Syed Shariq Mian1, 2* , Tajuddin1
, Tajuddin1 and Sukirti Upadhyay2
and Sukirti Upadhyay2
1Department of Saidla, Faculty of Unani Medicine, Aligarh Muslim University, Aligarh-202002, India
2Department of Pharmacognosy, Faculty of Pharmacy, IFTM University, Moradabad-244102, India
Corrresponding Author E-mail: Shariq.sl@myamu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2224
Abstract
Introduction: Arthritis (Wajaul Mafasil) is a condition which is growing worldwide due to lifestyle, environmental and genetic factors. In Unani literature, there are many herbs which are praised for treatment of Arthritis. So polyherbal formulation contains Ginger, Colchicum and Nux- Vomica is taken in combination for arthritis study. This combination is not previously reported but used by unani practitioners. Method: Three crude herbs (Ginger, Colchicum and Nux vomica) were extracted out in both aqueous and hydro-alcoholic solvent. LD-50 of all extracts (aqueous and hydro-alcoholic extract) was determined. Now respective extracts were mixed in effective dose ratio to obtain aqueous and hydro-alcoholic dosage form. Finally both effective combinations (aqueous and hydro-alcoholic) convert into tablet dosage form to determine its anti-arthritic activity by Carrageenan Induced Oedema Test, Cotton Pellet Induced Granuloma Test and Freund’s adjuvant Induced Arthritis Test. The efficacy of the Unani formulation was compared with reference drug (Diclofenac sodium). Result and Discussion: in Carrageenan Oedema Test, animals in high dose hydro-alcoholic, shows decrease in paw volume significantly after 3 hours of Carrageenan injection. In Cotton Pellet Induced Granuloma Test, animals in high dose hydro-alcoholic, shows reduction in granuloma formation significantly. In Freund’s Adjuvant Induced Arthritis Test, The significant reduction in paw volume was found in high dose hydro-alcoholic. Conclusion: Conclusively this study establishes anti-arthritic potential of polyherbal extract in unani literature. Thus these drugs possess synergistic anti-arthritic potential in combination against acute, sub-acute as well as chronic arthritis. So in future compatible dosages form may be prepared for treating arthritis.
Keywords
Anti-arthritic; Colchicum luteum; Strychnos nux-vomica; Wajaul Mafasil; Zingiber officinale
Download this article as:| Copy the following to cite this article: Mian S. S, Tajuddin T, Upadhyay S. Anti-Arthritic Evaluation of Ginger, Colchicum and Detoxified Nux-Vomica combination for Poly Herbal Unani Formulation. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Mian S. S, Tajuddin T, Upadhyay S. Anti-Arthritic Evaluation of Ginger, Colchicum and Detoxified Nux-Vomica combination for Poly Herbal Unani Formulation. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2TASQYQ |
Introduction
Unani system of medicine is very ancient, practiced for centuries in India. Its single as well as multi-preparations have great anti-inflammatory and anti-arthritic significance. Natural products in general have nearly all type of biological activity. According to WHO nearly five billion humans depend upon herbal medicine. Wajaul Mafasil (Rheumatoid arthritis) is an inflammation of one or more joints associated with pain (Sheikh et al., 2014). The area is still poorly studied, because only 7-12% plants have been screened for their pharmaceutical and therapeutically activities (Ahmad, Anwar, Khan and Kabir, 2015). Inflammation is a protective mechanism against noxious physical chemical and microbial stimulus. In response of external stimulus, body increases the secretion of various mediators such as prostaglandins, leukotrienes etc. at inflammation site. These mediators also increases the inflammation, thus inhibition of these mediators is necessary. Presently the inhibition of mediator is a purpose of anti-inflammatory compound. (Vane and Botting, 1998).
