Manuscript accepted on :26-07-2021
Published online on: 28-07-2021
Plagiarism Check: Yes
Reviewed by: Dr. Ameer Ali Shakr Hadi
Second Review by: Dr. Mohammad Barary
Final Approval by: Dr. Omar M. E. Abdel-Salam
Aakash Kewlani1 , Seema Bhalerao1
, Seema Bhalerao1 , Harshavardhan Bhide1
, Harshavardhan Bhide1 , Teja Deshpande1*, Abhijeet Tilak1
, Teja Deshpande1*, Abhijeet Tilak1 and Nilima Dharkar2
and Nilima Dharkar2
1Department of Pharmacology, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D.Y. Patil Vidyapeeth, Pune, Maharashtra, India
2Department of Rasashastra and Bhaishajya Kalpana, Dr. D. Y. Patil College of Ayurved and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra, India.
Corresponding Author E-mail: teja.deshpande@dpu.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2252
Abstract
Scope and Objective:Ocimum sanctum and Azadirachta indicaare known to be safe and effective anti-inflammatory agents in ayurveda. So, this study was planned to evaluate and compare anti-inflammatory activity of Ocimum sanctum, Azadirachta indica and combination of Ocimum sanctum + Azadirachta indica (COA) with Aspirin on acute inflammation in rats and also to assess mechanism behind their anti-inflammatory action. Materials and Methods: Wistar albino rats of either sex (150-250 g) were divided into 5 groups with six rats in each group. To induce inflammation, formalin (2.5%, 0.1 ml) was injected into sub-plantar region of left hind paw of rats. The study groups were administered orally with distilled water (3 ml), Aspirin (200 mg/kg), Ocimum sanctum (400 mg/kg), Azadirachta indica (500 mg/kg) and COA (400 mg/kgOcimum sanctum+500 mg/kgAzadirachta indica) half an hour before the formalin challenge. Effect of test drugs on acute inflammation was assessed by rat paw oedema test&mechanism behind their action was assessed using histopathological examination. Results:In rat paw oedema test, Ocimum sanctum, Azadirachta indica and COA groups showed significant reductionin oedema as compared to control; Azadirachta indica and COA groupsalso showed comparable effect to Aspirin group. In histopathological examination, Aspirin, Azadirachta indica and COA groups caused significant reduction in vasodilation, oedema, infiltration and margination of neutrophils while Ocimum sanctum group only caused significant reduction in oedema. Conclusion: This study revealedthat all test groups have significant anti-inflammatory efficacy;Azadirachta indica and COA also have comparable efficacy to Aspirin.
Keywords
Cellular changes; Digital plethysmometer; Formalin; Histopathology; Oedema; Vascular changes
Download this article as:| Copy the following to cite this article: Kewlani A, Bhalerao S, Bhide H, Deshpande T, Tilak A, Dharkar N. Comparison of Anti-Inflammatory Efficacy of Ocimum Sanctum, Azadirachta Indica and their Combination with Aspirin in Chemically Induced Acute Inflammation.. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Kewlani A, Bhalerao S, Bhide H, Deshpande T, Tilak A, Dharkar N. Comparison of Anti-Inflammatory Efficacy of Ocimum Sanctum, Azadirachta Indica and their Combination with Aspirin in Chemically Induced Acute Inflammation.. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3x9yPpW |
Introduction
Inflammation is the first response of the immune system that eliminates foreign invaders such as infectious pathogens & damaged tissues. It can be acute or chronic, which depends up on the nature of stimulus & effectiveness of reaction which eliminates stimulus or damaged tissues. [1,2,3]Acute inflammation is a stereotyped, nonspecific response and a planned mechanism of defence. [4]Aspirin (Acetylsalicylic acid) which is used as astandard drug for the treatment of inflammation. Aspirin produce its anti-inflammatory effects by inhibiting cyclooxygenase (COX) enzyme irreversibly by acetylating one of its serine residues leading to inhibition of prostaglandin synthesis. Though effective,aspirin also have some adverse effects like that of gastric mucosal damage & peptic ulceration. [5,6,7] Therefore, despite the current availability of anti-inflammatory drugs, a safer alternative is still required.Medicinal plants have been a source of variety of biologically active compounds for many centuries and are used as crude material or as pure compounds for treating various disease conditions.[8]The use of herbal medicines becoming popular due to toxicity and side-effects of allopathic medicines. [9]There is a history of use of herbal remedies for the treatment of inflammatory & painful conditions in Ayurveda & other systems of traditional medicines. Some of them are Neem (Azadirachta indica) and Tulsi (Ocimum sanctum).[10,11]
