Ruby George1 , Priti Mathur1*
, Priti Mathur1*  and Chandni Tandon2
and Chandni Tandon2
1Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow, India – 226 028
2Patanjali Research Institute, Haridwar, Uttarakhand, India 249405
Corresponding Author E-mail: pmathur@lko.amity.edu
DOI : https://dx.doi.org/10.13005/bpj/2272
Abstract
Background: Dinoxin B Withanolide was isolated from Datura inoxia and identified with its cytotoxic activity. But its antibacterial properties are not yet evaluated. We have previously reported the broad-spectrum antibacterial property of Dinoxin B Withanolide extracted from D.inoxia on standard strains. Objective: This research has focused to evaluate the efficacy of Dinoxin B Withanolide against infectious Staphylococcus aureus, including resistant strains. Methods: Electrospray Ionization-Mass Spectrometry is used to depict the presence of Dinoxin B withanolide from the chromatographic ethanolic leaf fraction. Antibacterial activity of different concentrations of Dinoxin B(12500-100000 μg/ml) was assessed using the agar diffusion, macro broth dilution, and time-kill assay methods. Docking studies and Drug likeness properties were analyzed. Result: Electrospray Ionization-Mass Spectrometry depicted the presence of Dinoxin B. All the isolates were susceptible to Dinoxin B within the range of 15±0.5mm to 24±0.5mm, and the bacteria were susceptible at a concentration rate of ≤12.5mg/ml. Time-kill assay showed that 25mg/ml of Dinoxin B displayed the highest inhibitory activity after four hours. The MBC values were compatible with the cidal concentration as seen in the time-kill study's growth curve. Computer-aided techniques resulted in a good Docking score towards Quorum-signaling Sar A protein (-7.82)and Penicillin Binding Protein(-6.9). Conclusion: Dinoxin B with its bactericidal properties and significant affinity towards Quorum-signaling Sar A protein and Penicillin Binding Protein can be considered as an effective bioactive compound against Methicillin Resistance Staphylococcus aureus.
Keywords
Antibacterial activity; Datura inoxia; Dinoxin B Withanolide; Molecular docking; Methicillin-Resistant; Staphylococcus aureus
Download this article as:| Copy the following to cite this article: George R, Mathur P, Tandon C. Dinoxin B Withanolide from Datura inoxia Mill as an Effective Phytocompound Against Urinary Tract Infection causing Staphylococcus aureus. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: George R, Mathur P, Tandon C. Dinoxin B Withanolide from Datura inoxia Mill as an Effective Phytocompound Against Urinary Tract Infection causing Staphylococcus aureus. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3uKy0Ve |
Introduction
Naturally occurring withanolides are steroids built on an ergostane skeleton with an oxidized form of lactone rings 1. They are mainly found in the genera of Withania, Physalis, and Datura of the Solanaceae family. Withanolides are often found as aglycones, but a few of them are reported as glycosides. Withanolides have a wide range of biological activity due to their complex and special structural skeletons, including anticancer, antimicrobial, anti-inflammatory, and immunoregulatory properties.
So far studies on withanolides are mainly focused on genera of Withania and very few cases reported from Physalis and Datura. Many species of Datura reported the presence of withanolides including D.stramonium2, D.ferox3, D. inoxia4, D. metel5, and D. fastuosa6.
D.inoxia Mill(Fig.1) is a perennial herb, grow to a height of 1 to 3 meters with serrated margins on hairy leaves, funnel-shaped white flowers, and pendulous spiny fruit with brown to orange seeds and funnel-shaped white flowers7. Philip Miller, an English botanist, was the first to classify the species in 1768. [8,9]. In different parts of the world, the plant is cultivated commercially due to its therapeutic properties10–12. As per the literature review, Dinoxin B13, Withametelinol A, Withametelinol B14, Withametelin11, Daturalacin, and Witharifeen 4 are the withanolides isolated from D.inoxia.
 |
Figure 1: Datura inoxia Mill |
Urinary tract infection (UTI) caused by Staphylococcus aureus are a globally common infection affecting more than hundred million people per year 15. They are one of the most common bacterial infection in all ages and groups with high risk in young women which results in significant morbidity and health care costs due to their Multi-Drug Resistance(MDR)16,17.
Many virulance factors regulates the actions of S.aureus including staphylococcal accessory regulator (SarA) and the accessory global regulator (AgrA) 18. Expression of Penicillin-binding proteins(PBPs) promoted with methicillin and other Beta-lactam antibiotics resistant strains19,20. Methicillin-resistant S. aureus(MRSA)and Multiple antibiotic resistance (MAR) shows varying mechanisms such as the development of biofilm, transformation into small colony variant, the evolvement of resistant genes, resistance to broad-spectrum efflux pumps; which limits treatment options and prompting to search for new compounds that can combat these strains19,21,22.