Zingiber officinale
Zingiberaceae (Ginger): It is well known medicinal herb in ayurvedic and unani literature. The rhizome is reported to contain over 400 different compounds. Major composition of ginger rhizomes are carbohydrates (50–70%), lipids (3–8%), terpenes and phenolic compounds. (Mao et al., 2019) Terpene components are zingiberene, α-farnesene, α-curcumene, β-bisabolene and β-sesquiphellandrene while phenolic components include gingerol, shogaol and paradols (Anonymous. 2007). These gingerols (23–25%) and shogaol (18–25%) are found in higher quantity than others. Besides these, amino acids, ash, protein, phytosterols, raw fiber, vitamins (e.g., nicotinic acid and vitamin A), and minerals are also present. (Gracia-Sancho and Salvado, 2017). Results from in vitro and animal studies demonstrate that ginger is likely to exert 5-HT3 antagonistic effects. (Abdulkhaleq et al., 2018)
Colchicum luteum
Liliaceae, in present study its corm is used. it is reported to contain Colchicines lumicolchicine, chlorogenic acid and 3,4,5,7-Tetrahydroxyflavone on the basis of different modern spectroscopic techniques However, colchicines were the main alkaloids reported (Ali et al., 2018). It Inhibits activation of the NLRP3 inflamma some in response to inflammatory microcrystals. Suppresses the expression of NF-κB (Dalbeth, Lauterio and Wolfe, 2014).
Strychnos nux-vomica
Loganiaceae (Nux vomica): It is a toxic drug, in this study it is detoxified as described. In literature the seed is reported to contain following compounds identified as: α-amyrin, vomicine, stearic acid, β-sitosterol,vanillin, ethyl gallate, methylgallate, novacine, strychnine, daucosterol, brucine chloro methochlorid, loganic acid, chlorometho chloride, brucine, geniposide and loganin. (Zhang and Tu, 2012). These are centrally-acting neurotoxins. Strychnine competitively antagonizes post-synaptic binding of the inhibitory transmitter glycine, which leads to heightened reflex excitability of muscle (Yin, Wang, Yin and Cai, 2003).
In the present study a combination of three crude herbs (Dried Rhizome of Ginger, dried corm of Colchicum and detoxified dried cotyledons of Nux vomica) is used for the treatment of arthritic, which is not mention in any literature previously but used many time in clinically by Prof, Tajuddin.
Detoxification of the Strychnos nux-vomica.
Nux-vomica seeds are soaked in water for 7 nights; fresh water is to be replaced every night. Then seeds further detoxified by boiling with cow milk in dolayantra (an assembly which contain boiling pan in which a cloth bundle is hanged) for 7 days, 3 hours daily. The seed coat and embryo are removed. The cotyledon shall be roasted in cow ghee and powdered well (Mohammad Taghizadeh Kashani et al., 2016).
Material and Methods
Collection of plant material
The crude herbs was procured from Dawakhana Tibbya college, Aligarh Muslim University, Aligarh and was acknowledged by literature available in books and authenticated by Prof. Abdul Latif. Ginger, Colchicum and Nux vomica with specimen voucher no. SC-0226/17, SC-0227/17 SC-0228/17 respectively was submitted in the museum of Department of Ilmul Advia (Pharmacy), Ajmal Khan Tibbya College, Aligarh Muslim University, Aligarh, for future reference. Report was found that all parameters are within range as in literature.
Formulation development method
Three crude herbs (Ginger, Colchicum and Nux vomica) were dry and grind by suitable methods in suitable equipments to convert it into powder form. Each powder drug is extracted out in both aqueous and hydro-alcoholic solvent. Thus we obtained aqueous and hydro-alcoholic extract of Ginger, Colchicum and Strychnos nux-vomica. These six extracts one by one is used to finding LD-50 in animal according to Annex 2a of OECD 423 guideline (oral toxicity). Now aqueous extracts were mixed in effective dose ratio to obtain aqueous extract dosage form (aqueous formulation) and hydro-alcoholic extracts were mixed in effective dose ratio to obtain hydro-alcoholic extract dosage form (Hydro-alcoholic formulation). Finally both aqueous and hydro-alcoholic effective formulations convert into tablet dosage form to evaluate its anti-arthritic activity by Carrageenan Induced Oedema Test, Cotton Pellet Induced Granuloma Test and Freund’s adjuvant Induced Arthritis Test. The efficacy of the Unani formulation was contrasted with the normal reference drug (Diclofenac sodium).
