa, Shigella spp, Salmonella spp, Proteus mirabilis, and Helicobacter pylori), viruses, parasites, and fungi have been documented 7, also it is much more efficacious than industrial antimicrobials and has low toxicity; as a consequence, can be used as an additional option for the treatment of numerous infections 8, 9, 10, 11. The antibacterial activity of garlic has been fundamentally attributed to the entity of allicin generated by the enzymatic activity of allinase on allicin12. Garlic is also efficacious toward antibiotic-resistant microorganisms, and combining garlic extracts with antimicrobial agents results in partial and total synergism [13]. The emergence of multidrug-resistant strains of Gram-negative andGram-positivebacteria is troubling for humans and animals, as well as the emergence of epidemic MRSA, as a consequence, garlic is a popular alternative substance for the treatment of MRSA 14. Hence current study aimed to determine the In-Vitro Antibacterial Activity of crude Garlic (Allium Sativum) extract against clinical isolate of Methicillin-Resistant Staphylococcus aureus (MRSA).
Materials and Methods
Study design
Across-sectional study was carried out during the period from April to June 2018 on a clinical isolate of MRSA from patients who were known to have MRSA after full microbiology identification methods, so specimens were collected from, wound, eye, sputum, and blood. Moreover Standard bacterial strains include American type culture collection(ATCC), S.aureus(ATCC 25923) used as control positive. And Sudanese garlic bulbs with small size and strong odor were obtained from the Khartoum Bahri market to determine their antibacterial activity. Study was approved by the College of High Graduate Studies ethical committee/Shendi University.
Sample size
A hundred Isolates were collected randomly, 73 MRSA isolated from wounds, 3 eye swabs, 21 from blood culture, and 3 isolated from sputum samples. Clinical isolates collected according to availability regardless of their type, and all isolates show resistance patterns to Oxacillin were included in the study.
Laboratory methods
Plant materials collection
The Garlic bulb (seeds) was collected from Central Sudan, Khartoum Bahri market in April 2018, and it was checked by the Medicinal and Aromatic Plants professional at Traditional Medicine Research Institute (TMRI), Khartoum, Sudan.
Preparation of crude extracts
Extraction was carried out for the seeds of garlic plant 100 g by using maceration techniques after weighting the grounded material by sensitive balance about 34g round material was macerated equal quantity for both aqueous and 70% ethanol extract.
For preparation of aqueous extraction, about 100 g of the garlic was soaking in 100 ml hot distilled water for about four hours with continuous steering. After cooled, extract was filtered using filter paper and the solvents were evaporated using freeze drier, and the yield percentage were calculated as follow: Weight of extract obtained / weight of plant sample X100.
For obtaining 70%extraction of Ethanol, 100 g of garlic was grinding by mortar and impregnating in 70% ethanol for 5 days with liquidation and steaming on daily basis. David calculated the given percentages after the solvent was evaporated under low pressure to arid and the extract was allowed to air dry completely, then multiply the weight of the extract by the weight of the sample. Finally, it was stored at 4°C in a tightly bottled glass flask, ready for use.
Table 1: Aqueous and 70% Ethanol Extraction
| SampleNo | Name of plant | Weight of planting | Weight of extract in Gram | Yield % |
| 1 | Garlic | 100 g | 6.8g | 6.8 % |
| 2 | Garlic | 100 g | 7.2g | 7.2 % |
Bacteriastrains
Under aseptic conditions, 100 clinical isolates of MRSA were obtained from El Ribat University hospital’s Microbiology laboratory in Khartoum, Sudan. The specimens were isolated from wound swabs, sputum, eye swabs, and blood culture and cultured in suitable media such as blood agar, chocolate blood agar, and McConkey agar and incubated aerobically at 370C over night.
Identification was achieved depending on colonial morphology, the feature of culture media, gram stain, biochemical reactions (Catalase, coagulase, DNAse test, and Mannitol Salt Agar), and susceptibility to Oxacillin discs (1 µg) using Mueller-Hinton agar MHA; when the zone of inhibition is more than 12 mm were identified as sensitive and 11-12 mm identified as intermediate 15, 16.
