Manuscript accepted on :26-07-2021
Published online on: 31-07-2021
Plagiarism Check: Yes
Reviewed by: Dr. Jyothsna Rao

Second Review by: Dr. Salman Ahmed

Final Approval by: Dr. Ian James Martin
Department of Biomedical Sciences, Sri Ramachandra Institute of Higher Education and Research, Porur, India.
Corresponding Author E-mail: lalithav@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2249
Abstract
The review is an overview of the features of growth factors involved in cellular signaling mechanisms regulating the wound healing process. Understanding the insights of this mechanism is significant for opening therapeutic and research avenues in wound healing. The review highlights the synergistic functioning of most of the growth factors which would enhance the possibility of these factors being the targets for wound care therapy.The significance of the onset and resolution of inflammation in the healing process is better understood clinically and a range of recombinant growth factors to combat this condition have been identified and used to accelerate healing process.The chemotactic and growth regulating factors act as triggers that take the cellular and biochemical components through the inflammation, proliferation, epithelialization, angiogenesis and tissue remodeling phases. Clinical conditions that create alteration in expression of these factors lead to slow and incomplete healing. The review emphasizes on the clinical use of synthetic and recombinant growth factors whose synergistic effects are remarkable. The review covers the specific signaling mechanisms involved in the regulation of these growth factor expressions, specifically the PI3K/AKT, RAS/MAP and JAK pathways; these could be potential targets for future research expansions in this field.
Keywords
Angiogenesis; Cytokines; Epithelialization; Inflammation; Growth factors; Wound healing
Download this article as:| Copy the following to cite this article: Vaidyanathan L. Growth Factors in Wound Healing – A Review. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Vaidyanathan L. Growth Factors in Wound Healing – A Review. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3k7DUL9 |
Introduction
This review discusses the basics of wound healing mechanisms and the significance of the growth factors in promoting regeneration of the wounded tissue. The synergistic effects of the growth factors in accelerating the healing process is explored. The growth factors take their role right from the inflammatory phase of the mechanism where their presence is both chemotactic for the infiltrating neutrophils and notable in resolution of inflammation, promoting the onset of macrophage increase at the wound site which is thought to be responsible to kick-start inflammation resolution. Hallmarks on the existence of growth factors in epithelialization and angiogenesis are also discussed.The review’s scope extends to the advancements in clinical usage of the external growth factors.The role of the growth factors in cellular signaling mechanisms like the PI3K/AKT pathway, the RAS/MAPK pathway and the JAK/STAT pathways as studies by pre-clinical and clinical findings is explored in the review. The use of synthetic recombinant growth factorsto achieve accelerated post-surgical healing and impaired clinical conditions like diabetic foot ulcers, obesity, malnutrition, medication-induced impairment, oncological treatment-induced impairments, surgical amputations warrants advancements. The content of this review has updations in technical development for basic science researchers and aids clinicians to take-up synthetic growth factor-based therapeutic interventionsto fight healing impairment that otherwise affects the patients life quality during and after the hospital stay.The list of triggers include chemotactic factors, inflammatory cytokines and growth promoting factors. These triggers take the cellular and biochemical components through the inflammation, proliferation, epithelialization, angiogenesis and tissue remodeling phases. Among the various triggers guiding the healing process, a number of growth factors function wholly or in synergy to induce the cellular signaling cascade, including Fibroblast Growth Factor, Epidermal Growth Factor, Platelet-derived Growth Factor, Vascular-endothelial Growth Factor, Transforming Growth Factor, Connective Tissue Growth Factor to name a few. These factors are said to make the structural healing more functional. Impaired healing results due to clinical conditions that influence the expression of any or most of these triggers; finally the impaired condition itself becoming a pathologically significant condition. Many synthetically derived and/or recombinant growth factors have experimentally proved effective and have been in clinical practice over years owing to their potency to overcome impairment and help accelerate healing as the case may need.
Role of growth factors in wound healing
Wound healing is a complex yet organized process of restoration of the functional structures lost during wounding, due to trauma, post-surgical complications, burns or otherwise. It is a natural repertoire of ordered overlapping series of cellular and vascular events, inflammation, proliferation, epithelialization, granulation tissue formation, maturation, matrix and tissue remodeling, which are triggered by a wide range of mediators from histamine to growth factors like TGF-β and vascular factors like VEGF4 from the wound microenvironment. On occurrence of a wound, a complex interplay of cells and proteins take place, the blood platelets aggregate and bind with collagen to prevent blood loss by forming a clot5. The incidence of the wound creates a hypoxic environment that promotes inflammation by releasing proinflammatory mediators, the molecular signals that manifold the inflammatory activities, example, by recruiting leukocytes and monocytes which differentiate into tissue macrophages6. Neutrophils are the predominant and prime inflammatory cells. Once activated they release enzymes like elastase, protease and collagenase which degrade and remove damaged tissues at the wound site.10 Platelets also initiate inflammation by producing EGF, PDGF, TGF β1 and IL-1. These attract neutrophils to the wound site; TGFβ induces selective differentiation of monocytes to macrophages which also contribute to the inflammation. Also the monocyte chemotactic protein, TGF-β1 recruits mast cells to the wound site which releases histamines, proteoglycans, proteases and platelet activating factor. Unresolved or prolonged inflammation leads to chronic non healing wounds. Once active the inflammatory cells synthesize TGFβ1 & IL-4 which in turn suppress them leading to reversal of inflammation (Fig. 1).
