Ahmed Algazeery1 , Ahmed H. Moustafa2
, Ahmed H. Moustafa2 , Ashraf S. El-Sayed3
, Ashraf S. El-Sayed3 , Marwa G. Rizk2
, Marwa G. Rizk2 and Norhan A. Sabbah4
and Norhan A. Sabbah4
1Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt
2Chemistry Department, Faculty of Science, Zagazig University, Zagazig, Egypt
3Botany and Microbiology Department, Faculty of Science, Zagazig University, Egypt
4Medical Biochemistry Department, Faculty of Medicine, Zagazig University, Egypt
Corresponding Author E-mail: asalgazeery@zu.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2234
Abstract
Background: Using synthetic drugs for treating liver fibrosis remains a challenge since, in contrast to natural products, are remarkably expensive and associated with several adverse effects. Herbs and plants showed strong antioxidant, anti-inflammatory, and antimicrobial properties. Aim: To investigate the hepatoprotective role of fresh chicory juice in delaying the immune response of hepatic cells to Carbon tetrachloride [CCl4]-induced fibrosis. Methods: Fresh chicory plant juice [50%] was given instead of drinking water to male albino rats [150-200 g]. Blood samples were collected for biochemical evaluation of liver and kidney function, antioxidant markers, lipid profile, and gene expression of TGF-ß by quantitative real-time quantification polymerase chain reaction [q PCR]. Liver tissue was removed and subjected to histopathological and genomic DNA fragmentation assay. Results: Measurements of liver enzymes, kidney function, lipid profile and levels of antioxidants confirmed the ability of chicory to protect the liver against CCl4-induced liver fibrosis by acting as a good inhibitor of TGF-ß. These results were confirmed by histopathological examination and DNA fragmentation. Conclusion: Administration of fresh chicory juice [50%] showed a significant protective role of chicory plant in delaying CCl4-induced liver fibrosis by decreasing TGF-ß.
Keywords
Collagen; Chicory; CCL4; DNA; Fibrosis; Liver; TGF-beta
Download this article as:| Copy the following to cite this article: Algazeery A, Moustafa A. H, El-Sayed A. S, Rizk M. G, Sabbah N. A. Evaluation of the Curative and Protective Role of Fresh Chicory Juice in Treatment of Hepatic Fibrosis in Male Albino Rats. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Algazeery A, Moustafa A. H, El-Sayed A. S, Rizk M. G, Sabbah N. A. Evaluation of the Curative and Protective Role of Fresh Chicory Juice in Treatment of Hepatic Fibrosis in Male Albino Rats. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2W3WGuU |
Introduction
The liver is a vital organ in the body working as the center of metabolism, and in detoxification of wastes, chemicals, and toxic agents 1. Reports confirmed that about 2 million people die with liver diseases per year worldwide 2. The primary causes of liver fibrosis are mostly alcohol and nonalcoholic fatty liver disease [NAFLD] in Western industrialized countries, while in the middle east viral infection e.g., HBV or HCV is the main cause, which could lead to hepatocellular carcinoma [HCC], as about 55%-85% of HCV-infected cases become chronic active cases and lead to the development of hepatic fibrosis, cirrhosis, and HCC 3.
In Egypt, predominance of hepatitis C virus [HCV] was mostly caused by antischistosomal 4 treatment, while about 24.3% of patients were infected through blood transfusion 5. Chronic hepatitis C [CHC] infection was reported to induce hepatic inflammation and stimulates liver fibrosis 6. Liver fibrosis is clinically silent, slowly progressive, and mostly asymptomatic disease. It is associated with the collapse of the hepatic parenchyma and its substitution with a collagen-rich tissue 7. The first symptoms of liver impairment in most of the cases are showing disease development into cirrhosis, which commonly occurs after 15–20 years, when the prognoses of survival and recovery are dramatically reduced. The only effective treatment for end-stage liver failure is liver transplantation 8. Synthetic drugs used to cure or prevent liver fibrosis have often proved life threatening and, therefore, the preference is being shifted to complementary and alternative medicines [CAM], which are either natural products or their derivatives 9.Chicory, Cichoriumintybus is a small perennial herb that is commonly found in nature, especially in the winter 10.