For the first time, Vermillion et al.; extracted Dinoxin B(Fig.2) and identified it with cytotoxic activities13. But its antibacterial properties are not yet evaluated. We observed the broad-spectrum antibacterial property of Dinoxin B Withanolide extracted from D.inoxia on standard strains23. Promising results of our experiment has encouraged us for studying the effect of Dinoxin B as an antibacterial, highlighting its inhibitory potentiality on Urinary tract infection causing S.aureus.
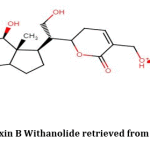 |
Figure 2: Structure of Dinoxin B Withanolide retrieved from PubChem(CID: 51041991) |
Methods
Collection of Sample and Preparation of extract
Leaves of Datura inoxia were obtained from Amity University Campus, Lucknow. The collected leaves were cleaned with distilled water and dried in shade.Ethanolic leaf extract was made using the fine leaf powder into a final concentration of 1mg/ml.
Fractionation of Extract through Column Chromatography
For fractionation of plant extract, a single solvent system was used through column chromatography 24. To fill up the column, Silica gel (60-120 mesh) was used and added with the sample and the collection of the fraction was done by pouring solvent at a flow rate of 1ml/minute until silica gel became visible as colorless. The final concentration of the collected fractions was retained as 1000µg/ml by using 10% DMSO25. Each isolated fraction was assessed for its antibacterial activity.
Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS)
Compound identification of fraction 4, which was the most active fraction in Zone of Inhibition analysis, LC-ESI-MS was performed from Central Drug Research Institute of India, Lucknow.
Test Organism used in the Study
To have a comprehensive understanding and learning, standard, as well as pathogenic strains of S. aureus were used; and denoted as (ATCC 25923) and isolates from urine samples of patients ( U-6151, U-6081, U-6090, and U-6089) in which U-6090and U-6089 are MDR and MRSA strains. All the isolates were obtained from Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow.
Agar Diffusion assay
Following the Kirby-Bauer diffusion technique26, conducted an agar well plate method to assess the antibacterial property against the standard as well as clinical strains of S.aureus.A spectrophotometer is used to check MacFarland standard turbidity. Bacterial inoculum was spread on Muller Hinton Agar plates and inoculated with 100 µl of fraction four of ethanolic leaf extract at different concentrations in 6mm sized wells and incubated at 370C for 24 hrs. The inhibition zones were measured(ZOI). Gentamicin (85mg) and DMSO (10%)were used as positive as well as negative controls. All the assays were done in triplicate, and the results were expressed as mean standard deviation.
Macrobroth Dilution for Determining MICs and MBCs
Macrobroth dilution approach is used to assess Minimum Inhibitory Concentration (MIC) and Minimum bactericidal concentration(MBC), of fraction four as per the protocol followed by Chandni et. al23. Different concentrations of fraction four are obtained through the two-fold serial dilution method and mixed with 100µl of the test organism (Staphylococcus aureus) to a final inoculum concentration of 5×105. The maximum dilution which inhibited bacterial growth was regarded as the MIC value. Bacterial inoculum without the tested fraction was used as the growth control, whereas bacterial inoculum itself was taken as the sterility control.
Subculturing from each tube of MIC without visible growth was used to measure MBC. Plates were incubated for 24 hours at 37°C. The lowest concentrations of the extract that did not generate any colony formation on the solid medium were considered as MBC.
Antibiosis Assessment
The MBC/MIC ratio was calculated to assess the antibiosis mechanism. Ratio ≤ 2 shows bactericidal effects and MBC/MIC ratio ≥4 is usually considered to be bacteriostatic 27.
Time-kill Assay
The most active fraction was subjected to a time-kill assay against the MRSA clinical strain28.An inoculum of approx 5×105cfu/ml,used in his study. The tested fraction was then added to the inoculum suspensions with final concentrations conforming to ½ x MIC, MIC, and 2 x MIC. Bacterial culture without a tested sample was used as a growth control in each trial. Gentamicin was used as an antibiotic control. These cultures were then incubated at 370C. Bacterial colony-forming unit (CFU) was determined at intervals of 0, 2, 4, 6, 18, and 24 hours by taking 1.0 ml of aliquotes. The procedure was repeated three times, and the log CFU/mL was plotted against time in a graph.
Susceptibility test of Antibiotic
The selected antibiotics for the antibiogram test comprised of three groups, namely aminoglycosides (Gentamycin); penicillins (Ampicillin), and quinolones (Ofloxacin)29. ZOI of each standard antibiotic against selected strains was evaluated after 24hrs of incubation at 37 °C.
Analytical statistics
Mean ± standard deviation is used to interpret all data. GraphPad software version 10 was used to examine statistical differences using One-way Anova. The mean is found statistically significant if the p-value is less than 0.05. Standard errors of the mean values were symbolized (±) and results were tabulated with standard error of the mean.