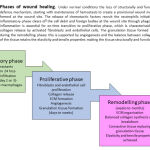 |
Figure 1: Phases of wound healing. |
Phases of wound healing
Under normal conditions the loss of structurally and functionally intact skin is attended by the body’s defence mechanism, starting with maintenance of hemostasis to create a provisional wound matrix which is contributed by the platelet plug formed at the wound site. The release of chemotactic factors recruit the neutrophils initiating the inflammation. The later stages of the inflammatory phase clears off the cell debri and foreign bodies at the wound site through phagocytosing macrophages. The early resolution of inflammation is essential for on-time transition to proliferative phase, which is characterised by formation of extracellular matrix through collagen release by activated fibroblasts and endothelial cells. The granulation tissue formed matures and is replaced by connective tissue during the remodelling phase; this is supported by angiogenesis and the balance between collagen synthesis and breakdown. The remodelling of the tissue retains the elasticity and tensile properties making the tissue structurally and functionally healed.
TNF-α and IL-1 produced by macrophages initiate and regulate proliferation of fibroblasts and endothelial cells. Upon activation the fibroblasts release collagen and other glycosaminoglycans which deposit with fibronectin and forms the ECM, the major component of the granulation tissue. The further growth of the granulation tissue requires angiogenesis, which is initiated by the bFGF and VEGF produced by the endothelial cell, keratinocytes and macrophages. PDGF released by the degranulating platelets holds the important role of increasing the structural integrity of blood vessels during angiogenesis. The balance between the collagen synthesis and breakdown forms an essential part of the remodeling of the collagen fibres. During remodeling the granulation tissue is slowly replaced by the connective tissue which contributes to the elasticity and tensile properties of the otherwise intact skin, the major contribution being from the connective tissue growth factors (Fig. 2).
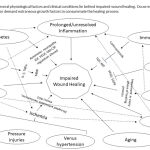 |
Figure 2: Impaired wound healing. |
Impaired wound healing
Several physiological factors and clinical conditions lie behind impaired wound healing. Occurrence of any of these individually or in combination demand extraneous growth factors to consummate the healing process
Epidermal growth factor (EGF)
Epidermal Growth Factor (EGF) is a growth factor that stimulates cell growth, proliferation and differentiation by binding to the Epidermal Growth Factor Receptor (EGFR). The protein was initially found among nerve growth factors extracted from mouse submandibular gland26. The family consists of other members including Transforming growth factor-α, heparin binding EGF (HB-EGF), amphiregulin, epiregulin, neuregulin and beta-cellulin7. EGF is a key regulator of epithelial cell motility thereby influencing the rate of re-epithelialization. It aids wound contraction by stimulating fibroblasts proliferation and migration and induces dermal maturation by binding with the EGFR in the cells at the wound site14. Topical application of EGF is considered to be a useful therapy for cutaneous wounds. Analysis of the wound fluid gave the first ever evidence of EGF at the wound site. It was studied that EGF exhibited a synergistic role with Insulin-like growth factor-1 (IGF-1) in stimulating in-vitro proliferation of keratinocytes7(Fig. 3).
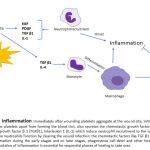 |
Figure 3: Role of growth factors in wound inflammation. |
Role of growth factors in wound inflammation
Immediately after wounding platelets aggregate at the wound site, initially to create a fibrin clot (hemostasis) to stop the blood flow from the damaged tissue. The platelets apart from forming the blood clot, also secretes the chemotactic growth factors like epidermal growth factor (EGF), platelet-derived growth factor (PDGF), transforming growth factor β1 (TGFβ1), Interleukin 1 (IL-1) which include neutrophil recruitment to the wound microenvironment, resulting in onset of inflammation which prolongs for 1-2 days. The neutrophils function by clearing the wound infection; the chemotactic factors like TGFβ and IL-4 mediate differentiation of monocytes to macrophages which contribute to inflammation during early stages and on later stages, phagocytose cell debri and other foreign bodies thus bringing about resolution of inflammation. The on-time start and early resolution of inflammation is essential for sequential phases of healing to take over.