Chicory is widely distributed in Africa, Asia-temperate, Asia tropical, Europe, Australia, Northern America, and Southern America 11. Approximately 70%–75% of the world’s population depends on herbal medicines for curing diseases because they are cost- effective, less toxic, and easily available little adverse effects and minor drug reactions 12,13. Chicory plant extracts were presented as an anti-inflammatory effect because of their sesquiterpene lactone in root 14. Recent studies by 15 have proved that dry chicory extract contains a phenolic complex of biologically active substances, namely, oxycoumarins, hydroxycinnamic acids, and flavonoids, these substances are an effective immune-correcting agent, which modulates the cell-mediated immune response, antibody response, and phagocytosis. In this study, we investigate the curative and protective anti-fibrotic activity of fresh chicory juice and its potential role in free radical scavenging and modulation of the genomic integrity associated with hepatic fibrosis. We designed the experiment by using the CCl4-induced hepatic fibrosis. The choice of CCl4 is based on its frequent environmental exposure since it occasionally released from industrial sites. CCl4 could reach hepatic tissue via inhalation, ingestion from air, drinking water, and foodstuffs or even soil 16,17.
Materials and Methods
This study was conducted with the permission of department of chemistry and Biochemistry faculty of science and the department of Medical Biochemistry faculty of human medicine Zagazig University–Egypt, at Zagazig Scientific Medical Research Center(ZSMRC).
Animals
In this study, 36 adult male albino rats [Rattusnorvegicus] of weight range from 150 to 200 g. The animals were obtained from the Faculty of Veterinary Medicine- Zagazig University. The animals were housed in the animal house of Zoology department, faculty of Science under standard conditions [26 ± 2 °C and relative humidity 30%-35%] in 12 h light and 12-h dark cycle. Animals were provided with water and standard rodent pellet diet. Only the chicory- treated animals were given fresh chicory juice [50%] instead of drinking water. The animals were accommodated to the laboratory conditions for two weeks before being treated. The study was approved by the Institutional Animal Care and Use Committee of Zagazig University [ZU-IACUC/3/F/75/2019].
Chemical
Carbon tetrachloride CCl4 was purchased from El Gomhoureya for Chemicals Trade & Medical Supplies [Zagazig, Egypt], and dissolved in olive oil [25%] [18], injected intraperitoneally [2mg/kg b. wt.] twice/week 45 days.
Plant
Fresh chicory plant was collected during October 2019 from “Zagazig, Sharkia, Egypt” where they grow. 250g of fresh chicory plant were washed with tap water, to remove dust, and then crushed within 500 mL-distilled water using an electric blender and the resulting mixture was filtered. The juice administered once per day instead of drinking water for 45 days.
Experimental Design
The study was conducted on 36 mature male albino rats [Rattusnorvegicus], rats were randomly divided into 6 main groups; each group comprised 6 rats.
Treatment schedule
The firstgroup [control group]
Animals were housed without treatment in a laboratory under slandered condition of nutrients and temperature for 12 weeks.
The second group [carbon tetrachloride–treated group]
Animals were injected with 25% carbon tetrachloride at a dose of [2 mL/kg b/wt.] dissolved in olive oil twice/week for 6 weeks.
The third group [a curative chicory group]
Animals were injected intra peritoneal with carbon tetrachloride at a dose of [2mL/kg b/wt.] twice/week for 6 weeks, then animals were administered 50% fresh chicory juice instead of drinking water once per day for another 6 weeks.
The fourth group [a protective chicory group]
We gave the animals 50% fresh chicory juice instead of drinking water once per day for other 6 weeks, then animals were injected intra peritoneal with 25% carbon tetrachloride at a dose of [2 mL/kg b. wt.] twice/week for other 6 weeks.
The fifth group [fresh chicory juice–treated group]
Animals were administered 50% fresh chicory juice instead of drinking water once per day for another 6 weeks.
The sixth group [olive oil treated group]
Animals were injected with olive oil at a dose of [2mL/kg b/wt.] twice/week for 6 weeks.
Blood sample collection
After 48h of the last dose of treatment, blood samples were collected by the removal of eyeball, animals killed by cut abdomen opening for liver tissue removal. Serum was harvested from blood without heparin by centrifugation at 3000 rpm and then serum samples were transferred into Eppendorf tubes and stored at −20 °C for measurement of biochemical parameters.
Tissue preparation
The liver was excised from each animal; slice samples were preserved in 10% formalin at room temperature and processed histopathological staining and subsequent light microscope examination. Slices of liver tissue were put into Eppendorf tubes and directly preserved in liquid nitrogen, then transferred to-80oC for genomic DNA fragmentation assay, and Quantitative Real-Time Quantification Polymerase Chain Reaction [qPCR].
