Shruti Chopra1 , Ajit Varma1
, Ajit Varma1 , Seema Jain2, Sangeeta Jain3, Devendra Choudhary1
, Seema Jain2, Sangeeta Jain3, Devendra Choudhary1
1Amity Institute of Microbial Technology, Amity University, Noida, U.P.,India
2Department of Obstetrics and Gynaecology, Vardhman Clinic, Rohini, Delhi,India
3Joy IVF Clinic, Jain Hospital, Pushpanjali Vikas Marg, Delhi, India
Corresponding Author E-mail: shrutimalhotra227@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2235
Abstract
Objective: To study the relationship between conventional semen parameters and sperm chromatin condensation (DNA fragmentation index) using aniline blue-eosin staining method among patients of different age groups visiting the In-vitro fertilization (IVF) clinic.Design: Retrospective study Setting: Tertiary care infertility centre Method: A total of 240 patient semen samples were studied between the period of May 2015 to May 2016 for conventional semen parameters (WHO criteria) and DNA fragmentation index (DFI) using aniline blue- eosin staining method. Patients were separated into three groups: <=30 years, 31-35 years and 36 years & above. Statistical analysis was performed using Pearson correlation co-efficient and regression tests on the groups. Main Outcome Measures: Sperm concentration (Millions /ml), motility(%), normal morphology(%), DFI (%). Result: In each age group, i.e., <=30years, 31-35 years and 36 years & above, there was a significant and negative correlation between DFI and sperm concentration (r= -0.50, r= -0.34, r= -0.49 respectively; P<0.05), motility(r= -0.69,r= -0.66, r= -0.54 respectively; P<0.05) and normal morphology (r= -0.86,r= -0.80, r= -0.75 respectively; P<0.05). Sperm DNA fragmentation index among the age groups was not statistically significantly (P>0.05). Conclusion: Our study demonstrated that age is not a predictor of DFI. Whereas, sperm concentration, sperm motility and normal sperm morphology showed a significant association with DFI in all the age groups i.e., better the conventional semen parameters, lower the DFI.
Keywords
Aniline Blue- Eosin; DFI; Sperm Chromatin Condensation, Sperm Concentration, Sperm Morphology, Sperm Motility
Download this article as:| Copy the following to cite this article: Chopra S, Varma A, Jain S, Jain S, Choudhary D. Sperm Dna Correlation between Fragmentation and Conventional Semen Parameters among Different Age Groups. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Chopra S, Varma A, Jain S, Jain S, Choudhary D. Sperm Dna Correlation between Fragmentation and Conventional Semen Parameters among Different Age Groups. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3uq7oZd |
Introduction
Semen analysis has been widely used as an essential test for predicting male fertility, however, it cannot detect sperm chromatin abnormalities. Various studies have been performed in the past predicting sperm chromatin abnormalities as one of the trigger for infertility in men 4. Sperm chromatin maturity is an essential factor for spermatozoa’s fertilization capacity and embryonic development 1-3.
Sperm chromatin abnormalitiescould occur at the time of spermiogenesis 5, where specific nucleoproteins pack the nuclear DNA tightly into a highly condensed chromatin. Sperm chromatin condensation abnormalities result in nuclear damage as DNA fragmentation/ denaturation that is associated with male infertility 6. However, the exactmechanism(s) by which chromatin abnormalities or DNA fragmentationoccur in human spermatozoa are not well understood.
Different methods have been used to evaluate sperm chromatinabnormalities or immaturity. Analysis of the sperm chromatin compaction is performed by aniline blue staining, which detects sperm chromatin immaturity 7-8 and differentiates between histones and protamines. Blue stain is taken up by lysine rich immature nuclei of sperm cells, whereas, nuclei of sperm cells that are mature remain unstained 8.
There has been a social trend of delaying family planning and parenthood among couples in today’s world. Spermatogenesis continues throughout life, compared to oogenesis, where the decline in ovarian follicle numbers marks the end of females reproductive cycle 9. Previous studies on Assisted Reproduction Technologies (ART) have shown that among couples seeking pregnancy, males were significantly elder in comparison to those who do not require ART (36.6 vs 33.5 years) 10.
Studies on sperm quality, DNA damage and their correlation with ageing has brought a lot of interest because of an increase in paternal age in the recent years.
This study was conducted to analyse the correlation of conventional semen parameters and DNA fragmentation index (DFI) for sperm chromatin compaction using Aniline blue-Eosin staining among different age group patients visiting the ART clinic.