Reports on therapeutic usage of the protein highlights the drugs containing the protein being used for treating diabetic and corneal ulcers in regions of South Korea and Belgium respectively. In early 1990s Okumura et al. demonstrated the use of EGF in combination with nafamostat, a protease inhibitor to treat open wounds in rat models. The treated models showed significant increase in the wound granulation tissue dry weight, hydroxyproline and uronic acid contents, suggesting the need for a protease inhibitor in place to stabilize the presence of EGF thereby establish its healing role15. A topical treatment of recombinant human epidermal growth factor (rhEGF) ointment was shown to be effective in promoting wound healing by increasing the rate of epidermal proliferation and wound contraction related to myofibroblast proliferation and collagen synthesis16. To face the challenge during transdermal delivery of EGF Kim et al. (2016) succeeded in using Hyaluronate (HA)-EGF conjugate in a patch type HA film. The conjugate allowed an extended residence time of EGF at the wound site and enhanced the regeneration of skin tissues17. Recombinant human EGF available commercially under the brand name Heberprot-P is used to treat diabetic foot ulcers (DFU)13. Recombinant EGFR also rescues the keratinocyte migration suppressed naturally by the shedding of the EGFR in the keratinocyte membranes on wounding35. Recombinant probiotic Escherichia coli Nissle 1917 expressing human EGF enhanced human enterocyte migration upon wounding in a murine monolayer model. The EGF secretion by the probiotic strain was ABC transporter mediated and the EGF secretion was studied to potentially activate, by phosphorylation, the ERK1/2 and AKT signals finally resulting in the enhancement of the healing process by multifaceted repertoire of cellular events like the proliferation, migration and reepethilialization36. High blood glucose upsets the EGFR/PI3K/AKT signaling pathway in a ROS-sensitive manner and interrupts epithelial wound healing in the cultured porcine cornea. The study by Xu et al. (2009) conclude that the therapeutic combination of EGFR ligands and antioxidants would be promising for diabetic keratopathy37.
Fibroblast growth factor (FGF)
An earlier study demonstrated growth stimulatory activity of a substance in fibroblasts which was later named as “fibroblast growth factor’’26.Basic fibroblast growth factor (bFGF) is reported to significantly upregulate the expression of epidermal stem cell markers and Notch1/Jagged1 signaling and down regulate the expression of myofibroblasts markers19. Delay in re-epithelialization of the full-thickness excision wounds in the absence of the factor breaks open the significance of the cellular mediator. The knockout of the FGF2 gene Sakamoto et al. (2017) have shown that the human cultured epidermis in combination with meshed skin grafts significantly reduced wound area by promoting granulation tissue formation and angiogenesis in a rat model by producing various growth factors like bFGF, IL-1α, PDGF, TGF and VEGF20. Among the various signaling pathways, viz., RAS/MAPK pathway, PI3 kinase/AKT pathway and PLCγ pathway, RAS/MAPK signaling pathway is found to predominate22. Farahpour et al. (2017) reported that the accelerative impact of wound healing agents were also due to modulating expression of fibroblast growth factor in BALB/c mice excision wound model23. The mitogenic and angiogenesis properties of the bFGF are said to induce tissue remodeling, wound healing and neovascularization as tested in animal models24.The rate of wound contraction in diabetic mice wound model was significantly reduced in the absence of FGF-7. The study suggested that the epithelial-mesenchymal interaction which governs the rate of wound closure is directly dependent on the expression levels of fibroblast growth factor25. Ortega et al. studied the ability of bFGF knockout mice to heal full-thickness excision skin wounds. The knockout mice delayed wound contraction and showed reduction in collagen deposition. bFGF promoted fibroblast migration in a dose-dependent manner. Therapeutically, the bFGF drugs had a contraindication in malignant tumor conditions owing to its cell proliferation potency26. mRNA expression studies in mice reveal the relationship between the decline in the FGF with age and impairment in cellular proliferation including keratinocytes, fibroblasts, preadipocytes etc. This decline is understood to be gradual starting from middle age proceeding at higher rates in the older mice28. Inhibition of the basic FGF receptor tyrosine kinase in rats resulted in dose-dependent delay in healing of experimentally induced tympanic membrane perforation34.
Transforming growth factor (TGF)
Human TGF-β superfamily consists of about 33 different proteins that play significant roles in cellular signaling mediated regulation of tissue homeostasis in multicellular organisms29. Platelets, macrophages, neutrophils, fibroblasts and few other cell types produce TGF-β in abundance. This is the key cytokine that initiates intracellular signaling pathways by binding with type II serine threonine kinase receptor influencing transcription of genes whose product affect all the phases of wound healing21. TGF-β signals through the transmembrane serine/threonine kinase receptors. Signal to the receptor induces cross phosphorylation among the two types of receptors which facilitates binding of the intracellular receptor-regulated Smad proteins. Smad knockout mice models lacked in TGF- β1 signaling which in turn resulted in a series of cellular events like increase in inflammation, increase in peripheral lymphocytes and immature neutrophils and insufficient keratinocyte migration and irregular contraction of fibroblasts and hence improper wound closure (Fig. 4).
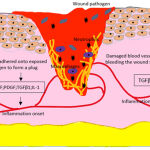 |
Figure 4: Hemostasis and Inflammatory Phase. |
Hemostasis and Inflammatory Phase
Immediately on occurrence of a wound, the platelets aggregate at the wound site to form a physical plug to shut the free flow of blood from the damaged vessels. The fibrin clot and the wound microenvironment releases pro-inflammatory mediators and other factors which recruit neutrophils to the wound site for clearing debri and wound infection, if any, and onset inflammatory phase. The late inflammatory phase is handled by the macrophages that are significant in clearing apoptotic wound tissue including the defensive leukocytes, an act that results in resolution of inflammation which is absolutely essential for proliferative cell migration. EGF, epidermal growth factor; PDGF, platelet derived growth factor; TGFβ1, transforming growth factor β; IL1, interleukin 1; IL4, interleukin 4.