Biochemical analysis
All the biochemical parameters of this study were estimated at Zagazig Scientific Medical Research Center ZSMRC.
Investigation of liver and kidney functions
The effect of fresh chicory juice administration on CCl4– induced fibrotic liver tissue is evaluated by measuring the levels of aspartate aminotransferase [AST] and alanine aminotransferase [ALT] in serum according to 19, by using a kit of Sigma-Aldrich, Cat. No. MAK055and MAK052, respectively. The level of alkaline phosphatase [ALP] was evaluated based on its kinetic activity according to the International Federation of Clinical Chemistry [IFCC] 20, by using a kit of Spectrum-Diagnostics, Cat. 217001. Evaluation of kidney function associated with induced hepatic fibrosis was analyzed by measuring the levels of urea based on Urease-UV a fixed rate method [21], by using a kit of Spectrum-Diagnostics, Cat. 321001. The level of creatinine was measured based on Creatinine Buffered Kinetic Jaffe reaction without deproteinization 22, by using a kit of Spectrum-Diagnostics, Cat. 234001 and the developed colors were measured spectrophotometrically at corresponding wavelengths given on kits.
Measurements of lipid profile
The effect of CCl4-induced hepatic fibrosis on lipid peroxidation of cellular membranes was mirrored in serum by measuring the triglycerides based on GPO–PAP Enzymatic colorimetric method 23 and by total cholesterol based on GHOD-PAP- Enzymatic colorimetric method 24, by using kits of Spectrum-Diagnostics, Cat. No. 314001 and 230001, respectively.
Measurement of Antioxidants
The status of oxidative stress was analyzed in serum by measuring the levels of superoxide dismutase activity 25 and reduced glutathione 26 by using Kits obtained from Sigma-Aldrich, Cat. No. 19160 and CS0260, respectively.
Histopathological examination
To evaluate the effect of treatment on morphological architecture, liver tissue was exposed to manual Hematoxylin and Eosin staining 27 ,28. The collagen area percentages were assessed by Masson’s Trichrome [MT] staining 29.
DNA fragmentation and agarose gel electrophoresis
Genomic DNA extraction of rat’s liver was extracted according to the purification protocol of genomic DNA from animal tissues [30] and following instructions of the QIAamp DNA Mini Kit Cat. No. 51304. The tissue was homogenized in 1-ml lysis buffer [20 mM Tris-Cl [pH 7.5], 0.15 M NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and 25 mM disodium pyrophosphate] at 37 °C for 1 h. Then, 0.4 ml of saturated NaCl was added to each set of cell lysates and were incubated on ice for 5 minutes and centrifuged at 3,000 ×g for 30 min. The DNA was precipitated using chilled ethanol, which was separated by centrifugation. Separated DNA was re-suspended in the TAE buffer [40 mM Tris-acetate and 1 mM EDTA], Gel preparation was done by using Molecular biology grade agarose to prepare 2% agarose gel containing 0.5 ug/ml ethidium bromide in 1 x TAE buffer according to [31] The power supply was turned on at 100 volts for 30-45 minutes to allow separation of DNA marker bands. The DNA bands were observed and photographed under a UV trans-illuminator.
Quantitative Real-Time Quantification Polymerase Chain Reaction [qPCR]
Each 100 mg of liver specimen from each group was ground after being frozen in liquid nitrogen. Total RNA was extracted using Trizol (Invitrogen; Thermo Fisher Scientific, Inc.). For evaluating the RNA quality, the A260/A280 ratio was analyzed using Nano Drop® ND–1000 Spectrophotometer (Nano Drop Technologies; Wilmington, Delaware, United States) and immediately reverse-transcribed into Complementary DNA (cDNA) using, a HiSenScript™ RH (-) cDNA Synthesis Kit (iNtRON Biotechnology Co., South Korea) .The resulting cDNA was preserved at −20 °C until used in subsequent PCR. Real-time RT-PCR was performed in a Rotor-Gene Q PCR System (Qiagen, Germany) using Top real SYPR Green qPCR Mix Plus (Enzynomics, Korea) following the manufacturer’s instructions. The PCR cycling conditions were initial denaturation at 95 °C for 12 min followed by 40 cycles of denaturation at 95 °C for 20 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 30 seconds. The oligonucleotide-specific primers were synthesized by Sangon Biotech (Beijing, China), the primer sequences are enlisted in table (1). The expression level of the target genes transforming growth factor beta (TGF-β1) was normalized using the mRNA expression of known housekeeping genes; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results are expressed as fold-changes compared to the control group following the 2-ΔΔCT method 32.