During our search we also found that these plants exhibit various properties. Ocimum sanctumhas shown to improve lipid profiles, prevent weight gain, hyperglycemia, hyperinsulinemia, protects blood vessels from atherosclerosis and protects liver, kidney, pancreatic islets from free radical damage and also possess antibacterial, antiviral and antifungal and anti-inflammatory activity etc. Its anti-inflammatory action of Ocimum. sanctum is mainly due to its eugenol and linoleic acid content causing inhibition cyclooxygenase and the lipoxygenase pathways of arachidonic acid metabolism. [12]Various parts of Azadirachta indicaexhibit many properties like its leaves are useful in treating leprosy, epistaxis, intestinal worms, inflammatory conditions, skin ulcers;its bark isused as analgesic, antipyretic and anti-inflammatory; its gum is effective against skin diseases like ring worms, scabies, wounds and ulcers andoil & seed pulp are used in leprosy and intestinal worm. One of the major constituents found in all the parts of Azadirachta indicawhich is responsible for its anti-inflammatory action is Nimbidin which suppresses the function of macrophages and neutrophils relevant to inflammation. [13]Leaves of Azadirachta indica are most commonly used for inflammatory diseases but few studies have reported toxic manifestation leading to death in guinea pigs, mice. [14]So, we chose to use bark of Azadirachta indicainstead of its leaves in this study. Hence, it was decided to prepare ethanolic extract of bark of Azadirachta indica& aqueous extract of leaves of Ocimum sanctumto evaluate their anti-inflammatory activity & compare it to a standard anti-inflammatory drug i.e. Aspirin. Anti-inflammatory efficacy of combination of Ocimum sanctum + Azadirachta indica(COA) was also evaluated in the present study.
Further, to the best of our knowledge no studies have been done so far, which could show that these medicines have serious systemic adverse effects like Aspirin. So, this study was planned to conduct a comparative study of Aspirin, Ocimum sanctum, Azadirachta indica and their combination (COA) for their anti-inflammatory efficacy. Rat paw oedema test was used in this study as it is one of the most common method of evaluating anti-inflammatory efficacy of any agent.[15] Histopathological examination was also done to assess mechanism involved behind their anti-inflammatory activity.
Materials and Methods
The study was commenced after IAEC(Institutional Animal Ethics Committee) approval was granted (IAEC No. CPCSEA/ DYPMC/ IAEC/ 01/2019) and was conducted in accordance with CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines. 16 Wistar albino rats of either sex were procured for experimentation from animal house of Dr. D. Y. Patil Medical college, Hospital and Research Centre, Pune. Food was stopped 24 hours prior to initiation of the study and water was stopped two hours before initiation of the experiment. Rats were housed in standard big polypropylene cages measuring 40 x 27.5 x 13.5 cm They were housed under standard condition of temperature (25 ± 5°C) and relative humidity (55 ± 10%) and 12/12-hour light/dark cycle.
Extraction of Plants
Plant extracts were prepared at Dr. D. Y. Patil College of Ayurved and Research Centre, Pune.
Ocimum sanctum
Fresh leaves of O. sanctum was collected, dried under shade for two days and powder was made from these leaves. 100 g of powder was macerated for two days in 1000 ml of distilled water,it was stirred frequently and 10 drops of chloroform was added per day. Then it was filtered twice using a double layered muslin cloth to remove insoluble material and was dried again. Finally, the brown-coloured semisolid residue was obtained and kept in water proof container kept in a refrigerator at 4ºC. Fresh preparations were made from it whenever required.[11]It was given in the dose of 400 mg/kg to each rat by dissolving them into distilled water.