Docking studies
To analyze the antibacterial mechanism of Dinoxin B Withanolide, docking studies were conducted using the Glide docking program of Maestro 12.4 Schrodinger software30. Proteins of S.aureus which promotes resistance to cause Urinary tract infection such as PBP [20,31,32], SarA Protein16,33–35, multidrug efflux pump protein36,37, AgrA Protein35,38, Topoisomerase and DNA gyrase39 were selected based on a Literature review. Protein structures were downloaded from the Protein Data Bank with PDB ID;3HUM(Penicillin-binding protein), 2FNP (SarA Protein), 4LLL(Multidrug efflux pump protein),4G4K(Accessory gene regulator Protein A),(4PLB)Topoisomerase and (2XCT) as DNA gyrase. Protein preparation of all selected proteins was done by using the Protein Preparation Wizard module of Glide.
Structure of ligand, Dinoxin B Withanolide(PubChem ID:51041991)as well as antibiotics used as comparative ligands such as Ampicillin, Ofloxacin, and Gentamycin were retrieved from PubChem with PubChem ID(s) 6249,6604200,3467respectively. Ligand preparation for all these molecules was done by using Lig Prep Wizard and after receptor grid generation docking was carried out.
Drug likeness properties
PreAdmet server was used to evaluate the Drug likeness of Dinoxin B based on Lipinski Rules of five[43,44].
ADMET property analysis
ADMET properties such as absorption, distribution, metabolism, excretion, and toxicity of Dinoxin B withanolide were determined using the PreAdmet server to ensure its effectiveness as an oral drug compound [40].
Results and Discussion
Compound Identification
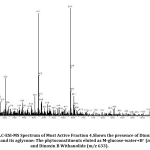
As we reported in the previous study[23], ethanolic leaf fraction four of Datura inoxia obtained through column chromatography is analyzed through LC-ESI-MS. This mass spectrum(Fig.3) also depicts the presence of Dinoxin B Withanolide and its aglycone. Phytoconstituents eluted in the spectrum of fraction four detect the cleavage of a glycosidic bond (Fig.4).
 |
Figure 4: Structure of phytoconstituents eluted in the spectrum of fraction four through Cleavage of a glycosidic bond. |
Agar Diffusion Assay
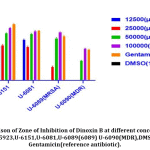
Inhibitory potential of Dinoxin B was observed using an agar well diffusion assay using different concentrations of fraction four in μg/ml (100000,50000,25000 and 12500)and compared to control(DMSO) and Gentamicin as reference antibiotic(Fig.5). As per the Kirby-Bauer test[41], S.aureus susceptibility based on Zone of Inhibition was evaluated (< 12mm (resistant); <13-14mm (intermediate), and >15mm (susceptible). As shown in Fig.6 clinical strains( U-6151, U-6081) isolated from urine samples, including MRSA( U-6089), MDR(U-6089), as well as Standard strain(ATCC 25923) of S. aureus, were showed significant activity(p < 0.05), which was comparable to the reference antibiotic, at higher concentration of Dinoxin B(100000 μg/ml,50000 μg/ml). Whereas MDR strain at 25000 μg/ml, 12500 μg/ml, and MRSA at 12500 μg/ml showed low levels of susceptibility. Susceptibility decreased with a decrease in concentration, which shows the impact of Dinoxin B in its higher concentration. Zone of inhibition varied(Table.1) in range of (mm) 0-15(1250μg/ml),0-18(25000 μg/ml),9.1-20.3(50000 μg/ml),14.6-23.3(100000 μg/ml),in which MDR(U-6090) showed higher resistance. Dinoxin B showed higher susceptibility to Methicillin-resistant strain(U-6089) than that of Gentamicin with a 22.5 mm zone of inhibition.
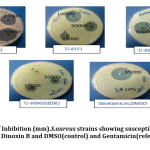 |
Figure 5: Zone of Inhibition (mm).S.aureus strains showing susceptibility to different concentrations of Dinoxin B and DMSO(control) and Gentamicin (reference antibiotic). |
Table 1: Zone of Inhibition(mm) for Dinoxin B. Datas are in triplicate and represented as mean ± SD.< 12mm (resistant); <13-14mm (intermediate)and >15mm (susceptible);shown as(R), (I)and(S).