Catalase test
A pure of 2-3 ml of hydrogen peroxide solution was added in a test tube, by sterile wooden stick several colonies of test organisms were put in hydrogen peroxide solution. The positive results indicated by immediate bubbling 15
Coagulase test
Coagulase test a colony of the test organism was emulsified in drop of physiological saline to make thick suspension and a drop of plasma was added to and mixed gently by rotating. The positive results indicated by producing clump within 10 seconds 15.
Deoxyribonuclease (DNAse)test
The test organism was cultured on a medium which contains DNA. After overnight incubation, the colonies were tested for DNAse production by flooding the plate with a weak hydrochloric acid solution. The acid precipitated hydrolyzed DNA. DNAse producing colonies were therefore surrounded by clear areas due to DNA hydrolysis 15.
Manitol Salt Agar (MSA)
A portion of colony was inoculated on manitol salt agar containing 75g/1sodium chloride and incubated aerobically at 37oC for 18-24hours. S. aureus ferments manitol producing yellow colonies 15.
Detection of Methicillin Resistance
MRSA identification was carried out using oxacillin screen plates following the guidelines of NCCLS. Briefly, a suspension equivalent to 0.5 McFarland standards, prepared from each strain, was inoculated homogenously on the entire surface of the Mueller-Hinton agar plate (Oxoid-UK) containing 4% NaCl and 6 μg/mL oxacillin, with the help of sterile swabs. All the plates were incubated at 35oC for 24 hrs. Indication of growth (>1 colony) identified the isolates as oxacillin/methicillin-resistant (Genç et al., 2008) ,when zone of inhibition present above 12mm was identified as sensitive and 11-12mm identified as intermediate.
Antibacterial susceptibility testing for extracts
The paper disc diffusion method was being used to evaluate the antimicrobial property of garlic extracts using MHA. The dilution factor of Bacterial suspension was done with a sterilephys iological solution to 108 CFU/ml(turbidity=McFarland standard 0.5). 100 µl of bacterial suspensions wabbed regularly on MHA and was allowed to dry for 5 minutes. Then filter paper discs (What man No.1, 6 mm india meter)was Sterilized in an oven at 80oC for 30 minutes, and placed on MHA and soaked with 20µl of a solution of each garlic extract with diluted different serial dilution as follow: 25%, 50%, 100%, 200% (0.25 mg/ml,0.5 mg/ml, 1 mg/ml, 2 mg/ml) respectively, next suspended by methanol for 70% ethanolic extract and the same concentration use dafter re-suspended by steriled istilled water for aqueous extract. The inoculated plates were incubated at 37°C overnight.
Data analysis
All data were analyzed using (SPSS) soft ware version 21, descriptive statistics (e.g., frequency and percentage), chi-square test, were used and the threshold for statistical significance was (p < 0.05).
Result
A total of 100 clinical isolates were collected from wound swab 73%, eyes wab 3%, sputum 3%, and blood for culture 21%. All isolates were positive for catalase, coagulase, Deoxyribonuclease (DNAse)test, and MSA as well as all isolates were showed growth on the Oxacillindisc, which indicates resistance.70% ethanol extract and their inhibition effect between MRSA clinical isolates and ATCC (25923) reference strains was significantly stated in higher concentration of ethanol 200%, and 100% (P-value=0.001, 0.038) respectively. No significant difference in aqueous extraction, result displayed in table 2 and 3.Different concentrations of 70% ethanol extract and aqueous extract and their inhibition effect related to gender, there is no significant P. value≥ 0.05 association between sex with different concentrations of ethanol extracts and their inhibition effect. All data are summarized in table 4.
Figures 1, 2 display the antibacterial activity of A.sativum 70% ethanol and aqueous extract tested on clinical isolates and ATCCC (25923) Staphylococcus aureus reference strain after re-suspended with distilled water as negative control respectively. The 70% ethanol extracts in all concentrations were sensitive when matched with oxacillin susceptibility test on MRSA strain, intermediate only in 200%(2mg/ml) aqueous extract,and other concentrations were resistant. The activity of A. sativumin both extracts increased with the concentration of it, Figure 3 display plates (a and c) showed antibacterial activity of 70% ethanol, and aqueous extract of A. sativum extract as well on clinical isolates, plate (b and d) showed antibacterial activity of 70% ethanol, and aqueous extract of A. sativum extract STD ATCC strain.