Azadirachta indica
Barks of A. indica were aerated by fan to dried them partially. Then they were heated for two days with oven to be fully dried. Powder was made from dried barks using grinder. Cold extraction method with ethanol was used to extract the whole powders for 3 days. Clean white cotton material was used to filter the whole mixture & later filtered through What man filter paper then filtrate was evaporated at 68ºC temperature by Rotary evaporator at 5 to 6 rpm. Freeze drier was used to dry the crude extract obtained after evaporation and which was later preserved at 4ºC. Fresh preparations were made from it whenever required.[10] It was given in the dose of 500 mg/kg to each rat by dissolving them into distilled water.
Combination of Ocimum sanctum+Azadirachta indica (COA)
After measuring required quantity (400 mg/kg of O. sanctum and 500 mg/kg of A.indica), both the extracts were dissolved together in distilled water and stirred using magnetic stirrer until a clear brown coloured solution with no residue was obtained and this solution was given to rats.
Standard drug
Aspirin obtained from Sigma-Aldrich, India was used as standard drug for comparison in the present study. It was given to rats by dissolving them into distilled water.
Chemical irritant used to produce inflammation
Formalin was obtained from Sigma-Aldrich, India.2.5% formalin was prepared freshly by adding 2.5 ml of 100% formalin to 97.5 ml of normal saline and was injected into left hind paw of the rats.
Study groups
Rats were divided into five groups with six rats in each group. Drugs were given to rats orally as:
Group I: Control (only 3 ml of distilled water)
Group II: Aspirin (200 mg/kg) [17]
Group III: O. sanctum (400 mg/kg) [11,18]
Group IV: A. indica (500 mg/kg) [10]
Group V: COA (400 mg/kg of O. sanctum + 500 mg/kg of A. indica)
Rat Paw oedema test 2,19,20
Freshly prepared formalin (2.5%) in a dose of 0.1 ml was injected into sub plantar region of left hind paw of rats. Test drugs were given to rats orally according to their body weight half an hour before formalin injection and control group was given distilled water. A mark was made at the ankle joint of each rat. Paw volume up to ankle joint was measured in all the groups just before injecting formalin and at 1, 2, 3, 4, 5 hours after the formalin injection using digital plethysmometer. Rat paws before and after formalin injection were immersed into cylinder of digital plethysmometer filled with water and reading of paw volume in ml was obtained into the screen of digital plethysmometer. Mean and standard deviation for each hour in every group was calculated and appropriate statistical test was applied to assess anti-inflammatory efficacy of treatment groups.
The percentage inhibition of oedema at each hour was calculated using the following formula[21]:
Histopathological examination [22,23]
To assess mechanism involved behind the anti-inflammatory activity of the test drugs, histopathological examination of inflamed paw tissues was done. So, after 5 h of formalin injection, rats were sacrificed, and the inflamed tissue of the paws were removed and sent to pathology laboratory where tissue samples were fixed in 10% formalin for 3 days, decalcified and then each sample was embedded in paraffin wax, sectioned into 4 μm tissue sections and stained with hematoxylin and eosin (H & E) stain. After staining, microscopic analysis of histopathological slides was done for observing inflammatory response with a ×10 objective.Parameters studied were vascular changes (vasodilation & oedema), cellular changes (infiltration & margination of neutrophils). Each parameter was graded from scale of 1+ to 5+ on the basis of their appearance in the histopathological slides. Where, 1+ to 5+grading implies changes in parameters from very mild to very severe. Mean, median and standard deviation were calculated using these grades and appropriate statistical test was applied to assess anti-inflammatory efficacy of treatment groups.
Statistical Analyses
Data was compiled in Microsoft excel 2016 spreadsheet and was analysed using IBM SPSS version 26 statistical software. Data passed the Shapiro-Wilk normality test for distribution. Results for rat paw edema test was analyzed by using Mauchly’s test of sphericity with necessary corrections followed by repeated measures ANOVA followed by Tukey’s test & results for histopathological examination were analyzed by using Kruskal-Wallis test followed by Dunn’s test. If the p-value was found to be less than 0.05, result was considered statistically significant.