| S.aureus | Different conc.of Fraction 4 (μg/ml) | Gentamicin (85000) (μg/ml) |
DMSO (10%) |
|||
| 12500 | 25000 | 50000 | 100000 | |||
| ATCC25923 | 15 ±0.5 (S) | 18±1(S) | 20.3±1.0(S) | 23.3±0.7(S) | 26.1 ±0.2(S) | 0 |
| U-6151 | 15.8±0.7(S) | 16.2±0.5(S) | 21.6±0.5(S) | 24 ±0.5 (S) | 25.8±0.5(S) | 0 |
| U-6081 | 18.6±0.5(S) | 15.4±0.5(S) | 21.3±0.5(S) | 22.5±0.5(S) | 22.8 ±0.28 (S) | 0 |
| U-6089(MRSA) | 0(R) | 3.4±0.5(R) | 19.3±0.5(S) | 22.5±0.5(S) | 22.8 ±0.28(S) | 0 |
| U-6090(MDR) | 0(R) | 0(R) | 9.1±0.28(R) | 14.6±.28(S) | 14.2±0.28(S) | 0 |
MIC, MBC, and MIC/MBC Ratio
Antibacterial effectiveness was evaluated through MIC assay, in which maximum dilution of Dinoxin B that slows down staphylococcal growth was noted. As shown in Table.2, Dinoxin B showed the same levels of MIC(12.5 ±0.00) against all strains of S.aureus except the MDR strain(50 ±0.00). With lower MIC(12.5 ±0.00) against UTIs, Dinoxin B can be considered as a potent phytocompound. The growth of bacteria was not inhibited in the negative controls. Minimum Bactericidal Concentration(MBC) of Dinoxin B was found to be 25±0.00mg/ml (Table.2), by the absence of bacterial colonies on fresh Muller-Hinton agar plates(Fig.7)
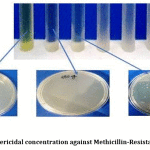 |
Figure 7: Minimum bactericidal concentration against Methicillin-Resistant S.aureus as 25mg/ml. |
Table 2: Result of MIC, MBC, and MIC/MBC. Data are in triplicate and represented as mean ± SD. nd-not determined.
| S.aureus Strains | MIC (mg/ml) |
MBC (mg/ml) |
MBC/MIC Ratio | Bactericidal(+) Bacteriostatic(-) |
| ATCC 25923 | 12.5 ±0.00 | 25.0 ±0.00 | 2 | + |
| U-6151 | 12.5 ±0.00 | 25.0 ±0.00 | 2 | + |
| U-6081 | 12.5 ±0.00 | 25.0 ±0.00 | 2 | + |
| U-6090(MDR) | 50 ±0.00 | >100 | nd | nd |
| U-6089(MRSA) | 12.5 ±0.00 | 25.0 ±0.00 | 2 | + |
| Gentamicin | 12.5 ±0.00 | 25.0 ±0.00 | 2 | + |
| DMSO | 0 | 0 | 0 | 0 |
The MBC/ MIC ratio ≤ 2 indicates bactericidal effects and MBC/MIC ratio ≥4 indicates bacteriostatic effect. Accordingly, Dinoxin B was found to be bactericidal effects against all tested isolates of S.aureus except MDR strain(U-6090), as shown inTable.2.
Time-kill assay
MRSA strain(U-6089) was tested for the time-kill assay using different concentrations of Dinoxin B ½ MIC,MIC and 2xMIC(6.25mg/ml, 12.5 mg/ml and 25 mg/ml). The results of the time-kill assay expressed changes in log10 CFU/ml[38]. The results obtained for the time-kill study shown in Fig.8. After 4 hours of incubation, the effect of MIC, and 2xMIC concentration of Dinoxin B on S. aureus growth inhibition was found to be almost identical. The fraction showed greater inhibitory action at 25mg/ml followed by 12.5 mg/ml and 6.25 mg/ml after eight hours of incubation. However, the bactericidal action of 2xMIC(25mg/ml) was observed after 18h incubation and of MIC(12.5mg/ml) after 22h of incubation. The bactericidal effect of Dinoxin B to MRSA was confirmed by the substantial reduction of bacterial colonies between 4 and 8 hours of incubation. Apart from that, the number of bacterial colonies on control plates increased as time increased, suggesting that the growth of S.aureus entered into the log phase. Present findings indicate that the fraction could yield a better inhibitory activity with an increase in the concentration and incubation time. The MBC values were compatible with the cidal concentration as seen in the time-kill study’s growth curve.
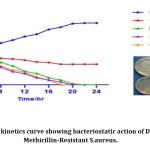 |
Figure 8: Time-kill kinetics curve showing bacteriostatic action of Dinoxin B against Methicillin-Resistant S.aureus. |
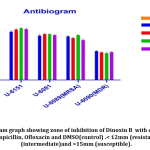
Results of antibiogram evaluated based on Kirby -Bauer scaling of Zone of inhibition; < 12mm (resistant); <13-14mm (intermediate)and >15mm (susceptible);41 and shown that all isolates possess comparatively similar susceptibility to Dinoxin B(100 μl) compared with the standard drugs as shown inFig.9. MDR strain showed resistance to Ampicillin and Ofloxacin while susceptible to Gentamicin and Dinoxin B. Effectiveness of Dinoxin B as an MRSA inhibitor is notable as it with a higher zone of inhibition(22.5mm) than that of referenced antibiotics.
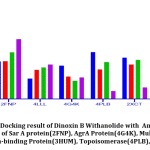
Docking Results
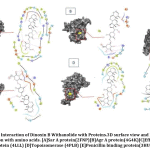
Resistant proteins of S.aureus that promote Multi-Drug resistance, which results in urinary tract infection were selected for docking studies. The results of docking interactions between Dinoxin B withanolide and targeted receptor proteins were shown in Table 3. Comparative docking results between Dinoxin B and selected antibiotics help to focus on its mechanism of action(Fig.10).