Table 1: Primers
| Gene | Forward primer [5′–3′] | Reverse primer [5′–3′] | Accession No | size |
| Rat TGFβ1 | CTGAACCAAGGAGACGGAAT | GGTTCATGTCATGGATGGTG | NM_021578.2 | 142 |
| Rat Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | NM_017008.4 | 143 |
Results
Evaluation of liver and kidney functions
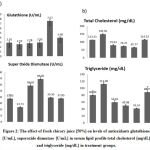
Measurements of liver enzymes, ALT, AST, and ALP in the CCl4-untreated group were 46 U/L, 45 U/L, and 330.08U/L, which showed a significant increase in ALT. The administration of 50% fresh chicory juice as a curative substance decreased the enzymes to 43 U/L, 39 U/L, and 294 U/L respectively, administration of fresh chicory juice as a protective agent, decreased them significantly to 41.7 U/L, 38.125 U/L, and 291.67 U/L, these results have proved that administration of fresh chicory juice is more effective as a protective agent than its application as a curative agent.
The effect of liver ailment on kidney function was evaluated by measuring the levels of creatinine and urea in the serum of the CCl4-untreated group, which were 0.96 mg/dL and 47.1 mg/dL recording a significant increase in both parameters, the administration of 50% fresh chicory juice decreased them to 0.615 mg/dL and 34.24 mg/dL in the curative group, respectively. They highly decreased in the protective group to 0.604 mg/dL and 29.1 mg/dL, which proved that fresh chicory juice was more effective in protection from renal dis function [Figure 1].
To mirror the effect of CCl4 administration on lipid peroxidation of hepatic cell membranes, the levels of triglycerides [TG] and total cholesterol were measured. They were highly increased in the CCl4-untreated group in case of TG to 112 mg/dL and 149.5 mg/dL in case of total cholesterol, which is highly significant. Administration of fresh chicory juice after CCl4 in the curative group highly decreased serum levels of TG and total cholesterol to 59.65 mg/dL and 79.63 mg/dL respectively while the administration of fresh chicory juice before CCl4 administration in the protection group highly decreased serum levels of TG and total cholesterol to 49.98 mg/dL and 74.25 mg/dL [Figure2], respectively, showing a significant decrease in serum level of total cholesterol in both protective and curative group.
To evaluate the status of oxidative stress, we measured the levels of antioxidant enzymes SOD and GSH in serum. Administration of fresh chicory juice after CCl4 in the curative group increased SOD and GSH significantly to 58.49 U/mL and 2.78 U/mL, respectively, while the administration of fresh chicory juice before CCl4 in the protective group highly increased SOD and GSH to 69.046 U/mL and 2.95 U/mL , Showing a significant increase in SOD [Figure2]. Furthermore, this confirms that chicory plants may act as a good antioxidant against oxidant stress of CCl4, especially as protective.
Histopathological analysis
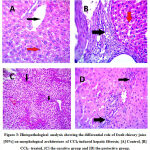
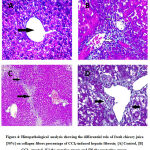
In CCl4-treated group histopathological analysis by hematoxylin and eosin stain [H&E] showed liver nodules surrounded by thick fibrous bands [Figure3].Masson’s Trichrome stain showed a significant increase in collagen fiber percentage, leading to liver cirrhosis F4 [Figure4]. In Curative group histopathological analysis by H&E showed thick fibrous bands extending from the central vein to the portal tract and from Porto-pre as a liver cloudy swelling [Figure3]. Masson’s Trichrome stain showed improved in collagen area percent F3 [Figure4].In protective group histopathological analysis by H&E showed short fibrous bands [Figure3].Masson’s Trichrome stain showed a significant decrease in collagen area percent F2 [Figure4].