Animal maintenance
The present study was designed to evaluate the anti-arthritic activity in healthy albino rat. Animals weighing 100 to 150 gm were selected. Six animals were grouped together in a cage where fresh water and foods was supplied. All procedures were performed in daylight between 9 to 14 hrs. Experimental protocols were in compliance with the NIH guidelines for the care and use of the laboratory animals. The animals were fed, Standard animal diet and water. All the experiments were performed by permission from animal ethical committee of Department of Ilmul Advia, A.K.T.C., A.M.U., Aligarh (registration no-1979/GO/Re/S/17/ CPCSEA/I).
Drugs and chemicals
Diclofenac sodium (Voveran, Novartis, India), Normal Saline (E. Merck Ltd, India), Strychnine, Colchicine and Brucine are procured by Sigma-Aldrich, USA, Carrageenan Type II (TCS chemical company, Japan), Freund’s adjuvant sample (Mycobacterium tuberculosis-heat-killed) was procured by Sigma-Aldrich, USA.
Determination of Anti-Arthritic Activity
Carrageenin Induced Oedema Test
Carrageenan induced paw oedema is a very common test which is used for determining acute inflammation. Only six animals were housed in a cage, and six groups were designed for study. Weighing 100-150 gm. Before giving the drugs, the volume of the left hind paw was plethysmo graphically measured. Six groups were arranged below. Winter et al. (1962).
Group I- Control (20 ml/kg distilled water + 0.1ml Carrageenan injection)
Group II- Standard (5 mg/kg Diclofenac Sodium orally + 0.1ml Carrageenan injection)
Group III- LDHA (290 mg/kg suspension of Hydro-alcoholic Extract formulation + 0.1ml Carrageenan injection)
Group IV- HDHA (580 mg/kg suspension of Hydro-alcoholic Extract formulation + 0.1ml Carrageenan injection)
Group V- LDA (550 mg/kg suspension of Aqueous Extract formulation + 0.1ml Carrageenan injection)
Group VI- HDA (1100 mg/kg suspension of Aqueous Extract formulation + 0.1ml Carrageenan injection)
0.1ml of 1% aqueous solution of Carrageenan was injected under plantar aponeurosis after one hour of test drug ingestion. The paw volume was measured regularly at one hour difference till 5 hour i.e. 2, 3, 4 and 5 hour after Carrageenan injection (Newbould 1963).
I= [1-(m-p)/(n-q)]×100
Here, I = Inhibition Percentage, m = Mean paw volume of test or standard group animals after injection, n = Mean paw volume of control group animals after injection, p = Mean paw volume of test or standard group animals prior to injection, q = Mean paw volume of control group animals prior to injection The results were also analyzed by a one-way ANOVA test followed by a pair-wise comparison of the different groups by LSD to determine the significance of the difference. The analysis was performed using the website’s software (Graphpad Instat Software).
Cotton Pellet Induced Granuloma Testg
Six groups were designed for this study. Only six animals house in each group weighing from 100 to 150gm. Each animal anaestheticized by ether after shaving off the fur. In the ventral region of rats, sterile, pre-weighted cotton pellets (20 ± 1mg) were implanted (Winter et al., 1957). Six groups were arranged below.
Group I- Control (20 ml/kg distilled water + Cotton pellet implant)
Group II- Standard (5 mg/kg Diclofenac Sodium orally + Cotton pellet implant)
Group III- LDHA (290 mg/kg suspension of Hydro-alcoholic Extract formulation + Cotton pellet implant)
Group IV- HDHA (580 mg/kg suspension of Hydro-alcoholic Extract formulation + Cotton pellet implant)
Group V- LDA (550 mg/kg suspension of Aqueous Extract formulation + Cotton pellet implant)
Group VI- HAD (1100 mg/kg suspension of Aqueous Extract formulation + Cotton pellet implant)
The test formulation was ingested 30 minutes prior to cotton pellet implantation. After 30 minutes each animal was anaesthetized and sterile cotton pellet implant with small incision. Test formulation continues till seven successive days. After 7th day of drug treatment, animal again anaesthetized and remove its cotton pellet. Remove all extraneous tissue from cotton pellet and weigh. If dry weight of cotton pellet increases, it means granuloma formed. Compare it with control group weight. The findings were also compared statistically by one way ANOVA Test followed by LSD.