Table 3: Docking Result Of Dinox in B With anolide: Docking result of Dinox in B with Sar A protein(2FNP), Multi-Drug Efflux pump Protein (4LLL), AgrA Protein(4G4K), Penicillin-binding Protein(3HUM), Topoisomerase(4PLB), and DNA Gyrase(2XCT).
| PDB ID | Docking score | Glide ligand efficiency | Glide score | Glide e-score |
Glide energy |
| 2FNP | -7.821 | 0.132 | -7.82 | -79.603 | -57.179 |
| 4LLL | -4.513 | -0.100 | -4.513 | -56.378 | -44.233 |
| 4G4K | -2.823 | -0.063 | -2.823 | -36.214 | -33.290 |
| 3HUM | -6.987 | -0.124 | -5.920 | -70.642 | -55.525 |
| 4PLB | -1.034 | -0.021 | -1.034 | -31.113 | -29.491 |
| 2XCT | -1.501 | -0.023 | -1.501 | -32.653 | -30.129 |
It was found that(Fig.10) Dinoxin B Withaolide showed a significant docking score with 2FNP (-7.82). Staphylococcal accessory regulator A (SarA) Protein(PDB ID:2FNP)in S.aureus, controls the modulation of the virulence factors [18]. SarA a known master controller of biofilm formation by regulating Quorum-signaling and promotes MRSA [42]. Dinoxin B can be considered as a Sar A selective therapeutic candidate, as its docking score is higher than all other selected antibiotics. Also, the review shows inhibition of Sar A proteins leads to bactericidal results[43], which in turn indicates that methicillin resistance is shown by Dinoxin B possibly by destroying the biofilm.
Varying expressions of PBPs are another reason for resistance to methicillin and other Beta-lactam antibiotics [19]. Drugs that regulate PBP activities are used to manage MRSA strains. Docking score(-6.987) of Dinox in B Withanolide and Penicillin Binding protein (PDB ID:3HUM); (Table.3 and Fig.10) is higher than the docking score of Ampicillin(-6.912), a beta-lactam antibiotic, Gentamicin(-6.151) and Ofloxacin(-5.301). The capacity to bind with PBPs inhibits the synthesis of the cell wall and thus promotes the bactericidal activity of ampicillin. The binding potentiality of Dinoxin B With anolide towards PBPs and similarity in docking score with that of Ampicillin highlights the efficacy of Dinoxin B Withanolide as a PBP inhibitor.
Docking score of Dinoxin B, as shown in Fig.10 with Multidrug efflux pump protein(4LLL) AgrA protein(4G4K), resulted in a moderate score (-4.513 and-2.823).In the case of Topoisomerase (4PLB) and DNA gyrase(2XCT), Dinoxin B Withanolide showed comparatively less docking scores(-1.034 and -1.501. This in turn indicates that Dinoxin B Withanolide shows better inhibition with cell wall proteins. Fig.11 shows the docking interaction of Dinoxin B to all selected proteins. It demonstrates the role of hydroxyl groups44 in protein-ligand interactions, which promote Dinoxin B’s inhibitory potential.
Drug likeness of Dinoxin B
Lipinski and colleagues suggested in 1997(52) that medically active compounds should match at least three of the observed criteria such as molecular weight less than 500 g mol−1, logP less than 5; the number of hydrogen bond acceptors less than10 and the number of hydrogen bond donors less than 10. The PreAdmet software was employed to study Lipinski’s rules for Dinoxin B and Withanolide and was found(Table 4) as an orally active compound, as it follows three parameters.
Table 4: Result showing physiochemical properties to predict the drug-likeness of Dinoxin B Withanolide as per Lipinski’s Rule.
| Physiochemical Properties | Lipinski Rule violation | |
| cLogP | 1.71 | No |
| Molecular weight | 632.75 | Yes |
| Hydrogen bond acceptors | 10 | No |
| Hydrogen bond donors | 5 | No |
ADMET properties of Dinoxin B
ADMET properties of Dinoxin B were predicted using the PreADMET tool. In vitro model of an oral drug, absorption was carried out by evaluating permeability through Caco-2 and MDCK (Madin-Darby canine kidney) cell model [45]. Dinoxin B shows(Table 4 ) moderate permeability with its predicted result(20.504).
The potentiality of drugs for oral delivery and transdermal delivery can be assessed through HIA (Human Intestinal Absorption) model and skin permeability model [46]. PreADMET can predict the percent of human intestinal absorption (%HIA). Obtained data shows that Dinoxin B Withanolide with 82.577% of HIA is a well-absorbed compound(HIA70-100%). A high intestinal absorption rate promotes its possibility as an oral drug. Negative skin permeability of Dinoxin B Withanolide predicts its poor transdermal property.