Observations and Results
Rat paw oedema test
Table 1: Paw volume in different groups at different hours.
| Treatment Group | Volume of paw oedema (in ml) at each hour – mean ± SD | |||||
| 0h | 1h | 2h | 3h | 4h | 5h | |
| Control | 1.1717 ± 0.6113 | 2.13 ± 0.15608 | 2.9233 ± 0.10053 | 3.7817 ± 0.17509 | 5.4833 ± 0.47990 | 6.25 ± 0.29759 |
| Aspirin | 1.1317 ± 0.04262 | 1.2433± 0.05125 | 1.32 ± 0.06603 | 1.5917 ± 0.07195 | 2.0433 ± 0.09501 | 2.1917 ± 0.09642 |
| O. sanctum | 1.2050 ± 0.09160 | 1.3033 ± 0.11343 | 1.6667 ± 0.07711 | 2.0433 ± 0.12675 | 2.56 ± 0.07874 | 2.9617 ± 0.05456 |
| A. indica | 1.1783 ± 0.06969 | 1.2133 ± 0.06186 | 1.2983 ± 0.05231 | 1.6617 ± 0.04665 | 2.1417 ± 0.08750 | 2.2333 ± 0.10558 |
| COA | 1.1833 ± 0.03670 | 1.2150 ± 0.04593 | 1.3083 ± 0.06047 | 1.6867 ± 0.08042 | 2.1700 ± 0.08390 | 2.2600 ± 0.08414 |
Table 2: Percentage reduction of paw oedema after each hour of the treatment.
|
Groups |
Time (in hours) | ||||
| 1h | 2h | 3h | 4h | 5h | |
| Control | 0% | 0% | 0% | 0% | 0% |
| Aspirin | 41.63% | 54.84% | 57.91% | 62.74% | 64.93% |
| O. sanctum | 38.81% | 42.99% | 45.97% | 53.31% | 52.61% |
| A. indica | 43.03% | 55.58% | 56.06% | 60.94% | 64.26% |
| COA | 42.95% | 55.25% | 55.40% | 60.43% | 63.84% |
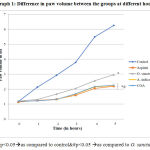 |
Graph 1: Difference in paw volume between the groups at different hours. |
When reduction in paw volume as observed in rat paw oedema test in different groups were compared, all the test groups& Aspirin showed significant effect as compared to control, O. sanctum did not show significant effect as compared to Aspirin, A. indica and COA but A. indica and COA had comparable effect with Aspirin & each other.
Histopathological examination
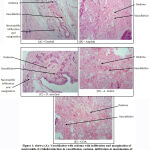
One histopathological slide from each group is displayed in the following figure.
Table 2: Vascular change (vasodilation) in different groups
| Group | Mean | Median | Standard deviation |
| Control | 2.83 | 3.00 | 1.169 |
| Aspirin | 0.50 | 0.50 | 0.548 |
| O. sanctum | 1.83 | 2.00 | 0.408 |
| A. indica | 0.83 | 1.00 | 0.408 |
| COA | 0.83 | 1.00 | 0.408 |
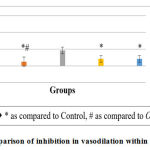 |
Graph 2: Comparison of inhibition in vasodilation with in different groups. |
When inhibition of vasodilation as observed in histopathological slides of different groups were compared, O. sanctum did not show significant effect whereas A. indica and COA showed significant effect as compared to control. None of the test groups showed significant effect as compared to Aspirin while Aspirin showed significant effect as compared to control and O. sanctum.
Table 3: Vascular change (oedema) in different groups
| Group | Mean | Median | Standard deviation |
| Control | 1.50 | 1.50 | 0.548 |
| Aspirin | 1.00 | 1.00 | 0.000 |
| O. sanctum | 1.00 | 1.00 | 0.000 |
| A. indica | 1.00 | 1.00 | 0.000 |
| COA | 1.00 | 1.00 | 0.000 |
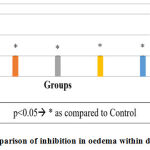 |
Graph 3: Comparison of inhibition in oedema within different groups. |
When inhibition of oedema as observed in histopathological slides of different groups were compared, all the test groups and Aspirin showed significant effect as compared to control and comparable effect with each other.