DNA fragmentation assay
Genomic DNA samples were extracted from untreated and treated animals. The CCl4– injected animals [lane 3] were extensively fragmented, associated with significant internucleosomal DNA fragmentation in CCl4– induced hepatic cells, in contrast to the control group, which gave a single intact un-fragmented band on lane 2 [Figure 5]. To investigate the curative effect of fresh chicory juice in lane 4, a slight improvement in DNA integrity appeared compared to lane 3. The possible protective effect of fresh chicory juice was shown in lane 5 as an obvious DNA band like the controls. The DNA fragmentation pattern on the gel explains a marked difference between the curative and protective applications associated with a potential modulator role of fresh chicory juice [50%] upon the underlying genomic DNA integrity and might be of value in medicinal plants industry.
Quantitative Real-Time Quantification Polymerase Chain Reaction [qpcr]
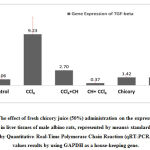
In CCl4-treated group expression of TGF-ß increased to 9.23 showing a significant increase compared to control group 1.059. Administration of fresh chicory juice after CCl4 decreased expression of TGF-ß to 2.703 , administration of fresh chicory juice before CCl4 highly decreased expression of TGF-ß to 0.368 , recording significant decrease compared to CCl4-treated group and these two groups became semi-similar to control group , this may confirm that caffeic acid derivatives that found in chicory plant may act as a good inhibitor of TGF-ß and administration of fresh chicory before CCl4 was more effective than administrating it after CCl4 and there was none –significant difference between control group, chicory only group and olive oil group as shown in [Figure 6 ] .
Discussion
The liver is a vital organ; it supports metabolism, immunity, digestion, and detoxification of wastes. Liver cells contain many enzymes, which may be released into bloodstream. Damaged liver cells increase the levels of secreted enzymes in blood, which is used as an indicator of liver damage. The exposure to fibrotic agents such as chemicals, viral infection, or toxins could induce subsequent cellular changes and finally convert healthy hepatic cells into fibrous bands. Of these agents CCl4 arises as a harmful chemical occasionally released from industrial activities, it is converted to trichloromethyl free radicals leading to lipid peroxidation, which degenerate lipid membrane that causes damage to the cell membrane of hepatocytes and liberation of liver enzymes into blood 33 . Strategy adopted for treating damaged hepatic cells depends on its ability of either reducing the harmful effects or preserving the normal physiological function, which has been disturbed by hepatotoxic agents. However, these anti-fibrotic drugs are remarkably expensive and associated with several adverse effects. Herbs and plants may play an important hepatoprotective effect in controlling of various liver disorders [34]. This study was designed to investigate the hepatoprotective role of fresh chicory juice in delaying the immune response of hepatic cells to fibrotic agents. Our results revealed that the use of fresh chicory in foods could be more effective as a protective than curative against liver fibrosis. Our results investigating hepatic and renal functions showed that the administration of 50% fresh chicory juice significantly decreased liver and renal enzymes compared to untreated CCl4 rats, implying its role in treatment of hepatic fibrosis. Here we proved that the use of this plant before exposure to fibrotic agents could present better readings as a protective mean supporting hepatic cells and delaying the process of fibrogenesis. Histopathological examination revealed that the administration of fresh chicory juice [50%] instead of drinking water showed a significant recovery, which agrees with 34. Its use as a pretreatment shielded liver hepatocytes by averting the oxidation in liver cells and prevented the liver damage caused by CCl4 35. The curative and protective role of chicory plant in liver fibrosis was previously reported 36,37,38. The use of chicory plant as a natural production in treatment and protection against hepatic fibrosis could be explained by measuring the status of oxidative stress. Therefore, we measured the levels of enzymatic antioxidants in serum GSH and SOD, which was significantly increased after and before the exposure of fibrotic agents; however, our data showed that fresh chicory juice has amazingly increased the levels of SOD and GSH in protection than treatment. Reports confirming that the oxidative stress could play an important role in the initiation of fibrosis by increasing harmful cytokines such as transforming growth factor-β [TGF-ß] 39.It was found that the CCl4 exposure associated with the release of free radicals, which stimulate lipid peroxidation of cell membrane. This could consequently cause severe damage of hepatic cells showed by elevation in levels of total cholesterol and triglyceride in serum 40. The measurement of the levels of triglycerides and total cholesterol levels in serum showed high elevation in untreated CCl4 animals that