Table 5: Result showing ADMET Properties of Dinoxin B using and Pre-ADMET Prediction
|
ADMET |
Dinoxin B | Pre-ADMET Prediction |
| CaCO2 | 20.5044 | 4 – 70 Middle permiabilty |
| MDCK | 0.072 | < 4 Good permiabilty |
| HIA | 82.5778 | 70-100%;wellabsorbedcompounds |
| Skin permiability | -2.212 | < 0 Poor Skin permiability |
| BBB | 0.004 | < 0 CNS inactive compound |
| PPB | 91.01 | >90 Chemicals strongly bound |
| CYP 2C19 inhibition | Non-inhibitor | Promote metabolism |
| CYP 2C9 inhibition | Non Inhibitor | Promote metabolism |
| CYP 2D6 inhibition | Non Inhibitor | Promote metabolism |
| CYP 3A4 inhibition | Non Inhibitor | Promote metabolism |
| CYP 3A4 substrate | Substrate |
Promote metabolism |
| Ames test | Non-mutagen | Non-Carcinogenic |
BBB(Blood-Brain Barrier) prediction helps to identify the distribution potentiality of compounds, which may affect the Central Nervous system(CNS) 47. Dinoxin B Withanolide with 0.004 BBB permeability(less than 0), can be considered as a CNS inactive compound.
As only free drugs can cross membranes and link to their desired targets, determining the amount of drug bound to plasma proteins (PPB)is important in drug discovery48. PPB values greater than 90% indicate that they are strongly bound to plasma proteins, and Dinoxin B Withanolide showed remarkable(91.01%) PPB efficiency.
Fifty to ninety percent of therapeutic molecules are the substrate of five major isoforms (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4)of P45049. Inhibition of these isoforms is undoubtedly one of the most common causes of pharmacokinetics-related undesirable side effects. Result indicate Dinoxin B as a substrate or non-inhibitor of most of the evaluated isoforms. The Ames test is a method for determining a compound’s mutagenicity that was proposed by Dr.Ames 50 and the result showed Dinoxin B Withanolide as a non-mutagen.
Conclusion
Dinoxin B Withanolide was found in ethanolic leaf extract of Datura inoxia, which had strong antibacterial properties, according to our previously published research and current research. Dinoxin B was confirmed to be bactericidal to UTI-causing S.aureus using different methods. Its significant inhibition to methicillin-resistant strains has been proven in both dry and wet test results. In silico findings revealed that it has a high binding potential against Sar A proteins (biofilm regulators) and Penicillin-binding proteins, which are modern-day threats as they promote Methicillin Drug Resistance. The interaction of dinoxin B with cell wall proteins is proven to be the mechanism of action, according to observed results. As the experiments demonstrated, if this phytocompound can compete with the currently used antibiotics, it can undoubtedly be considered as a drug candidate (green antibiotics) for treating UTI-causing S.aureus.
Acknowledgment
The authors are thankful to Pro-Vice-Chancellor; Amity University Uttar Pradesh, Lucknow Campus; Prof (Dr.) J K Srivastava; Head of Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow Campus, Prof (Dr.) Nuzhat Husain; Director Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Dr.Manodeep Sen; Associate professor Department of Microbiology Dr. Ram Manohar Lohia Institute of Medical Sciences, and Mr.Vinod Devaraj; Application Scientist Schrodinger Inc. for providing the necessary facilities for experiments and their constant support and encouragement.
Conflict of Interest
None
Funding Source
This research is not supported by any funding agency.
References
- Glotter. Withanolides and related ergostane-type steroids. Nat. Prod. Rep.,1991; 415–440
CrossRef - N. Tursunova, V.A. Maslennikova, N.K. Abubakirov. Withanolides of Datura stramonium II. Withastramonolide. Chem. Nat. Compd.,1978;14:73-75.
CrossRef - S. Veleiro, A.M. Cirigliano, J.C. Oberti, G. Burton. 7-Hydroxywithanolides from Datura ferox. J. Nat. Prod.,1999;7:62.
CrossRef - S. Siddiqui, S. Arfeen, F. Afshan, S. Begum. Withanolides from Datura innoxia. Heterocycles., 2005;65:857-863.
CrossRef - X. Kuang, B.Y. Yang, Y.G. Xia, Q.H. Wang, Two new withanolide lactones from flos daturae, Molecules.,2011;16:5833-5839.
CrossRef - Zhang, C.M. Cao, R.J. Gallagher, B.N. Timmermann. Antiproliferative withanolides from several solanaceous species. Nat. Prod. Res., 2014;28:1941-1951.
CrossRef - O. Ayuba, T.O. Ojobe, S.A. Ayuba. Phytochemical and proximate composition of Datura innoxia leaf, seed, stem, pod and root. J. Med. Plants Res.,2011;5:2952-2955.
- D. Meikle, C.B. Heiser. A Digest of DwalesNightshades: The Paradoxical Plants. Kew Bull., 1972.