TGF-β1 enhances angiogenesis by promoting the endothelial progenitor cells to facilitate blood supply to the wound site30. TGF-β1 plays a major role in myofibroblast differentiation which is a potent target for treating hypertrophic scars and keloids. Binding of these and other isoforms to TGF-β receptors is found to induce the overproduction of collagen I, collagen III, fibronectin and other cytokines18. Hayashi et al. (1989) investigated the effect of the peptide in corneal healing of a Vitamin A deficient rat model and observed the infiltrated acute inflammatory cells in the peripheral stroma which gradually spread to the central cornea indicating the role in reepithelialisation, collagen remodeling and neovascularization. The significance of TGF-β expression in cellular growth differentiaition and function was understood when Clark and Coker (1998) studied the TGF-β1 knock out mice. 50% of these mice died in utero and the rest suffer from uncontrolled inflammation after birth28. Exogenous TGF-β 1 implantation in male albino rabbits with a standard surgical incision in the diestema region regulated the oral mucosal wound healing process better than the controls through alteration in the production levels of Nitric Oxide (NO).
Platelet derived growth factor (PDGF)
Platelet derived growth factor (PDGF) is a helping hand in regulating a lot of cellular activities related to wound healing process including mitogenesis of fibroblasts and many cells, angiogenesis and chemotaxis. The protein is made of A and B polypeptide chains, combining to generate three isoforms, AA, AB and BB. Located on the chromosomes 7 and 22, the genes for A and B chains express to generate proteins of varying degrees of affinities to cell surface receptors belonging to the tyrosine kinase family38. The expression if stimulated by conditions like low oxygen tension, thrombin levels and presence of other growth factors or cytokines. PDGF binds to the specific receptors and activate them through ligand-induced dimerization of α and β receptors, forming homo- and heterodimers of different signaling strengths. Receptor dimerization induces autophosphorylation of intracellular receptor components, change in conformation in-turn and activation39. Activation of the PDGF receptors tend to induce chemotaxis in certain cell types, however PDGF α receptors are studied to inhibit chemotaxis of a few selected cell types like the fibroblasts and the smooth muscle cells. Both the receptor types induce increase in intracellular Ca2+concentrations. In connection with this the receptors are understood to inhibit communication between cells through gap junctions and present an anti-apoptotic effect. A group of intracellular adaptor molecules involve in interacting with the receptor molecule including PI3-K, Src, Stat5, Grb2, Grb7, Nck, Crk etc. These molecules contain the conserved SH2 domain which binds with a phosphorylated tyrosine, which is achieved in this case by ligand dimerization40.
PDGF treated incisional and excisional wounds at 20-200 picomoles concentration by accelerating the infiltration of inflammatory cells and fibroblasts, extracellular matrix deposition and collagen formation; the effect was reported to be an exaggerated one resulting in accelerated healing process as compared to the untreated models41.Earlier Lynch et al. (1987) reported that the platelet-derived growth factor worked better as a partially purified fraction when compared with a pure fraction in terms of inducing fibroblasts migration, collagen and glycosaminoglycan production and DNA synthesis. Purified PDGF in combination with equal volumes of epidermal growth factor (EGF) showed 44% increase in width of the epidermal layer with a thicker keratinocyte layer. The growth factor in combination with insulin-like growth factor (IGF-I) resulted in 2.5 fold wider connective tissue layer. These effects were not seen when either of the synergistic growth factors were added alone to the surgical wound created in Yorkshire pigs42. Similar synergistic role of PDGF was described by Giselle Hosgood (1993) when exogenously administered in combination with transforming growth factor (TGF-β). Though both factors work with a different model of action, synergistically they induce chemotaxis of the inflammatory cells involved in the healing process. In 2008, the US Food and Drug Administration approved indications for use of Regranex Gel, a human recombinant platelet-derived growth factor for topical application for treating diabetic foot ulcers; however serious mortality rate was recorded and warning information were disseminated among the users and medical professionals (Fig. 5, 6).