decreased significantly in chicory-administered animals, confirming its antioxidant properties in scavenging released free radicals and hepatoprotective role in the process of fibrogenesis. The role of fresh chicory juice in treatment and prevention of hepatic fibrosis was investigated at the molecular level by analysis of genomic integrity via DNA fragmentation test , which showed that CCl4 exposure could degrade the genomic DNA into variable nucleosomal fragments increasing the susceptibility of mutagenesis and disturb cell regulation. While in treatment and protection groups, a marked integrity of DNA band, which appeared as a single intact band slightly like control rats. Also, we found that the administration of chicory sustains DNA and help in protection against fibrotic agents. This may add a potential modulator role of fresh chicory juice [50%] upon the underlying genomic DNA 41, which might be of value in medicinal plants industry. It was found that liver fibrosis could consequently affect kidney functions 37. Here we found a significant decrease in levels of both creatinine and urea in fresh chicory juice administered protective groups than curative groups compared with untreated CCl4-induced fibrotic liver. This observation of agreement with Li et al. and El-Masry et al. who revealed a direct depletion of urea and creatinine levels after administration of chicory extract on nanoparticles- damaged kidney 37,42. TGF-ß signaling is considered an important pathway leading to hepatic cell proliferation and fibrogenesis 43, high levels of TGF-ß are contributing to chronic liver damage 44. TGF-β is synthesized as a latent ancestor by a variety of cells including endothelial cells, macrophages, hepatocytes, and platelets were recently identified as an important source of TGF-β in the liver 45. Our results showed that CCl4 recorded highly increase in expression of TGF-β because of formation of Reactive Oxygen Species [ROS] where Roehlen et al [46] has proved that ROS and lipid peroxides exposure activate HSC which produce TGF-β, administration with fresh chicory juice has highly decreased expression of TGF-β, but fresh chicory juice was more efficient in case of administration before CCl4 , it helped preventing prolongation of fibrosis, as recommended above according to histopathological examination , CCl4 group was in F4 stage of fibrosis and emerging to cirrhosis, and reversed to F3 in case of administration after CCl4 and reversed to F2 in case of administration before CCl4 according to modified form of stages of liver fibrosis by 4. In accordance with our results a study 48 has proved that Caffeine inhibited TGF-β activation by lung epithelial cells, where phytochemical analysis proved that chicory plant contains caffeic acid derivatives [chiroric acid, chlorogenic acid, isochlorogenic acid, dicaffeoyl tartaric acid] 49,50 , which may also help in reducing the activation of TGF-β expression in liver, flavonoids and polyphenol compounds that act as good antioxidants. In addition 51 has proved that TGF-β signal transduction pathway may be one of the key mechanisms contributing to anti-fibrosis effect.
Conclusion
Chicory may effectively protect against CCl4-induced hepatic fibrosis in rats in both biochemical and histological analysis as it contains polyphenolic and flavonoid compounds, which have significant antioxidant activities that may protect the liver against free radical injury by preventing lipid peroxidation of the cell membrane and it is a promising anti-fibrotic therapeutic agent.
Acknowledgment
We greatly appreciate the partial financial support from Zagazig University, Egypt.
Conflinct of interests
The authors declares there is no conflict of interest.
Funding Source
We appreciate the partial funding from Faculty of Science, Zagazig University, Egypt.
References
- Al-Harbi NO, Imam F, Nadeem A., Al-Harbi M.M., Iqbal M., Ahmad S.F. Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. IntImmunopharmacol. 2014; 21, 383–388.
CrossRef - Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S.Burden of liver diseases in the world. J Hepatol. 2019; 70[1],151–71.
CrossRef - El-Ghitany E. M.: Hepatitis C Virus Infection in Egypt: Current Situation and Future Perspective. Journal of High Institute of Public Health. 2019; 49[1]:1-9.
CrossRef - Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, et al.The present and future disease burden of hepatitis C virus [HCV] infection with today’s treatment paradigm. J Viral Hepat. 2014; 21 Suppl 1:34-59.
- Rao MR, Naficy AB, Darwish MA, Darwish NM, Schisterman E, Clemens JD, et al.Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC infect dis. 2002; 2:29.
CrossRef - Sebastiani G., Gkouvatsos K., Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014;20:11033–11053.
CrossRef - Popper H, Uenfriend Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am. J. Med. 1970; 49:707–721.
CrossRef - Bansal R., Nagórniewicz B., Prakash J. Clinical Advancements in the Targeted therapies against liver fibrosis. mediators of Inflammation, 2016.16.
CrossRef - UzmaLatief and Riaz Ahmad. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs, J Tradit Complement Med. 2018; Jul; 8[3]: 352–360.