Freund’s Adjuvant Arthritis Test
It represents the chronic inflammation process. Seven groups were designed for this study. Only six animals house in each group weighing from 100 to 150gm. The animals provided the regular diet and tap water, were maintained at a uniform temperature. All the animals were injected with 0.075 ml of Freund’s Adjuvant (1.0 mg of heat-killed and dried Mycobacterium tuberculosis in 0.85 ml of paraffin oil) (F-5881, Sigma Aldrich, USA) in right paw except plain control group. The paw volume was measured with plethysmographically on 1st day prior to Freund’s adjuvant injection. Paw volume continuously measured alternatively from 11th day to 17th day i.e. on day 11, 13, 15, 17. Ankle thickness was also measured as same schedule with Micrometer Screw Gauge. The animals in all the groups were given oral treatment once a day for 5 days (Persico et al 1988) Seven Groups are as below
Group I- Plain control (No F. Adjuvant + 20ml/ kg distilled water)
Group II- Control (0.075 ml F. Adjuvant + 20ml/ kg distilled water)
Group III- Standard (0.075 ml F. Adjuvant + 5mg/kg Diclofenac Sodium)
Group IV- LDHA (0.075 ml F. Adjuvant + 290 mg/kg suspension of Hydro-alcoholic extract Formulation)
Group V- HDHA (0.075 ml F. Adjuvant + 580 mg/kg suspension of Hydro-alcoholic extract Formulation)
Group VI- LDA (0.075 ml F. Adjuvant + 550 mg/kg suspension of Aqueous extract Formulation)
Group VII- HDA (0.075 ml F. Adjuvant + 550 mg/kg suspension of Aqueous extract Formulation)
The final measurements were made on the closing day, i.e. day 17, immediately after administration of the treatment. The animals were sacrificed by cervical dislocation after one and a half hours of treatment. The percentage of inhibition was found out by the following formula:
I= [1-(m-p)/(n-q)]×100
Here, I = Inhibition Percentage, m = Mean paw volume of test or standard group animals on 17th day, n = Mean paw volume of control group animals on 17th day, p = Mean paw volume of test or standard group animals on 1st day, q = Mean paw volume of control group animals on 1st day The mean paw volume / ankle joint thickness was also compared statistically by ANOVA Test followed by LSD.
Statistical analysis
All results were representing in Mean ± SEM, for six observations. The results were also tested by one way ANOVA to assess the significance difference followed by pair wise comparison of different groups by LSD. The analysis was performed by using Graphpad Instat software.
Results
On the basis of OECD 423 guidelines the LD50 value of different extracts were obtained. After pilot study, LD-50 of each drug is obtained as:
| Extract Name | LD-50 of each drug | 1/10th (High Dose) | 1/20th (Low Dose) |
| Aqueous extract of Ginger | 5000 mg/Kg b.w. | 500 mg/Kg b.w. | 250 mg/Kg b.w. |
| Aqueous extract of Colchicum | 5000 mg/Kg b.w. | 500 mg/Kg b.w. | 250 mg/Kg b.w. |
| Aqueous extract of Strychnos nux-vomica | 1000 mg/Kg b.w. | 100 mg/Kg b.w. | 50 mg/Kg b.w. |
| Hydro alcoholic extract of Ginger | 5000 mg/Kg b.w. | 500 mg/Kg b.w. | 250 mg/Kg b.w. |
| Hydro alcoholic extract of Colchicum | 500 mg/Kg b.w. | 50 mg/Kg b.w. | 25 mg/Kg b.w. |
| Hydro alcoholic extract of Strychnos nux-vomica | 300 mg/Kg b.w. | 30 mg/Kg b.w. | 15 mg/Kg b.w. |
High and low dose tablets of both extract is prepared by combining three drugs in effective dose ratio. Thus four tablets are formed.