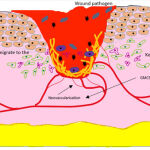 |
Figure 5: Proliferative Phase. |
Proliferative Phase
Overlapping with the inflammatory phase, the phase continues with proliferation and migration of keratinocytes and fibroblasts towards the wound site, various growth factors initiate and accelerate the migration at the wound margins. Angiogenesis favours capillary sprouting and tissue regeneration during this reparative process, all of these triggered by growth factors, singly or in synergy. TNFα, tumor necrosis factor α; IL1, interleukin 1; FGF, fibroblasts growth factor, PDGF, platelet derived growth factor, TGFβ1, transforming growth factor β1, EGF, epidermal growth factor; IGF, insulin-like growth factor
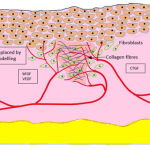 |
Figure 6: Remodelling Phase. |
Remodelling Phase
by the numerously growing new blood vessels. The fibroblasts migrated to the wound site, the collagen and the vascular networks all together form new connective tissues to reconstruct and replenish the granulation tissue, to retain the original structurally and functionally efficient skin. The transition between the various collagen types mediated by the connective tissue growth factor (CTGF) brings back the tensile strength of the tissue at its pre-wounded state; however the completion of remodelling spans different time durations according to the wounded tissue type and intensity of wounding. bFGF, basic fibroblasts growth factor; VEGF, vascular endothelial growth factor
Vascular endothelial growth factor (VEGF)
Angiogenesis, a key event in various natural cellular processes including wound healing is induced by an interesting proangiogenic factor called vascular endothelial growth factor (VEGF). All the seven members of the VEGF family share a homology domain and are thought to transduce signals by binding to specific vascular endothelial growth factor receptors. Activated platelets at the wound site release VEGF-A which through binding with VEGFR-1, on the inflammatory cells, attracts circulating neutrophils and monocytes. The recruited neutrophils further bring into the loop various proinflammatory cytokines like IL-1β and TNF-α. In addition the VEGF-A is studied to have a hand in regulating the plasminogen activator production during the re-epithelialization phase44. VEGF induces local vascular regeneration in radius fracture model of rabbits. It is studied to be a very important factor to promote vascularity and closure in hypoxic chronic wounds. The impaired healing of diabetic wounds is studied to be due to diminished production of VEGF and thus decreased angiogenesis. In addition to increasing and maintaining vasculature in the experimental full-thickness mice wound models, the vascular permeability factor, VEGF, also accelerates wound healing by attracting the inflammatory cells at the site of injury and inducing migration and proliferation of the endothelial cells45. The expression of VEGF and their receptors is thought to be regulated by hypoxia, a condition created during traumatic wound or ischemic injury. Developmental studies in mouse embryos have revealed that loss of at least a single allele of VEGF gene results in abnormal blood vessel formation and embryonal death. The absence of VEGF receptors result in failed differentiation of haemangioblast precursor cells to endothelial cells, a crucial cellular developmental process46. The importance of VEGF in wound healing was understood from the Cheng et al’s. (2016) effort to review the use of anti-VEGF agents to control wound healing process in patients who have undergone glaucoma filtration surgery, done to manage intraocular pressure, as scarring during regular wound healing process in these patients would lead to failure in the surgery47.
Granulocyte macrophage colony stimulating factor (GM-CSF)
The keratinocytes, upon injury, secrete the granulocyte macrophage colony stimulating factor (GM-CSF) which acts in an autocrine manner to enhance epidermal proliferation. The significance of GM-CSF in normal wound healing was understood when GM-CSF antagonists over-expressing mice were experimentally observed; the mice showed delayed epithelialization and neovascularization48. GM-CSF enhances keratinocyte’s migratory capabilities thereby supporting the influx of these cells at the wound site, a remarkable event that ends up in epithelialization and granulation tissue formation49. GM-CSF deficiency resulted in defective vascular collagenous matrix production suggesting the significance in maintaining vascular integrity. In diabetic mice exogenous GM-CSF enhanced wound healing by increasing production of IL-6 and monocyte chemoattractant protein-1 production. Topical application of GM-CSF was found to treat decubital ulcers in cancer patients at a concentration of 200µg/mL50. At a lesser concentration, Gulcelike et al. (2006) proved a local injection of GM-CSF helped enhance healing of an incisional wound in the Adriamycin-treated rats51. In addition GM-CSF happens to be chemotactic for a wide range of inflammatory cells and mediators, indirectly contributing to the wound inflammation and repair. GM-CSF thus meeting all the requirements to be an active agent accelerating wound healing mechanisms, achieved interests among scientists to be synthesized in lab by recombinant technology. rHuGM-CSF has been through clinical trial cases, intradermal infiltration of the same resulted in complete healing of the chronic plebostatic ulcerative lesion in about eight weeks of treatment. Experimental studies have also recorded GM-CSF has a stimulatory effect on the phagocytotic and bactericidal properties of macrophages52. Local injections of GM-CSF healed sacral pressure ulcer in a patient with bilateral hemiplegia in an observational case study. A firm granulation tissue formed within few days; biopsy of the granulation tissue showed inflammatory cells and fibroblasts, the site of injections showing higher infiltration. Randomised control trials in patients with diabetic foot ulcers evidenced the role of GM-CSF in increasing the release of neutrophil endothelial progenitor cells from bone marrow thus being significant in infiltrating the inflammatory cells. Systemic administration of GM-CSF has proved to be effective even in healing dystrophic epidermolysis bullosa wounds in a pilot trial study. At molecular level, binding of recombinant GM-CSF to its specific receptor creates a signaling complex that activates Janus Kinase (JAK) and signal transducer and activator of transcription (STAT) proteins; through a stream of signaling molecules like MAPK and c-fos and c-jun genes the factor influences regulation of hematopoietic differentiation53-56.