Table 4: Cellular changes (infiltration & margination of neutrophils) in different groups
| Group | Mean | Median | Standard deviation |
| Control | 4.67 | 5.00 | 0.516 |
| Aspirin | 0.83 | 1.00 | 0.408 |
| O. sanctum | 1.83 | 2.00 | 0.408 |
| A. indica | 0.83 | 1.00 | 0.408 |
| COA | 0.83 |
1.00 |
0.408 |
 |
Graph 4: Comparison of inhibition in cellular changes within different groups. |
When inhibition of cellular changes as observed in histopathological slides of different groups were compared, Aspirin, A. indica&COAshowed significant effect as compared to control &comparable effect with each other while O. sanctumdid not show significant effects as compared to control.
Discussion
Most commonly used phlogistic agents like formalin and carrageenan which almost have similar efficacy of producing inflammation and show a peak effect at 4th hour after their administration [24] therefore percentage inhibition oedema at and after 4th hour after formalin injection are major determinants of the anti-inflammatory activity of these extracts.In this study, Ocimum sanctumproduced 53.31% and 52.61% inhibition of oedema, Azadirachta indicaproduced 60.94% and 64.26% inhibition of oedema, COAproduced 60.43% and 63.84% inhibition of oedema and Aspirin produced 62.74% and 64.93% inhibition of oedema at 4th& 5th hour respectively after injection of formalin. This study reports that all the test groups had significant (p<0.05) anti-inflammatory activity. Aspirin, Azadirachta indica& COAalso possess better anti-inflammatory efficacy compared to Ocimum sanctum while they themselves have comparable effect to each other.
A similar study was conducted by Kalabharathi HL et al (2011) where they compared paste ofOcimum sanctumleaves (500 mg/kg) with indomethacin (100 mg/kg) which reported that Ocimum sanctumproduced 67% inhibition of oedema while Indomethacin produced 76% inhibition of oedema and they observed that Ocimum sanctumproduced 88.15% reduction in oedemato that of Indomethacin. [25] Another study was conducted by Hannan JMA et al (2011) where they compared ethanolic extract of leaves of Ocimum sanctum(500 mg/kg) with Diclofenac (10 mg/kg) and reported that Ocimum sanctumproduced 30.32% inhibition of oedema while diclofenac produced 66.57% inhibition in oedema 4 hours after injection of carrageenan. [26]Another study was conducted by Mirje MM et al (2015) with aqueous extract of leaves of Ocimum sanctum (400 mg/kg) andcompared its efficacy in various doses with indomethacin (25 mg/kg). Percentage inhibition of oedema observed in their study at 4thand 5th hour after formalin injection by Ocimum sanctum were 95.12% and 97.25% & by Indomethacin were 78.56% & 84.67%. 15
Emran et al (2015) conducted a study where they compared efficacy of ethanolic extract of bark ofAzadirachta indica(500 mg/kg) with diclofenac (2.5 mg/kg) in which Azadirachta indicaextract produced 43.06% and 22.22% inhibition of oedema while diclofenac produced 52.05% and 28.77% inhibition of oedema at 4th and 8th hour after injection of carrageenan.[9] A study conducted by Kumar S et al (2015) compared anti-inflammatory efficacy of Azadirachta indica leaf extract to aspirin. They observed that Azadirachta indica (500 mg/kg) produced 63.01% and 66.31% inhibition of oedema while aspirin (200 mg/kg) produced 72.05% and 66.31% inhibition of oedema at 4thand 6th hour after injection of carrageenan. [27] All the previous studies report that both Ocimum sanctum and Azadirachta indica possess potent anti-inflammatory activity, however their anti-inflammatory activity is less as compared to standard anti-inflammatory agents.