Materials and Method
Patient Selection
A total of 240 patient semen samples were studied between the period of May 2015 to May 2016 for conventional semen parameters (WHO criteria) and DNA fragmentation index(DFI) using aniline blue- eosin staining method. Patients selected for our study were less than 40 years old and were divided into three groups based on their age into <=30 years, 31-35 years and 36 years & above. Patients with history of any pathogenic infection in the past 2 months, with OAT’s, Azoospermia, Necrozoospermia, Hematospermia, Pyospermia and smokers were ruled out. This study was carried at a Tertiary care infertility centre and permission to conduct the experiments was taken from the hospital ethical committee, also informed written consents were obtained from each patient.
Semen Analysis
Patients provide semen samples for analysis by masturbation after an abstinence of 3 to 6 days. After 30 minutes of liquefaction at room temperature, every sample was analyzed for the conventional semen parameters (12) and DFI using the Aniline blue- eosin staining method.
In this technique,sperms were stained with AnilineBlue-eosin as describedin a previous report (5,11,13).10 μL of raw semen sample was smeared on a slide and subsequently air dried for each patient. Slides were fixed at room temperature in 4% formalin for 5 minutes, then rinsed with water and air dried. 5% aqueous aniline blue solution (HIMEDIA) (pH 3.5) was used to stain the slides for 5 minutes followed by rinse with water and air drying. Slides were then counter stained for 1 minute in 0.5% eosin (Merck), again rinsed and air dried.
Slide examinations were carried under bright field microscope at X 1000 magnification using oil immersion. Immature sperms stained dark blue, whereas the eosin counter stain, stained the mature sperms red pink. The percentage of abnormalsperm chromatin condensation or DNA fragmentation index (DFI) was recorded as the ratio of thenumber of dark-blue sperm to total number of sperm cells observed and multiplied by 100. A minimum of 200 sperm cells were observedfor every slide.
Statistical Analysis
Statistical analysis was performed using Pearson correlation co-efficient and regression tests on the groups.
Results
A total of 240 patient samples were studied and the average age of patients was 31.9 years. Patients were divided into three groups based on their age into <=30 years, 31-35 years and >=36 years.
The percentage of sperm DNA fragmentation index(DFI) and semen parameters studied in the selected pool of patients showed a significant and negative correlation.
Table 1 shows that sperm concentration and DFI are negatively correlated to each other with a correlation co-efficient(r) of -0.50, -0.34 and -0.49 in the three groups.
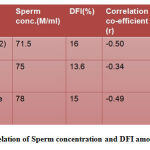 |
Table 1: Correlation of Sperm concentration and DFI among age groups |
In table 2 sperm motility and DFI between the different age groups shows significant and negative correlation with a correlation co-efficient(r) of -0.69, -0.66 and -0.54 in the three groups.
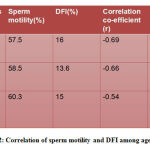 |
Table 2: Correlation of sperm motility and DFI among age groups |
Table 3 also shows that sperm morphology and DFI are negatively correlated to each other with a correlation co-efficient(r) of -0.86, -0.80 and -0.75 in the three groups.
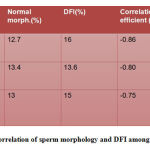 |
Table 3: Correlation of sperm morphology and DFI among age groups |
Also, our study demonstrated that sperm DNA fragmentation index between the different age groups was not statistically significantly (P>0.05). As shown in graph 1 where DFI among different age groups shows a linear pattern depicting that DFI is not related to age.
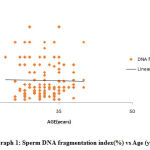 |
Graph 1: Sperm DNA fragmentation index(%) vs Age (years) |
Discussion
The results of our study demonstrated that some of DNA fragmentation index (DFI) has negative correlation to some of the semen parameters studied including sperm concentration, sperm motility and sperm morphology as shown in other studies (13,20-22), in contrast to studies that show positive correlation (15-16,17-19). Hammadehet al.(8)studied sperm chromatin condensation using AB staining to assess male fertility and reported no correlation between sperm chromatin condensation and sperm count, motility and morphology.
H.S.Kimet al. (13) reported a negative correlation between abnormal sperm chromatin condensation and strict morphology. Dadoune et al.(14) observed that normal morphology sperms had a higher percentage of normal sperm chromatin condensation heads than sperms with subnormal morhology.