CrossRef - Kim M and Shin HK.The water-soluble extract of chicory reduces glucose uptake from the perfused jejunum in rats. J Nutr1996; 126[9]:2236-2242.
CrossRef - USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network-[GRIN]. National Germplasm Resources Laboratory, Beltsville, Maryland. 2015: http://www.ars-grin.gov.4/cgibin/npgs/html/taxon.
CrossRef - Khan SA, Rasool N, Riaz M, Nadeem R, Rashid U,Rizwan K, Zubair M, Bukhari IH, Gulzar T., Evaluation ofAntioxidant and Cytotoxicity Studies of Clerodendruminerme. Asian J Chem 2013; 13: 7457-7462.
CrossRef - Bae M, Park, Y. K., & Lee, J. Y.Food components with anti-fibrotic activity and implications in prevention of liver disease. The Journal of Nutritional Biochemistry, 2018; 55, 1–11.
CrossRef - Bischoff T., Karchesy Y., Laurantos M., Nguyen D.Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolate from Cichorumintybus L, J Ethnopharmacol. 2004; 95[3], 455.
CrossRef - Saybel OL, Rendyuk TD, Dargaeva TD, Nikolaev SM, Khobrakova VB. Phenolic Compounds and Immunomodulating Activity of Chicory Cichoriumintybus Extrac. Pharmacogn J. 2020;12[5]:1104-7.
CrossRef - International Program on Chemical Safety [IPCS]. Carbon Tetrachloride. Environmental Health Criteria. 1999; 208. WHO. Geneva.
- Agency for Toxic Substances and Disease Registry [ATSDR] Toxicological Profiles for Carbon Tetranscholoride” Department of Health and Human Services, Public Health Services, 2005 [Update], Atlanta G.A Us.
- Li Z, Wei W, Chen B, Cai G, Li X, Wang P, Tang J, Dong W. The Effect of rhCygb on CCl4-Induced Hepatic Fibrogenesis in Rat. Sci Rep. 2016, 23:6:23508.
CrossRef - El-Sayed ASA, Ali, DMI, Yassin MA, Zayed RW, Ali GS (2019): Sterol inhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process Biochemistry, 76:55-67.
CrossRef - El-Sayed AS, Ali GS,Aspergillus flavipes is a novel efficient biocontrol agent of Phytophthora parasitica. Biological Control. 2020; 140:104072
CrossRef - Tietz, N.W., Finley, P.R. and Pruden, E.L. Clinical Guide to Laboratory Tests. 2nd Edition, W.B. Saunders, Philadelphia, 1990; 304-306.
- Tietz N.W.1986: Textbook of clinical chemistry. WB Saunders, philadelphia, pp 1271- 1281.
CrossRef - McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983 Mar;29[3]:538-42. PMID: 6825269.
CrossRef - Ellefson RD and Caraway 1976:Fundamental of clinical chemistry. Ed Tietz NW P506.
- Nishikimi, M., Roa, N.A. and Yogi, K. The Occurrence of Supeoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochemical Biophysical Research Communications, 1972; 46, 849-854.
CrossRef - Tietze, F.Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Analytical biochemistry, 1969; 27 [3], 502-522.
CrossRef - National Society for Histotechnology [U.S.]. Guidelines for hematoxylin and eosin staining, 2001; http://bit.ly/SQWR0w.
- Carson F.L., Hladik C. Histotechnology: A self-instructional text, 3rd ed.ASCP Press, Chicago. 2009.
- Carson F.L. Histotechnology A Self-Instructional Text, 1990; 1st Ed, pp 142-143, ASCP, Ill.
- Kuo JH, Jan MS, Jeng J, Chiu HW. Induction of apoptosis in macrophages by air oxidation of dioleoylphosphatidylglycerol.J Control Release. 2005 108: 442–452.
CrossRef - Patel J.S., Vitoreli, A., Palmateer A.J., El-Sayed A.S.A., Norman D.J., Goss E.M., Brennan M., Ali G.S. 2016. Characterization of Phytophthora Isolated from ornamental plants in Florida. Plant Diseases. 100; 2:500-509.
CrossRef - Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C[T]] Method. Methods. 2001; 25[4]:402-8.
CrossRef - Shay, J.E.S.; Hamilton, J.P. Hepatic fibrosis . Avenues of investigation and clinical implications. Clin. Liver Dis. 2018; 11, 111–114.