Connective tissue growth factor (CTGF)
Connective tissue growth factor is a protein regulating a number of biological processes like cell proliferation and differentiation, adhesion and angiogenesis. Exogenous CTGF has been extensively researched in animal and human trials for healing potency. Streptozotocin- induced diabetic Sprague-Dawley rats were used as diabetic wound models with experimentally created full-thickness excision wounds. Topical administration of recombinant CTGF for a period of seven days showed a difference in wound closure in the diabetic group though not statistically significant56,57. Another experimental excision wound models were observed after long-term administration of recombinant CTGF which revealed enhanced fibroblasts proliferation and collagen deposition at the granulation tissue. The vascularity was better in the treated model compared to the controls. The wound cellular microenvironment was influenced by the CTGF treatment, especially in enhancing the macrophage counts. The macrophage population is inevitable for onset and resolution of wound inflammation. In addition CTGF expression was observed in the early stages of acute burn injury; however experimental evidences for its role need to be deduced. Collagen biosynthesis and deposition determines the extent of healing and thus the quality of healing58. CTGF treated diabetic wounds markedly showed an increase in collagen IV, which is significant to take the healing forward to a remodeling phase. Since biosynthesis of collagen involves formation of hydroxyproline, the measure of the later serves as an indication of tissue collagen. Radio-active proline injected into the wound site traces the net rate of collagen synthesis and deposition in experimental rat models. Reepithelialisation though occurred in the CTGF knockout mice was found to be impaired; CTGF inhibition delayed wound closure and stromal scarring. CTGF apart from being chemotactic for fibroblasts, also regulates scarring. CTGF is understood to be a part of the positive feedback loop, inducing thrombospondin to convert the latent TGF-β precursor to active form59,60.
Conclusion
Managing impairment in regular healing mechanism and improving life quality, in patients with specific surgical or clinal conditions, is a major challenge for clinicians. The therapeutic use of synthetic extraneous growth factors like EGF, FGF, VEGF, GM-CSF etc have been increasing since the past few years. Recombinant proteins replaced the synthetic preparations to overcome the drawbacks associated. Future research and pre-clinical testing can target molecular interactions between intracellular proteins triggered by these signal; mechanisms like PI3K/AKT pathways and JAK/STAT pathway could be considered to customize these growth factors on a case to case basis.
Acknowledgement
The support from Sri Ramachandra Institute of Higher Education and Research is duly acknowledged.
Conflict of interest
No competing interests exist.
References
- Manjunatha BK. Vidya SM, Rashmi KV, Mankani KL, Shilpa HJ, Singh SJ. Evaluation of wound-healing potency of Vernonia arborea Hk. Indian J Pharmacol 2005;37:223-6
CrossRef - Gardner S, Sidisunthorn P and Anusarnsunthorn V. A field guide to Forest Trees of Northern Thailand. Kobfai Publishing Project. Bangkok. Thailand.2000
- Krishna Kumari GN, Masilamani S, Ganesh MR, Aravind S, Sridar SR. Zaluzanin D: A fungistatic sesquiterpene fromVernonia arborea. Fitoterapia 2003;74:479-82.
CrossRef - Wahl S.M. “Transforming growth factor beta.” in Inflammation: Basic Principles and Clinical Correlates, Third Edition, J. Gallin and R. Snyderman, eds., Lippincott-Raven Publishers, Philadelphia. 1999:883-892.
- Rajan V and Murray RZ. The duplicitous nature of inflammation in wound repair. Wound Practice and Research 2008;16(3):122-129.
- Koh TJ and DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Review in Molecular Medicine 2013;1-14.
- Werner S and Grose R. Regulation of wound healing by growth factors and cytokines. Physiological Reviews 2003;83:835-870.
CrossRef - Peter TD and Kenneth KI. Growth Factors for Wound Healing. Biotechnology 1989;7:793-798.
CrossRef - Sephra NR. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012;12:12347-12360.
CrossRef - Traversa B and Sussman G. The role of growth factors, cytokines and proteases in wound management. Primary Intention 2001;9(4):161-167.
- Stephan B, Olivera S, Michael SG, Harold B and Marjana TC. Growth factors and cytokines in wound healing. Wound Repair and Regeneration 2008;16:585-601.
CrossRef - Zhang SZ, Lipsky MM, Trump BF, Hsu IC. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes.Cell Biol Toxicol. 1990;6(2):219–34.
CrossRef - Berlanga, J.; Fernández, J. I.; López, E.; López, P. A.; del Río, A.; Valenzuela, C.; Baldomero, J.; Muzio, V.; Raíces, M.; Silva, R.; Acevedo, B. E.; Herrera, L. (2013). “Heberprot-P: a novel product for treating advanced diabetic foot ulcer”.MEDICC Review. 15 (1): 11–15.
CrossRef - Richard JB. Epidermal growth factor and Epidermal growth factor receptor: The Yin and Yang in the treatment of cutaneous wounds and Cancer. Advances in Wound Care. 2013;2(1):24-29.
CrossRef - Okumura K, Kiyohara Y, Komada F, Iwakawa S, Hirai M and Fuwa T. Improvement in wound healing by epidermal growth factor (EGF) ointment. 1. Effect of nafamostat, gabexate or gelatin on stabilization and efficacy of EGF. Pharmaceutical Research. 1990;7(12):1289-93.
CrossRef - Kwon YB, Kim HW, Roh DH, Yoon SY, Baek RM, Kim JY, Kweon H, Lee KG, Park YH and Lee JH. Journal of Veterinary Science. Topical application of epidermal growth factor accelerates wound healing by myofibroblast proliferation and collagen synthesis in rat. 2006;7(2):105-9.
CrossRef - Kim H, Kong WH, Seong K-Y, Sung DK, Jeong H, Kim JK, Yang SY and Hahn SK. Hyaluronate-Epidermal growth factor conjugate for skin wound healing and regeneration. Biomacromolecules. 2016;17(11):3694-3705.