Table 2: 70% ethanol extract and their inhibition effect between MRSA clinical isolates and ATCC (25923) reference strains.
| Con% | MRSA Clinical isolates | ATCC reference strain |
P. value |
| Mean± SD | Mean± SD | ||
| 200% | 17.59±0.880 | 14.56±1.095 | 0.001 |
| 100% | 17.96±1.095 | 12.24±0.632 | 0.038 |
| 50% | 15.41±0.714 | 13.62±0.805 | 0.761 |
| 25% | 14.33±0.614 | 12.25±0.47 | 0.798 |
Table 3: aqueous extract and their inhibition effect between MRSA clinical isolates and ATCC (25923) reference strains.
| Con% | MRSA Clinical isolates | ATCC reference strain | P. value |
| Mean± SD | Mean± SD | ||
| 200% | 11.91±0.293 | 10.88±0.206 | 0.329 |
| 100% | 10.65±0.555 | 9.51±0.465 | 0.642 |
| 50% | 9.59±0.659 | 9.44±1.501 | 0.571 |
| 25% | 8.57 ±0.716 |
8.12±0.474 |
0.422 |
Table 4: Different concentrations of 70% ethanol and aqueous extract and their inhibition effect related to gender.
| Con% | Male n=54
Mean± SD |
Female n=46
Mean± SD |
P. value |
| 70% Ethanol Extract | |||
| 200% | 17.59±0.880 | 17.96±1.095 | 0.74 |
| 100% | 17.96±1.095 | 16.54±0.836 | 0.388 |
| 50% | 15.41±0.714 | 15.41±0.805 | 0.971 |
| 25% | 14.33±0.614 | 14.37±0.771 | 0.798 |
| Aqueous Extract | |||
| 200% | 11.91±0.293 | 11.96±0.206 | 0.329 |
| 100% | 10.665±0.555 | 10.70±0.465 | 0.642 |
| 50% | 9.59±0.659 | 9.46±1.501 | 0.571 |
| 25% | 8.57 ±0.716 | 8.67±0.474 | 0.422 |
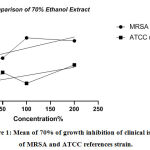 |
Figure 1: Mean of 70% of growth inhibition of clinical isolates of MRSA and ATCC references strain. |
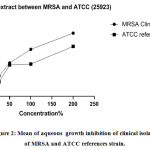 |
Figure 2: Mean of aqueous growth inhibition of clinical isolates of MRSA and ATCC references strain. |
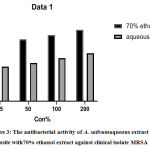 |
Figure 3: The antibacterial activity of A. sativum aqueous extract opposite with 70% ethanol extract against clinical isolate MRSA. |
Discussion
Antibiotics’ massive use in the treatment of infectious diseases has resulted in the emergence and development of resistant strains, which is now a significant cause of failure in the treatment of infectious diseases 17. In this study, 70 % ethanol extract of crude A. sativum showed remarkable antibacterial activity against standard strains of S.aureus and clinical isolatesmethicillin-resistant that agreed with Janan et al., 18, while aqueous extract showed little activity, these were different from that obtained by Hadir et al. 19,
may be referred to using the homogenization aqueous extraction technique. However,the opposite results of extract do not indicate less activity of bioactive constituents because the active ingredient (s) could be existent in inadequate amounts. , but the use of boiled water in the maceration method of the extract for garlic may bean effect on the stability of allicin and other bioactive components that agreed with Strika et al.,20.Besides that, this could be due to various main: hydrogen bonding of water to the reactive oxygen atom in allicin can minimize its instability; and/or there could be water-soluble elements in minced garlic that destabilize the chemical compound that Lawson mentioned 21.