Present study supports the result found in above mentioned studies that Ocimum sanctum and Azadirachta indica have a potent anti-inflammatory efficacy but also suggests non-inferiority of Azadirachta indica to that of Aspirin.
The morphologic hallmarks of acute inflammation are small blood vessels dilation, leukocytes accumulation (mainly neutrophils) & fluid in the extra vascular tissue (oedema).1 From histopathology slides, it can be said that mechanism of action of Ocimum sanctum is not clear but it significantly reduced oedema grossly as well as microscopically whereas Aspirin, Azadirachta indica and COA caused significant reduction in vasodilation, oedema (grossly & microscopically), infiltration & margination of neutrophils.A study conducted by Kaur G et al (2004) reported that Azadirachta indica causes inhibition of infiltration of neutrophils and macrophages.28 To the best of our knowledge, no such study was done in past which assessed mechanism of Ocimum sanctum using histopathological examination.
Present study implies that herbal medicines like Azadirachta indica&Ocimum sanctumpossess a potent anti-inflammatory activity against acute inflammatory conditions and can prove to be effective medicines when given along with other standard drug therapies. This study has its own limitations, plants extracts were tested for their efficacy only against acute inflammation and not against chronic inflammation and it was done in small number of animals, there could be a more significant result when large number of animals and different species of animals are included in this study. Mechanism of action of extracts were assessed using histopathological examination, a more precise information could be obtained about their mechanism when other investigations like assessment of inflammatory mediators will also becarried out.
Conclusion
This study revealed that both Ocimum sanctum and Azadirachta indica and their combination (COA) have significant anti-inflammatory effect on acute inflammation. Azadirachta indica and COAproved to be a better anti-inflammatory agent compared to Ocimum sanctum and showed comparable effect to Aspirin while Ocimum sanctum though potent was not a better anti-inflammatory agent than Aspirin. From theabove results, it could be considered that COA is having its anti-inflammatory effect mainly due to its Azadirachtaindica content therefore, Azadirachta indicac an prove to beaneffective add-on therapy for the treatment of acute inflammation but more studies are required to validate its anti-inflammatory effect in clinical settings.
Acknowledgement
We acknowledge Dr. Archana Buch, Professor, Department of Pathology, Dr. D. Y. Patil Medical College, Hospital & Research Centre, Pune for helping with the histopathological examination of tissues. We Also acknowledge Dr. S. L. Jadhav, Professor, Department of Preventive & Social Medicine, Dr. D. Y. Patil Medical College, Hospital & Research Centre, Pune for helping with the statistical Analysis of the data.
Funding Source
None
Conflict of Interest
None
References
- Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology. 10th Philadelphia: Elsevier Inc; 2018.
- Arzi A, Olapour S, Yaghooti H, Karampour NS. Effect of Royal Jelly on Formalin Induced-Inflammation in Rat Hind Paw. Jundishapur J Nat Pharm Prod.2015;10(1). doi: 10.17795/jjnpp-22466.
CrossRef - Damjanov I. Harsh Mohan Textbook of Pathology. 7th New Delhi. Jaypee Brothers Medical Publishers (P) Ltd. 2015.
- Ryan GB, Majno Acute inflammation. A review. Am J Pathol. 1977; 86(1): 183–276.
CrossRef - Grosser T, Smyth EM, FitzGerald GA. Pharmacotherapy of Inflammation, Fever, Pain and Gout. In: Bruton LL, Dandan RH, Knollmann BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th New York: McGraw Hill Education publication; 2018, 685-710.
- Tripathi KD. Essentials of Medical Pharmacology. 8th New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2019.
- Borazan NH, Furst DE. Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, drugs used in Gout. In: Katzung BG, Trevor AJ. Basic & Clinical Pharmacology, 13th New Delhi: McGraw Hill Education (India) Pvt. Ltd.; 2015, 618-41.
- Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS et al. NaturalProducts-antifungal agents derived from plants. J Asian Nat Prod Res. 2009; 7:621-638.
CrossRef - Dasilva EJ. Medicinal plants: a reemerging health aid. Electron J Biotechnol. 1999; 2: 57-70.