CrossRef - Jianglin T and Jun W. Current progress in understanding the molecular pathogenesis of burn scar contracture. Burns Trauma. 2017;5:14.
CrossRef - Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z, Chen L, Zhao J, Liu X, Qi S, Xiong K and Xie J. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Research and Therapy. 2017;8:114.
CrossRef - Sakamoto M, Morimoto N, Inoie M, Takahagi M, Ogino S, Jinno C, Suzuki S. Cultured human epidermis combined with meshed skin autografts accelerates epithelialization and granulation tissue formation in a rat model. Annals of Plastic Surgery. 2017;78(6):651-658.
CrossRef - Jagajeevan J, Ardeshir B. Transforming growth factor beta (TGFβ) and keloid disease. International Journal of Surgery. 2007; 5(4): 278-285.
CrossRef - Ye-Rang Yun, Jong Eun Won, Eunyi Jeon, Sujin Lee, Wonmo Kang, Hyejin Jo, Jun-Hyeog Jang, Ueon Sang Shin and Hae-Won Kim. Fibroblast growth factors: Biology, function and application for tissue regeneration. Journal of Tissue Engineering. 2010; 1(1): 677-686.
CrossRef - Mohammad Reza Farahpour, Milad R. Vahid and Ahmad Oryan. Effectiveness of topical application of Ostrich oil on the healing of Staphylococcus aureus and Pseudomonas aeruginosa-infected wounds. Connective Tissue Research. 2017; 6.
CrossRef - Sadanori Akita, Kozo Akino and Akiyoshi Hirano. Basic fibroblast growth factor in scarless wound healing. Advances in Wound Care. 2013; 2(2): 44-49.
CrossRef - Peng C, Chen B, Kao HK, Murphy G, Orgill DP and Guo L. Lack of FGF-7 further delays cutaneous wound healing in diabetic mice. Plastic and Reconstructive Surgery. 2011; 128(6): 673e-84e.
CrossRef - Keisuke Okabe, Ruka Hayashi, Noriko Aramaki-Hattori, Yoshiaki Sakamoto, Kazuo Kishi. Wound treatment using growth factors. Modern Plastic Surgery. 2013; 3: 108-112.
CrossRef - Antonio Medeiros Dantas Filho, José Lamartine de Andrade Aguiar, Luís Reginaldo de Menezes Rocha, Ítalo Medeiros Azevedo, Esdras Ramalho and Aldo Cunha Medeiros. Effects of the basic fibroblast growth factor and its anti-factor in the healing and collagen maturation of infected skin wound. Acta Cirúrgica Brasileira. 2007; 22(1): 64-71.
CrossRef - Akiko Komi-Kuramochi, Mitsuko Kawano, Yuko Oda, Masahiro Asada, Masashi Suzuki1, Junko Oki and Toru Imamura. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. Journal of Endocrinology. 2005; 186: 273-289.
CrossRef - Clark D and Coker R. Molecules in focus Transforming growth factor-beta (TGF-β). International Journal of Biochemistry and Cell Biology. 1998; 30(3): 293-298.
CrossRef - Chen W and Dijke P. Immunoregulation by members of the TGF-β superfamily. Nature Reviews Immunology. 2016; 16: 723-740.
CrossRef - Pakyari M., Farrokhi A., Maharllooei M.K., Ghahary A. Critical role of Transforming Growth Factor Beta in different phases of wound healing. Advances in Wound Care. 2013; 2(5): 215-224.
CrossRef - Coskun S., Peker E.G.G., Balabanli B., Ahiska S. and Acarturk F. Effect of transforming growth factor beta 1 (TGF-beta 1) on nitric oxide production and lipid peroxidation in oral mucosal wound healing. Medicinal Chemistry Research. 2011; 20(1): 23-28.
CrossRef - Hayashi K., Frangieh G., Wolf G. and Kenyon K.R. Expression of transforming growth factor-beta in wound healing of Vitamin A-deficient rat corneas. Investigative Ophthalmology & Visual Science. 1989; 30(2): 239-47.
- Holger Kaftan, Lars Reuther, Barbel Miehe, Werner Hosemann, Achim Beule. Inhibition of fibroblast growth factor receptor 1: influence on tympanic membrane wound healing in rats. European Archives of Oto-Rhino-Laryngology. 2012; 269(1): 87-92.
CrossRef - Saveria Pastore, Francesca Mascia, Valentina Mariani, Giampiero Girolomoni. The epidermal growth factor receptor system in skin repair and inflammation. Journal of Investigative Dermatology. 2008; 128(6): 1365-1374.
CrossRef - Hye Jin Choi, Jung Hoon Ahn, Seong-Hwan Park, Kee Hun Do, Juil Kim, Yuseok Moon. Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via Human Epidermal Growth Factor Receptor in human intestinal epithelial cells: Therapeutic implication using recombinant probiotic. Infection and Immunity. 2011: 1079-1087.
CrossRef - Ke-Ping Xu, Yanfeng Li, Alexander V. Ljubimov, Fu-Shin X. Yu. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol-3 kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009; 58: 1077-1085.
CrossRef - Westermark B, Heldin CH. Platelet-derived growth factor. Structure, function and implications in normal and malignant cell growth. Acta Oncologica. 1993; 32(2): 101-5.