| Effective Dose (Tablet Form) | Drugs | Ratio | Suspension of Tablet |
| High dose aqueous extract tablet (HDA) | Ginger: Colchicum: Strychnos nux-vomica | 500:500:100 | 1100 mg/kg |
| Low dose aqueous extract tablet (LDA) | Ginger: Colchicum: Strychnos nux-vomica | 250:250:50 | 550 mg/kg |
| High dose hydro alcoholic extract tablet (HDHA) | Ginger: Colchicum: Strychnos nux-vomica | 500:50:30 | 580 mg/kg |
| Low dose hydro alcoholic extract tablet (LDHA) |
Ginger: Colchicum: Strychnos nux-vomica | 250:25:15 | 290mg/kg |
These are administered orally in the animals after shaking the suspension well with the help of the feeding canula.
In the Carrageenan oedema test, standard drug, higher and lower doses of both formulations were found to decrease the paw volume significantly three hours after Carrageenan injection. The percentage inhibition of oedema was found to be 64.70% in the standard drug group. The inhibition was found to be 62.74%, 76.47%, 58.82% and 62.74% in LDHA, HDHA, LDA and HDA respectively. All results are shown in Table-1 and a comparison of percentage inhibition among all groups was shown in Figure-1.
Table 1: Effect of Test Formulation on Carrageenan Induced Paw Oedema Model.
| Group | 1 hr after Carr. Inj. | 2 hr after Carr. Inj. | 3 hr after Carr. Inj. | 4 hr after Carr. Inj. | 5 hr after Carr. Inj. |
| Percentage of inhibition
(%) |
Percentage of inhibition
(%) |
Percentage of inhibition
(%) |
Percentage of inhibition
(%) |
Percentage of inhibition
(%) |
|
| Control | – | – | – | – | – |
| Standard (Diclofenac
5mg/kg) |
48.7805 | 56.814 | 64.7059 | 56.5217 | 48.8372 |
| LDHA
(290mg/Kg) |
46.3415
|
54.545
|
62.7451
|
54.3478
|
37.2093
|
| HDHA
(580mg/Kg) |
51.219
|
59.0909
|
76.4706
|
58.6957
|
51.1628
|
| LDA
(550mg/Kg) |
43.9024
|
52.2727
|
58.8235
|
52.1739
|
44.186 |
| HDA
(1100mg/Kg) |
41.4634
|
54.5455
|
62.7451
|
47.8261
|
41.8605
|
| F-Value | 1.56 | 1.85 | 3.93 | 2.18 |
n=6 x =Against Control, 1 = p < 0.05, 2 = p < 0.01, 3 = p < 0.001
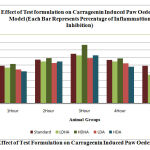 |
Figure 1: Effect of Test Formulation on Carrageenin Induced Paw Oedema Model. |
In the cotton pellet induced granuloma test, standard drug group, higher and lower doses of both extract formulations were found to reduced granuloma formulation significantly. The percentage of inhibition of granuloma was found to be 53.91% in standard drug group. The inhibition was found to be 37.79%, 50.57%, 33.41% and 39.44% in LDHA, HDHA, LDA and HDA respectively. All results are shown in Table-2 and a comparison of percentage inhibition among all groups was shown in Figure -2.
Table 2: Effect of Test Formulation on Cotton Pellet Induced Granuloma Test
| Group | Percentage Inhibition of Granuloma (%) |
| Control | ___ |
| Standard (Diclofenac 5mg/kg) | 53.91 |
| LDHA (290mg/Kg) | 37.79 |
| HDHA (580mg/Kg) | 50.57 |
| LDA (550mg/Kg) | 33.41 |
| HDA (1100mg/Kg) | 39.44 |
n=6 x=Against Control, 1=p<0.05, a=Against LDHA, 2 = p < 0.01, b = Against HDHA, 3 = p < 0.001 c = Against LDA, d = Against HAD
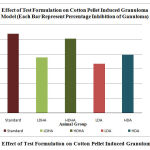 |
Figure 2: Effect of Test Formulation on Cotton Pellet Induced Granuloma Model. |
In Freund’s adjuvant Induced Arthritis Test, On 17th Day the increase in paw volume was significantly reduced with standard drug group. Increase in paw volume was also significantly reduced in LDHA, HDHA, LDA and HDA. The percentage of inhibition of increase in paw volume was found to be 64.19% with standard drug group. The inhibition was found to be 59.25%, 71.60%, 40.74% and 62.96% in LDHA, HDHA, LDA and HDA. All results are shown in Table-3 and a comparison of percentage inhibition among all groups was shown in Figure -3.