Our study also shows that DNA fragmentation index (DFI) based on sperm chromatin compaction/condensation is an important factorto predict male fertility, along withthe routinely studied conventional semenparameters (8,13,22) among different age groups.
Sperm chromatin structure consists of a tightly packaged DNA and nuclear protein whi Sperm chromatin structure consists of a tightly packaged DNA and nuclear protein which are highly condensed, reducing the nuclear volume and head size 16. Sperm chromatin condensation is an important process which involves the replacement of somatic histones by protamines during the phase of spermiogenesis 17 which are responsible for the final condensation and the dense packaging of the sperm genome into unique tightly coiled “doughnut-loop” sub-units 23-24. Sperm DNA degradation has been found to be associated with the presence of persistent histones as shown by various studies 25-26 which affects male fertility. Therefore, it is important to evaluate sperm chromatin condensation during routine semen analysis in order to provide a better treatment option to patients with male factor and unexplained infertility.
Our study observed that DFI for sperm chromatin condensation had no relationship to ageing in men as shown in other studies 27-28. A study by H.S. Kim et al. 13 did not show any statistical significance of age of men on morphology and abnormal sperm chromatin condensation.Also, Nijset al. 29 studied that there was no influence of age of men on sperm concentration, motility or morphology, also no significant increase in DNA fragmentation was observed in their study of 278 patients that underwent their first IVF or ICSI cycle. In contrast, studies have shown that men with advanced age are at higher risk of having children with chromosomal defects 30.
Conclusion
Our study demonstrated that age is not a predictor of DFI. Whereas, sperm concentration, sperm motility and normal sperm morphology showed a significant and negative correlation with DFI in all the age groups i.e., better the conventional semen parameters, lower the DFI. It’s important to understand that DFI based on sperm chromatin compaction/condensation is a very important parameter for the assessment ofmale fertility.
Acknowledgement
We would like to thank the technical staff at Joy IVF and Amity University for their support.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Sakkas, Urner F.,Bizzaro D. et al. Sperm nuclear DNAdamage and altered chromatin structure: effect on fertilizationand embryo development.Human Reproduction 1998;vol. 13, no. 4,supplement, pp. 11–19.
CrossRef - E. Hammadeh, M. Stieber, G. Haidl, andW. Schmidt. Associationbetween sperm cell chromatin condensation, morphologybased on strict criteria, and fertilization, cleavage andpregnancy rates in an IVF program. Andrologia1998; vol. 30, no. 1,pp. 29–35.
CrossRef - D. Esterhuizen, D. R. Franken, J. G. H. Lourens, E. Prinsloo,and L. H. Van Rooyen. Sperm chromatin packaging as anindicator of in-vitro fertilization rates.Human Reproduction2000; vol. 15, no. 3, pp. 657–661.
CrossRef - Sakkas D, Mariethoz E, Manicardi G, et al.Origin ofDNA damage in ejaculated human spermatozoa. RevReprod1999; 4: 31–7.
CrossRef - Wong A, Chuan SS, Patton WC, Jacobson JD, Corselli J, Chan PJ. Addition of eosin to the aniline blue assay to enhance detectionof immature sperm histones. FertilSteril 2008; 90:1999-2002.
CrossRef - Agarwal and T.M. Said. Role of sperm chromatin abnormalities and DNA damage in male infertility. Human Reproduction Update 2003; vol.9 no. 4, pp. 331-345.
CrossRef - Auger, M. Mesbah, C. Huber, and J. P. Dadoune. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men.International Journal of Andrology1990; vol. 13, no. 6, pp. 452–462.
CrossRef - E. Hammadeh, T. Zeginiadov, P. Rosenbaum, T. Georg, W. Schmidt, and E. Strehler. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Archives of Andrology2001; vol. 46, no. 2, pp. 99–104.
CrossRef - Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl2008; 29: 469-487.
CrossRef - Engel W, Sancken U, Laccone F. Paternal age from a genetic point of view. J ReproduktionsmedEndokrinol2004; 1: 263-267.
- Park YS, Kim MK, Lee SH, Cho JW, Song IO, Seo JT. Efficacy of testicularsperm chromatin condensation assay using aniline blueeosinstaining in the IVF-ET cycle. ClinExpReprod Med 2011; 38:142-7.