CrossRef - Sadeghi H, Nikbakht MR; Ghaitasi I and Sabzali S .Hepatoprotective effect of Cichoriumintybus on CCl4- induced liver damage in rats. African Journal of Biochemistry Research Vol.2 [6], 2008; pp. 141-144. ISSN 1996-0778.
- Khalid A, Shahid S, Khan SA, Kanwal S, Yaqoob A, Rasool ZG, Rizwan K. Antioxidant activity and hepatoprotective effect of Cichoriumintybus seed extract against carbon tetrachloride-induced liver toxicity in rats.Tropical J.Pharmac. Research. 2018; 17: 1531-1538.
CrossRef - Belal N.M. Hepatoprotective Effect of Feeding Celery Leaves Mixed with Chicory Leaves and Barley Grains to Hypercholesterolemic Rats. Asian Journal of Clinical Nutrition.2011; 1992-1472.
- Li GY, Gao HY, Huang J, Lu J, Gu JK, Wang JH. Hepatoprotective effect of Cichoriumintybus L., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol. 2014 28:16:753-60.
CrossRef - Chen Y.J.L., Chou P.C., Hsu C.L., Hung J.F., Wu Y.C., Lin J.G.Fermented Citrus Lemon Reduces Liver Injury Induced by Carbon Tetrachloride in Rats. Evid Based Complement Alternat Med. 2018; 20, 6546808.
CrossRef - El-Sayed A.S.A, Abdel-Azeim S, Ibrahim H.M., Yassin M.A., Abdel-Ghany S.E., EsenerS.,Ali G.S. Biochemical stability and molecular dynamic characterization of Aspergillus fumigatus cystathionine γ-lyase in response to various reaction effectors. Enzyme and Microbial Technology. 2015;81, 31- 46.
CrossRef - Helal E, Abd El-Wahab S, Sharaf AM and Zedan G. Effect of Cichoriumintybus L. on fatty liver induced by oxytetracycline in albino rats. The Egyptian Journal of Hospital Medicine. 2011; 45: 522-535.
CrossRef - Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory [Chichoriumintybus L.] fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food ChemToxicol. 2014;72:138-46.
CrossRef - El-Masry T, Altwaijry N, Alotaibi B, Tousson E, Alboghdadly A and Saleh A.Chicory [Cichoriumintybus L.] extract ameliorates hydroxyapatite nanoparticles induced kidney damage in rats.Pakistan Pharmaceutical Sci.2020; 33,3:1251-1260.
CrossRef - Dewidar, B.; Meyer, C.; Dooley, S.Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis. Cells. 2019; 8,
CrossRef - Holt, A.P.; Salmon, M.; Buckley, C.D.; Adams, D.H. Immune interactions in hepatic fibrosis. Or “Leucocyte-stromal interactions in hepatic fibrosis”. Clin. Liver Dis. 2008; 12, 861.
CrossRef - Ghafoory, S.; Varshney, R.; Robison, T.; Kouzbari, K.; Woolington, S.; Murphy, B.; Xia, L.; Ahamed, J. Platelet TGF-_1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018; 2, 470–480.
CrossRef - RoehlenN, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020; 3;9[4]:875.
CrossRef - Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2009; 49:1335-74.
CrossRef - Tatler AL, Barnes J, Habgood A, Goodwin A, McAnulty RJ, Jenkins G. Caffeine inhibits TGFβ activation in epithelial cells, interrupts fibroblast responses to TGFβ, and reduces established fibrosis in ex vivo precision-cut lung slices. Thorax. 2016;71[6]:565-7.
CrossRef - Mushtaq A, Ahmad M and Jabeen Q.: Pharmacological role of Cichoriumintybus as a hepatoprotective agent on the elevated serum marker enzymes level in albino rats intoxicated with nimesulide. Int J Curr Pharm Res. 2013; 5[3]: 25-30.
- Shad MA, Nawaz H, Rehman T, and Ikram N.: Determination of some biochemicals, phytochemicals and antioxidant properties of different parts of Cichoriumintybus: A comparative study. J. Anim. Plant Sci. 2013; 23: 1060-1066.
- Qin D, Wen Z, Nie Y, Yao G. Effect of CichoriumGlandulosum Extracts on CCl4-Induced Hepatic Fibrosis. Iran Red Crescent Med J. 2013; 15,12: e10908.
CrossRef