The limitation of this strategy is that allicin can develop diallyl di-sulfide once it reacts with water 22, 23, which may not have the same degree of antimicrobial effect as allicin. The yield percentage of ethanolic extract was noted 7.2 % slightly increased than aqueous 6.8%, and that due to the time of extract according to the methodology of technique, in aqueous 4 hours only needed and then filtrated the final product in one day,but the ethanolic extract needed 5 days with daily filtration. This effect of time on yield and in sufficient quantity has a role in antibacterial activity. The most probable explanation for these differences between results of zone inhibition mm in this study compared with other studies maybe due to the different bacterial strains tested, and the antibacterial activity methods, method of extract, and time, plus the concentration of organosulfar compounds, which vary greatly between garlic species around the world 24. A study by Bayan L et al. 25 noted that one negative aspect in ascertaining the antimicrobial property of Garlic Extract is the inability to detect Methicillin Resistance results, the scientists used standardization in their methodologies, and this results in significant differences in the outcomes.As a result, it is crucial to establish policies and procedures for all guidelines used to assess the antimicrobial property of garlic extract.One major benefit of garlic is that bacteria need not appear to emerge resistant to it, as they do to several advanced antibiotics: “garlic does not seem to produce such resistant strains” Erdogrul, 26, 27. With greatly increased extract concentration, the mean diameter of the growth inhibition zone of standard and clinical isolates of bacteria enhanced. This out come is in contact with the report of Mahfuzul Hoque et al. MD 28.The best inhibition zone obtained by 70% ethanol extract of crude garlic was 20 mm in diameter against clinical isolates of MRSA strains at the concentration of 4 mg\ 2ml,(200%conc).And the minimum inhibition zone was 13 mm in diameterat 0.5 mg/ml(25%conc) on aqueous. Despite the fact that garlic has been postulated to have a synergistic or additive impact with regular antimicrobial agents against E. coli and S. aureus just a few studies have been conducted to demonstrate the effect of garlic when used in combination with standard antimicrobials 29, 30.
Limitations of study and Prospective view
The extract’s active garlic ingredient (Allicin) compounds are crucial for its antimicrobial properties. More research is required to verify these results and for evaluating the combination of garlic with other commercial antibiotics. so Broad extraction projects for garlic plant components is crucial to determine the best active ingredients by an ideal standard methods. Alternative methods to extract processing by solvents must be more used and tested as a squeezing technique to avoid the loss of bioactivity of components. one of the limitation of current study is that a commercial /clinical antibiotic) to show the efficacy of the garlic extracts were not used in this study.
Conclusion
The findings demonstrate that 70%ethanol extract of crude Alliumsativum has significantly inhibitory effect on methicillin-resistant Staphylococcus aureus is better than aqueous extract. From this, we can conclude that the potential of using alcohol – related extract of garlic as potential antimicrobial agents. This study does not undermine the value of antibiotic use, but instead the probability of using them in low dosage to minimize their negative consequences.
Acknowledgment
This publication was supported by the Deanship of scientific research at Prince Sattam Bin Abdul Aziz University. Authors appreciated to Faculty of Medical Laboratory Sciences, Shendi University, Sudan. We would like to express our heartfelt thanks to Traditional Medicine Research Institute for assisting with methods. We would like to thank the National Center for Research, Khartoum-Sudan, and Microbiology department El-ribat University for the collaboration during the recruitment process and provided surveillance data.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding Source
None
References
- Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. 2013;16(10):1031-1048.
- Saranraj,P.andSivasakthi,S.MedicinalPlantsanditsAntimicrobialProperties:AReview.GlobalJournalofPharmacology.2014 8(3):316-327.
- Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, Mavumengwana V, Li HB. Bioactive Compounds and Biological Functions of Garlic (Allium sativum). Foods. Jul 2019 5;8(7):246.
CrossRef - Josling PA. The heart of garlic Nature’s aid to healing the humanbody,HEC Publishing, ChicagoIllinois. 2005
- Pedrazza-Chaverri J, Tapia E, Medina-Campos ON, de los AngelesGranados M, Franco M.Garlic prevents hypertension induced bychronicinhibitionofnitricoxidesynthesis.LifeSci.2006 62:71-77.
CrossRef - Theanalysisofonionandgarlic.J.Chromat.2006 A.12(1):3- 22.
- JaberMA,Al-MossawiA.Susceptibilityofsomemultipleresistantbacteria to garlic extracts. J. Biotechnol. 2007 6(6):771-776.
- Tepe B, Daferera D, Sokmen M, Polissiou M, Sokmen A. In vitroantimicrobial and antioxidant activities of the essential oils and variousextractsof thymus. J.Agric.FoodChem.2004 52:1132-1137.
CrossRef - BajpaiM,Pande A, Tewari SK, Prakash D.Phenolic contents andantioxidantactivityofsomefoodandmedicinalplants.Int.J.FoodSci.Nutr. 2005 56(4):287-291.