CrossRef - Carl-Henrik Heldin and Bengt Westermark. Mechanism of action and invivo role of platelet-derived growth factor. Physiological Reviews. 1999; 79(4): 1283-1316.
CrossRef - Carl-Henrik Heldin and Johan Lennartsson. Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harbor Perspectives in Biology. 2013; 5: a009100.
CrossRef - Pierce GF, Mustoe TA, Altrock BW, Deuel TF and Thomason A. Role of platelet-derived growth factor in wound healing. Journal of Cellular Biochemistry. 1991; 45(4): 319-26.
CrossRef - Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proceedings of the National Academy of Sciences. 1987; 84: 7696-7700.
CrossRef - Hosgood G. Wound Healing. The role of platelet-derived growth factor and transforming growth factor beta. Veterinary surgery. 1993; 22(6): 490-495.
CrossRef - Ann Hoeben, Bart Landuyt, Martin S. Highley, Hans Wildiers, Allan T. van Oosterom and Ernst A. De Bruijin. Pharmacological Reviews. 2004; 56(4): 549-580.
CrossRef - Robert D. Galiano, Oren M. Tepper, Catherine R. Pelo, Kirit A. Bhatt, Matthew Callaghan, Nicholas Bastidas, Stuart Bunting, Hope G. Steinmetz and Geoffrey C. Gurtner. The American Journal of Pathology. 2004; 164(6): 1935-1947.
CrossRef - Michael J. Cross and Lena Claesson-Welsh. FGF and VEGF function in angiogenesis: signaling pathways, biological responses and therapeutic inhibition. TRENDS in Pharmacological Sciences. 2001; 22(4): 201-207.
CrossRef - Cheng J.W., Cheng S.W., Wei R.L. and Lu G.C. Anti-vascular endothelial growth factor for control of wound healing in glaucoma surgery. Cochrane Database of Systemic Reviews. 2006; 1: CD009782.
- Mann A., Niekisch K., Schirmacher P. and Blessing M. Granulocyte macrophage colony stimulating factor is essential for normal wound healing. Journal of Investigative Dermatology. 2006; 11: 87-92.
CrossRef - Schirmacher P., Mann A., Breuhahn K. and Blessing M. Keratinocyte-macrophage colony stimulating factor accelerates wound healing: stimulation of keratinocyte proliferation, granulation tissue formation and vascularization. Journal of Investigative Dermatology. 2001; 117(6): 1382-1390.
CrossRef - Raderer M., Kornek G., Hejna M., Koperna K., Scheithauer W. and Base W. Topical granulocyte-macrophage colony stimulating factor in patients with cancer and impaired wound healing. Journal of the National Cancer Institute. 1997; 89(3): 263.
CrossRef - Gulcelik M.A., Dinc S., Dinc M., Yenidogan E., Ustun H., Renda N. and Alagol H. Local granulocyte macrophage colony stimulating factor improves incisional wound healing in adiramycin-treated rats. Surgery Today. 2006, 36(1): 47-51.
CrossRef - Castrogiovanni P., Ventimiglia P. and Imbesi R. Wound healing: experience with rHuGM-CSF. Wounds. 2010; 22(10).
- El Saghir N.S., Bizri A.R.N., Shabb N.S., Husami T.W., Salem Z. and Shamseddine A.I. Pressure ulcer accelerated healing with local injections of granulocyte macrophage colony stimulating factor. Journal of Infection. 1997, 35(2): 179-182.
CrossRef - Cruciani M., Lipsky B.A., Mengoli C. and Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Systemic Review. 2013.
CrossRef - Fine J.D., Manes B. and Frangoul H. Systemic granulocyte colony-stimulating factor (G-CSF) enhances wound healing in dystropic epidermolysis bullosa (DEB): results of a pilot trial. 2015, 73 (1): 56-61.
CrossRef - Ramazani Y., Knops N., Elmonem M.A., Nguyen T.Q., Arcolino F.O., Van de Heuvel L., Levtchenko E., Kuypers D. and Goldschmeding R. Connective tissue growth factor (CTGF) from basics to clinics. 2018, 68-69: 44-66.
CrossRef - Alfaro M.P., Deskins D.L., Wallus M., DasGupta J., Davidson J.M., Nanney L.B., Guney A.M., Gannon M. and Young P.P. A physiological role for connective tissue growth factor in early wound healing. 2013, 93(1): 81-95.
CrossRef - Henshaw F.R., Boughton P., Lo L., McLennan S.V. and Twigg S.M. Topically applied Connective Tissue Growth Factor/CNN2 Improves Diabetic Preclinical Cutaneous Wound Healing: Potential Role for CTGF in Human Diabetic Foot Ulcer Healing. 2015, 2015: 236238.
CrossRef - Smith Q.T. Collagen Metabolism in Wound Healing. Trauma. 1975: 31-45.
CrossRef - Daniel J.G., Liya P., Sriniwas S., Cong M., Bryon E.P., Edward W.S., Andrew L., Gregory S.S. Conditional knockout of CTGF Affects Corneal Wound Healing. Cornea. 2014, 55(4): 2062-2070.
CrossRef