CrossRef - F. Blakeslee, A.G. Avery, S. Satina, J. Reitsama. The genus Datura., 1959.
- Dafni, Z. Yaniv. Solanaceae as medicinal plants in Israel. J. Ethnopharmacol.,1994.
CrossRef - W. Baig, B. Nasir, D. Waseem, M. Majid, M.Z.I. Khan, I. ul Haq. Withametelin: a biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J.,2020;12:1526-1537.
CrossRef - Kaushik, P. Goyal. In vitro evaluation of Datura innoxia (thorn-apple) for potential antibacterial activity. Indian J. Microbiol.,2008; 353–357.
CrossRef - Vermillion, F.O. Holguin, M.A. Berhow, R.D. Richins, T. Redhouse, M.A.O. Connell, J. Posakony, S.S. Mahajan, S.M. Kelly, J.A.
Simon.Dinoxin B, a Withanolide from Datura inoxia Leaves with Specific Cytotoxic Activities.2011; 267–271.
CrossRef - S. Siddiqui, I. Ali Hashmi, S. Begum.Two New Withanolides from the Aerial Parts of Datura innoxia. Heterocycles.,2002;57:715.
CrossRef - C. Kaul, R. Wadhwa. Science of Ashwagandha: Preventive and Therapeutic Potentials. Sci. Ashwagandha. Prev. Ther. Potentials.,2017; 1–508.
- Peng, L. Zeng, H. Jin, L. Yang, Y. Xiao, Z. Lan, Z. Yu, S. Ouyang, L. Zhang, N. Sun. Discovery and antibacterial study of potential PPK1 inhibitors against uropathogenic E. coli. J. Enzyme Inhib. Med. Chem.,2020;35:1224–1232.
CrossRef - Singh, N. Singh, R. Singh. Isolated of Staphylococcus and gram-negative bacteria from the hospitalized area and screening bacteria against various plant extract. Int. J. Med. Sci., Clin. Invent., 2018;5:3619–3624.
CrossRef - Liu, A.C. Manna, C.H. Pan, I.A. Kriksunov, D.J. Thiel, A.L. Cheung, G. Zhang. Structural and function analyses of the global regulatory protein SarA from Staphylococcus aureus.Proc. Natl. Acad. Sci. U. S. A. ,2006;103: 2392–2397.
CrossRef - J. Peacock, G.K. Paterson. Mechanisms of Methicillin Resistance in Staphylococcus aureus . Annu. Rev. Biochem.,2015;84: 577–601.
CrossRef - E. Zurenko, B.H. Yagi, R.D. Schaadt, J.W. Allison, J.O. Kilburn, S.E. Glickman, D.K. Hutchinson, M.R. Barbachyn, S.J. Brickner. In vitro activities of U-100592 and U-100766:novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother.,1996;40: 839–845.
CrossRef - Aiola, G. Amico, P. Battaglia, E. Battistelli. The Large-Scale Polarization Explorer (LSPE). Clin. Microbiol. Rev.,2002;15:167–193.
- M. Luna, E. Rodríguez-Noriega, L. Bavestrello, E. Gotuzzo. Treatment of methicillin-resistant Staphylococcus aureus in Latin America. Brazilian J. Infect. Dis.,2010:14.
CrossRef - Tandon, P. Mathur, M. Sen, S. Kanojiya,.Identification of an antibacterial withanolide (Dinoxin b) from leaf of datura inoxia mill. Int. J. Phytomedicine.,2016;8:1–12.
- D. Sarker, Z. Latif, A.I. Gray.Natural Products Isolation: an overview.Nat. Prod. Isol.,2006;864:1–25.
CrossRef - I. Okeke, C.U. Iroegbu, E.N. Eze, A.S. Okoli, C.O. Esimone. Evaluation of extracts of the root of Landolphia owerrience for antibacterial activity. J. Ethnopharmacol.,2001;78: 119–127.
CrossRef - Hudzicki. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol Author Information. Am. Soc. Microbiol.,2012;1–13.
- Venkateswarulu, K. Srirama, I. Mikkili, M. Nazneen Bobby, J.B. Dulla, V.N. Alugunulla, D. Sweety, A.P. Karlapudi. Estimation of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Antimicrobial peptides of Saccharomyces boulardii against Selected Pathogenic Strains.Karbala Int. J. Mod. Sci.,2019;5: 266–269.
CrossRef - Prabavathy, R. Niveditha. Antibacterial activity of Scoparia dulcis extract on uropathogenic Escherichia coli and Staphylococcus aureus. Res. J. Pharm. Biol. Chem. Sci. ,2015;6:621-626.
- M. Assis, M. Nedeljković, A. Dessen.New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus.Drug Resist. Updat.,2017;31:1–14.
CrossRef - Manual, Schrödinger Release 2019-3: Glide, Schrödinger. LLC, New York, NY., 2019., Schrödinger Release 2018-3 LigPrep, Schrödinger. LLC, New York, NY.,2018.