CrossRef - Emran TB, Uddin MMN, Rahman A, Uddin Z, Islam M. Phytochemical, Antimicrobial, cytotoxic, Analgesic and AntiInflammatory Properties of Azadirachta Indica: A Therapeutic Study. J Bioanal Biomed. 2015. doi:10.4172/1948-593X.S12-007.
CrossRef - Mirje MM, Zaman SU, Ramabhimaiah S. Evaluation of the antiinflammatory activity of Ocimum Sanctum Linn (Tulsi) in albino rats. Int J Cur Microbiol App Sci. 2014; 3(1): 198-205.
- Cohen MM. Tulsi- Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med. 2014; 5(4): 251-9.
CrossRef - Gupta A, Ansari S, Gupta S, Narwani M, Gupta M, Singh M. Therapeutics role of neem and its bioactive constituents in disease prevention and treatment. J Pharmacogn Phytochem. 2019; 8(3): 680-91.
- Biswas K et al. Biological activities and Medicinal Properties of neem (Azadirachta indica). Curr Sci. 2002; 82(11): 1336-45.
- Vogel HG. Drug discovery and evaluation: Pharmacological assays, 2nd ed. Berlin: Springer-Verlag Berlin Heidelberg; 2002.
- CPCSEA guidelines for laboratory animal facility. [cited 5 july 2020] Available from: http://icmr.nic.in/bioethics/final_cpcsea.pdf
- Swayeh OA, Clifford RH, Soldato PD, Moore PK. A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br J Pharmacol 2000; 129(2): 343–50.
CrossRef - Mirje MM, Kumbar S, Moharir G. Anti-inflammatory activity of Ocimum Sanctum (linn) in formalin induced acute models of albino rats. Int JClin Biomed Res. 2015;1(1): 1-4.
- Motevalian M, Shiri M, Shiri S, Shiri Z, Shiri H.Anti-inflammatory activity of Elaeagnus angustifolia fruit extract on rat paw edema. Basic Clin Physiol Pharmacol. 2017. doi: 10.1515/jbcpp-2015-0154.
CrossRef - Tyagi P, Pore S, Prakash J. Anti-inflammatory agents. In Gupta SK. Drug Screening Methods. 3rd New Delhi. Jaypee Brothers Medical Publishers Pvt. Ltd.;2016, 495-513.
CrossRef - SinghM, KumarV, SinghI, GauttamG, Kalia Anti-inflammatory activity of aqueous extract of Mirabilis jalapaLinn. Leaves. Pharmacogn. Res., 2010; 2 (6): 364-67.
CrossRef - Chang CW et al. Analgesic and Anti-Inflammatory Activities of Methanol Extract of Cissus repens in Mice. EvidBased ComplementAlternat Med. 2012; doi:10.1155/2012/135379.
CrossRef - Sadeghi H et al. In vivo anti-inflammatory properties of aerial parts of Nasturtium officinale. Pharm Biol 2014; 52(2): 169–74.
CrossRef - Aceto HW, Porreca F and Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990: 40(2): 229-38.
CrossRef - Kalabharathi H L, Suresha R N, Pragathi B, Pushpa V H And Satish A M. Anti-Inflammatory Activity Of Fresh Tulsi Leaves (Ocimum Sanctum) In Albino Rats. Int J Pharm Bio Sci, 2011; 2 (4):45-50.
- Hannan JMA, Das BK, Uddin A, Bhattacharjee R, Das B, Chowdury HS et al. Analgesic and anti-inflammatory effects of Ocimum sanctum (linn) in laboratory animals. Int J Pharm Sci Res, 2011; 2 (8): 2121-25.
- Kumar S, Vandana UK, Agrawal D, Hansa J. Analgesic, Anti-inflammatory and Anti-Pyretic Effects of Azadirachta indica (Neem) Leaf Extract in Albino Rats. Int J Sci Res, 2013; 4 (8): 713-21
- Kaur G, Alam MS, Athar M. Nimbidin suppresses functions of macrophages and neutroplils: relevance to its antiinflammatory mechanisms. Phytother Res. 2004; 18(5): 419-24.
CrossRef