Table 3: Effect of Test Formulation on Freund’s adjuvant Induced Arthritis Model (Established Type) (Paw Volume)
| Group | On day 17 after Freund’s adjuvant administration |
| Percentage of inhibition (%) | |
| Control | ___ |
| Standard (Diclofenac 5mg/kg) | 64.1975 |
| LDHA (290mg/Kg) | 59.2593 |
| HDHA (580mg/Kg) | 71.6049 |
| LDA (550mg/Kg) | 40.7407 |
| HDA (1100mg/Kg) | 62.963 |
| F-value | 4.26 |
n=6 x = Against Adjuvant 1 = p < 0.001
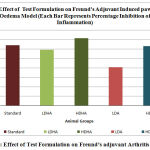 |
Figure 3: Effect of Test Formulation on Freund’s adjuvant Arthritis Model. |
Discussion
In Carrageenan induced oedema test, the study shows that both doses of both extracts of formulation have anti-inflammatory activity. This test was performed in a multiple test interval i.e. inhibition was also observed at 1, 2, 3, 4 and 5 hours after Carrageenan injection. In anti-inflammatory process, multiple inflammatory mediators predominate in different phases of acute inflammation. Histamine secretion is maximum at 1 hour while prostaglandins at 3 to 4 hours, after the inflammatory stimulus (Abdulkhaleq et al., 2018). The present study shows that at all test intervals, all test drugs exerted inhibition of inflammation. The inhibitory effect was lower at 1 hour and highest at 3 hrs. Hydro-alcoholic extracts were more effective at 3 hours. These results suggest that HDHA, LDHA, LDA and HDA inhibits the action of most acute inflammatory mediators, in which HDHA have the greatest inhibition at 3 hours on Prostaglandin.
The Cotton pellet granuloma model is a proliferative inflammation process indicator. In this procedure granuloma or inflammation was formed over several days. Vascular changes, exudation of fibrous fluids, large numbers of neutrophiles, fibroblast, plasma cells and histiocytes present at granuloma site. (Mescher, 2017). Weight loss in granuloma suggests that formulation decreases the proliferation condition. The results also suggest that a HDHA decreases the weight of cotton pellets significantly (p<0.01) compared to a lower dose, due to the dose-dependent response to the test formulation.
In Freund’s adjuvant Arthritis Test the standard drug, all test Formulations were found to decrease the paw volume significantly (p<0.001) than that of control group. Therefore, the study shows that all test Formulations have significant anti-arthritic activity. The results clearly show that the HDHA has a good protective activity against arthritis, which is in close to the effect of standard drug. (Al-Hejjaj, Numan, Al-Sa’ad and Hussain, 2011).
The Radiographic Study of right hind paw was suggested that there is minimum sclerosis of bones in treated groups as compare to untreated groups. The present study show that high dose of hydro-alcoholic extract formulation highly minimize radiographic arthritic changes, therefore more osteoblastic activity of formulation. All radiographic pictures with anterior and lateral view are shown in Figure-4
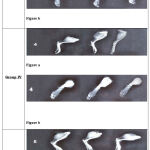 |
Figure 4: X- Ray: Lateral (a) and anterioposterior(b) view of left hind paw of Albino Rats |
Conclusions
HDHA possesses significant activity against acute, sub-acute and chronic inflammation. The study offers an improvement in Unani health care by showing the more convenient Tablet form to be effective, which may replace the existing usage of powder form which is more in quantity and less convenient to use.