CrossRef - Wojdylo A, OszmianskiJ, Czemerys R.Antioxidant activity andphenoliccompoundsin32selectedherbs.FoodChem. 2007;105:940-949.
CrossRef - Tsao SM, Yin MC.In vitro antimicrobial activity of four diallyl sulphides occurring naturallyin garlic and Chinese leek oil.J.Med.Microbiol.2001;50:646-649.
CrossRef - Alam, R., Fawzi, E. M., Alkhalf, M. I., Alansari, W. S., Aleya, L., & Abdel-Daim, M. M “Anti-Inflammatory, Immunomodulatory, and Antioxidant Activities of Allicin, Norfloxacin, or Their Combination against Pasteurellamultocida Infection in Male New Zealand Rabbits. Oxid Med Cell Longev. 2018;2018:1780956.
CrossRef - Yadav S, Trivedi NA, Bhatt JD. Antimicrobial activity of fresh garlic juice: An in vitro study. Ayu. 2015;36(2):203-207.
CrossRef - Sharma, P.C., Yelne, M.B. and Dennis,T.J. Database on MedicinalPlantsusedinAyurveda. Vol.6.CCRAS: NewDelhi. 2005 pp.420-440.
- Chees brough,M.Distric laboratory Practice in Tropical countries. Second Edition, Part2. NewYork ,United States; 2005. Cambridge university Press.
CrossRef - Genc, Y., Özkanca, R. &Bekdemir, Y. Antimicrobial Activity Of Some Sulfonamide Derivatives On Clinical Isolates Of Staphyl ococus Aureus. Annals Of Clinical Microbiology And Antimicrobials, 2008.7,
CrossRef - Ibrahim,T.A., Opawale, O. andOyinloye, J.M.A. Antibacteria l activity of herbal extracts against multi drug resistant of bacteria from clinic alorigin. Journal of life Science Leaflets.2011;15:490-498.
- Janan M, Alaa T, and Ismaeil M. A. Antibacterial activity of garlic against multi drug resistant staphylococcus aureus and enter ococcusfaecalis University of Duhok journal, 201619(1) :114-122.
- Hadir, M. , Alaa, H. , Ibrahim, A. ,O.A. aljeferi , and R.Ralhendi. Inhibitory effect of garlic extract multi drug resistant organisms.Egyptian journal of medical microbiology,2013; 22 , 1.
- Strika ,I., Basic, A., Halilovic , N. antimicrobial effects of garlic (Allium Sativum).Bulletin of the chemists and technologists of Bosnia and Herzegovina, 2017; 47:17-20.
- Lawson LD. The composition and chemistry of garlic cloves and processed garlic. In: Koch HP, Lawson LD, eds. Garlic: the science andtherapeutic application of Allium sativum L and related species(2nd edn).Baltimore: WilliamsandWilkins,1996:37–107.
- TheorganosulphurchemistryofthegenusAllium:implications for the organic chemistry of sulphur. AngewChemIntEdEngl;1992; 31: 1135–78.
CrossRef - LawsonLD,WangZYJ.Changesintheorganosulphurcompoundsreleased from garlic during aging in water, dilute ethanol or dilute aceticacid.J.Toxicol.14:214.
- Arora S.D., Kaur J.Antimicrobial activity of spices. J AntimicrobAgents., 1999; 12:257-262.
CrossRef - Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4(1):1-14.
- Erdogrul OT. Antibacterial activities of some plant extracts used infolkmedicine.PharmBiol, 2002; 40:269-273.
CrossRef - Rangan C, Barceloux DG: Food additives and sensitivities. DisMon, 2009; 55:292-311.
CrossRef - MahfuzulHoque MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Antibacterial activity of guava (Psidiumguajava L.) and Neem (Azadirachtaindica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis. 2007 Winter;4(4):481-8.
CrossRef - Eja ME, Arikpo GE, Enyi-Idoh KH, Ikpeme EM. An evaluation of the antimicrobial synergy of garlic (Allium sativum) and Utazi (Gongronemalatifolium) on Escherichia coliand Staphylococcus aureus. Malays J Microbiol. 2011;7:45–9
- Pillai R, Trivedi NA, Bhatt JD. Studies on in vitro interaction of ampicillin and fresh garlic extract against Staphylococcus aureus by checkerboard method. AncSci Life. 2013;33(2):114-118.
CrossRef