- J. Alves, H.J.C. Froufe, A.F.T. Costa, A.F. Santos, L.G. Oliveira, S.R.M. Osório, R.M.V. Abreu, M. Pintado, I.C.F.R. Ferreira. Docking studies in target proteins involved in antibacterial action mechanisms: Extending the knowledge on standard antibiotics to antimicrobial mushroom compounds. Molecules. 2014;19: 1672–1684.
CrossRef - Gajdács. The continuing threat of methicillin-resistant Staphylococcus Aureus.Antibiotics. 2019;8:52.
CrossRef - Arya, R. Ravikumar, R.S. Santhosh, S.A. Princy.SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections. Front. Microbiol., 2015;6: 1–12.
CrossRef - Lu, W. Hu, Z. Tian, D. Yuan, G. Yi, Y. Zhou, Q. Cheng, J. Zhu, M. Li.Developing natural products as potential anti-biofilm agents. Chinese Med.U.K.,2019;14: 1–17.
CrossRef - Ravichandiran, K. Shanmugam, K. Anupama, S. Thomas, A. Princy. Structure-based virtual screening for plant-derived SdiA-selective ligands as potential antivirulent agents against uropathogenic Escherichia coli. Eur. J. Med. Chem.,2012;48: 200–205.
CrossRef - J. Foster. Antibiotic resistance in Staphylococcus aureus: Current status and future prospects. FEMS Microbiol. Rev.,2017;41: 430–449.
CrossRef - Dantas, T.M. de Aquino, J.X. de Araújo-Júnior, E. da Silva-Júnior, E.A. Gomes, A.A.S. Gomes, J.P. Siqueira-Júnior, F.J.B. Mendonça Junior. Aminoguanidine hydrazones (AGH’s) as modulators of norfloxacin resistance in Staphylococcus aureus that overexpress NorA efflux pump. Chem. Biol. Interact., 2018;280: 8–14.
CrossRef - M.A. El-Baky, T. Sandle, J. John, G.E.D.A. Abuo-Rahma, H.F. Hetta.A novel mechanism of action of ketoconazole: Inhibition of the nora efflux pump system and biofilm formation in multidrug-resistant staphylococcus aureus. Infect. Drug Resist.,2019;12: 1703–1718.
CrossRef - Wang, D. Zou, K. Xie, M. Xie. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep., 2014;10: 2341–2345.
CrossRef - Lin, D. Sahakian, S. de Morais, J. Xu, R. Polzer, S. Winter. The Role of Absorption, Distribution, Metabolism, Excretion and Toxicity in Drug Discovery. Curr. Top. Med. Chem., 2005;1:277-351.
- M. Bauer AW, Kirby WM, Sherris JC, A. Bauer, W. Kirby, J. Sherris, M. Turck. Susceptibility testing by a standardized single disc method. Am J Clin Pathol.,1966;45: 493–496.
CrossRef - Balamurugan, M. Hema, G. Kaur, V. Sridharan, P.C. Prabu, M.N. Sumana, S.A. Princy.Development of a biofilm inhibitor molecule against multidrug resistant Staphylococcus aureus associated with gestational urinary tract infections.Front. Microbiol.,2015;6: 1–13.
CrossRef - P. Durán, F.H. Kayser, B. Berger-Bächi. Impact of sar and agr on methicillin resistance in Staphylococcus aureus. FEMS Microbiol. Lett.,1996;141: 255–260.
CrossRef - B. Pisano, A. Kumar, R. Medda, G. Gatto, R. Pal, A. Fais, B. Era, S. Cosentino, E. Uriarte, L. Santana, F. Pintus, M.J. Matos. Antibacterial Activity and Molecular Docking Studies of a Selected Series of Hydroxy-3-arylcoumarins. Molecules.,2019;24: 1–14.
CrossRef - Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, H. Tokuda. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm., Sci.,2000;5:195-204.
CrossRef - H. Zhao, J. Le, M.H. Abraham, A. Hersey, P.J. Eddershaw, C.N. Luscombe, D. Boutina, G. Beck, B. Sherborne, I. Cooper, J.A. Platts.Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure – Activity relationship (QSAR) with the Abraham descriptors. J. Pharm. Sci.,2001;90:749-784.
CrossRef - Ajay, G.W. Bemis, M.A. Murcko. Designing libraries with CNS activity. J. Med. Chem., 1999;24;4942-51.
CrossRef - Keen. Effect of Binding to Plasma Proteins on the Distribution, Activity and Elimination of Drugs.Concepts Biochem. Pharmacol., 1971;213-233.
CrossRef - R.O. de Montellano. Cytochrome P450: Structure, mechanism, and biochemistry, fourth edition., 2015.
- N. Ames, E.G. Gurney, J.A. Miller, H. Bartsch. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens., Proc. Natl. Acad. Sci. U. S. A.,1972.