Acknowledgment
The authors are grateful to the, Dept. of Ilmul Saidla, F/o Unani Medicine, A.M.U., Aligarh for providing support to carry out this work.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abdulkhaleq L, Assi M, Abdullah R, Zamri-Saad M, Taufiq-Yap Y, Hezmee M. The crucial roles of inflammatory mediators in inflammation: A review. Veterinary World. 2018; 11(5):627-635.
CrossRef - Ahmad S, Anwar N, Khan M, Kabir H. Effect of detoxification (tadbeer) in content of toxic metabolites of Strychnos nux-vomica: A Unani approach for its use in human. Journal of Pharmacy and Bioallied Sciences. 2015;7(4):314.
CrossRef - Al-Hejjaj W, Numan I, Al-Sa’ad R, Hussain S. Anti-inflammatory activity of telmisartan in rat models of experimentally-induced chronic inflammation: Comparative study with dexamethasone. Saudi Pharmaceutical Journal. 2011;19(1):29-34.
CrossRef - Ali R, Kamil S, Rehman M, Mir M, Amin U. A Review on Pharmacological Properties of Colchicum luteum – A Himalayan Herb. International Journal of Livestock Research. 2018; 8(5):14.
CrossRef - Anonymous 2007. The Unani pharmacopoeia of India. New Delhi: Government of India, Ministry of Health & Familiy Welfare, Dept. of Indian Systems of Medicine & Homeopathy; 2007.
- Dalbeth N, Lauterio T, Wolfe H. Mechanism of Action of Colchicine in the Treatment of Gout. Clinical Therapeutics. 2014; 36(10):1465-1479.
CrossRef - Gracia-Sancho J, Salvado J. Gastrointestinal tissue. Academic Press; 2017.
- Graphpad Instat software. Statistical analysis tool
- Mao Q, Xu X, Cao S, Gan R, Corke H, Beta T et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185.
CrossRef - Mescher A. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017; 4(2):39-53.
CrossRef - Mohammad Taghizadeh Kashani L, Hatami A, Safaei A, Shirzad M, Ahmadian-Attari M. Different Traditional Methods of Nux-Vomica Detoxification Have Therapeutic Rationales. Jundishapur Journal of Natural Pharmaceutical Products. 2016; 11(1).
CrossRef - Newbould B. Chemotherapy of Arthritis Induced In Rats by Mycobacterial Adjuvant. British Journal of Pharmacology and Chemotherapy. 1963; 21(1):127-136.
CrossRef - Persico F, Pritchard J, Fisher M, Yorgey K, Wong S, Carson J. Effect of tolmetin glycine amide (McN-4366), a prodrug of tolmetin sodium, on adjuvant arthritis in the rat. J Pharmacol Exp Ther. 1988; 247(3):889-96.
- Sheikh H,. F, Jabeen A, Siddiqui M. Management of Waja Ul Mafasil (Arthritis) In Unani System of Medicine: A Review. International Journal of Research in Ayurveda & Pharmacy. 2014; 5(1):60-64.
CrossRef - Vane J, Botting R. Mechanism of Action of Nonsteroidal Anti-inflammatory Drugs. The American Journal of Medicine. 1998;104(3):2S-8S.
CrossRef - Winter C, Porter C. Effect of Alterations in Side Chain upon Anti-inflammatory and Liver Glycogen Activities of Hydrocortisone Esters. Merck Institute for Therapeutic Research, West Point, Pa. Journal of the American Pharmaceutical Association (Scientific ed). 1957; 46(9):515-519.
CrossRef - Winter C, Risley E, Nuss G. Carrageenan-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Experimental Biology and Medicine. 1962; 111(3):544-547.
CrossRef - Yin W, Wang T, Yin F, Cai B. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnos nux-vomica. Journal of Ethnopharmacology. 2003; 88(2-3):205-214.
CrossRef - Zhang J, Tu P. Chemical constituents from processed seeds of Strychnos nux-vomica. Journal of Chinese Pharmaceutical Sciences. 2012;21(2).
CrossRef