CrossRef - World Health Organization. WHO laboratory manual for the examinationand processing of human semen [Internet]. 5th ed.Geneva: World Health Organization; c2013 [cited 2013 Mar 4].Available from: http://www.who.int/reproductivehealth/ publications/infertility/9789241547789/en/index.html.
CrossRef - S. Kim, M. J. Kang, S. A. Kim et al.The utility of sperm DNAdamage assay using toluidine blue and aniline blue staining inroutine semen analysis. Clinical and Experimental Reproductive Medicine 2013; vol. 40, no. 1, pp. 23–28.
CrossRef - Dadoune JP, Mayaux MJ, Guihard-Moscato ML. Correlation between defects in chromatin condensation of human spermatozoa stained by aniline blue and semen characteristics. Andrologia 1988; 20:211-7.
CrossRef - Kazerooni, N. Asadi, L. Jadid et al.Evaluation of sperm’schromatin quality with acridine orange test, chromomycin A3and aniline blue staining in couples with unexplained recurrentabortion. Journal of Assisted Reproduction and Genetics 2009; vol. 26,no. 11-12, pp. 591–596.
CrossRef - W. Aoki, L. Liu, and D. T. Carrell. Identification and evaluation of a novel sperm protamine abnormality in a population ofinfertile males. Human Reproduction 2005; vol. 20, no. 5, pp. 1298–1306.
CrossRef - Hofmann and B. Hilscher. Use of aniline blue to assess chromatin condensation in morphologically normal spermatozoa innormal and infertile men. Human Reproduction 1991; vol. 6, no. 7,pp. 979–982.
CrossRef - R. Franken, C. J. Franken, H. de la Guerre, and A. de Villiers. Normal sperm morphology and chromatin packaging: comparison between aniline blue and chromomycin A3 staining. Andrologia1999; vol. 31, no. 6, pp. 361–366.
CrossRef - A. Lazaros, G. A. Vartholomatos, E. G. Hatzi et al.Assessment of sperm chromatin condensation and ploidy status usingflow cytometry correlates to fertilization, embryo quality andpregnancy following in vitro fertilization. Journal of AssistedReproduction and Genetics 2011; vol. 28, no. 10, pp. 885–891.
CrossRef - Salsabili, A. Mehrsai, B. Jalalizadeh et al.Correlation ofsperm nuclear chromatin condensation staining method withsemen parameters and sperm functional tests in patients withspinal cord injury, varicocele, and idiopathic infertility. UrologyJournal 2006; vol. 3, no. 1, pp. 32–37.
- Sadek, A. S. A. Almohamdy, A. Zaki, M. Aref, S. M. Ibrahim,and T. Mostafa. Sperm chromatin condensation in infertilemen with varicocele before and after surgical repair. Fertilityand Sterility 2011; vol. 95, no. 5, pp. 1705–1708.
CrossRef - Sellami,, N.Chakroun,, S. Ben Zarrouk, et al. Assessment of Chromatin Maturity inHuman Spermatozoa: Useful Aniline Blue Assay forRoutine Diagnosis of Male Infertility. Advances in Urology 2013; Article ID 578631, 8 pages.
CrossRef - Zhao M, Shirley CR, Hayashi S, Marcon L, Mohapatra B, Suganuma R,et al. Transition nuclear proteins are required for normal chromatin condensation and functional sperm development. Genesis 2004; 38:200–13.
CrossRef - Ward WS, Zalensky AO. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr 1996; 6:139–47.
CrossRef - Foresta C, Zorzi M, Rossato M, Varotto A. Sperm nuclear instability andstaining with aniline blue: abnormal persistence of histones in spermatozoa in infertile men. Int J Androl1992; 15:330–
CrossRef - Laberge RM, Boissonneault G. Chromatin remodeling in spermatids:a sensitive step for the genetic integrity of the male gamete. Arch Androl 2005;51:125–33.
CrossRef - Thomas W., Bernd R.et.al. The correlation between male age, sperm quality and spermDNA fragmentation in 320 men attending a fertilitycenter. .J Assist Reprod Genet 2009; 26:41–46.
CrossRef - Lutjens CM, Rolf C. et.al. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17:1826–32.
CrossRef - Nijs M, De Jonge C, Cox A, Janssen M, Bosmans E, Ombelet W. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia 2011; 43:174-9.
CrossRef - Sloter ED, Marchetti F., et al. Frequency of human sperm carrying structural aberrationsof chromosome 1 increases with advancing age. FertilSteril. 2007; 87:1077–86.
CrossRef








