Sabari Anadh J V1 , Swapna R Nayaka3
, Swapna R Nayaka3 , Usha N S3
, Usha N S3 , Subha V4
, Subha V4 , Manimekalai K1
, Manimekalai K1 and Mushraf Syed2
and Mushraf Syed2
1Department of Pharmacology, Faculty cum Research Scholar, MGMCRI, Puducherry. India.
2Department of Pharmacology, Melaka Manipal Medical College (Manipal Campus), MAHE, Manipal-576104, Karnataka, India.
3Department of Pharmacology, MVJ Medical College and Research Hospital, Hoskote.Karnataka, India.
4Animal Biotechnologist, MGMCRI (Department of Pharmacology), Puducherry. India.
Corresponding Author E-mail: syed.mushraf@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2265
Abstract
Purpose: The study aimed to assess the acute toxicity and anti-diabetic activity of Halimedagracilis (green marine alga). Methods: The Halimedagraciliswere collected from the coastal area of the Gulf of Mannar biosphere reserve and shade dried. Methanolic extract of Halimedagracilis (MEHG) was prepared and it was screened for acute toxicity and anti-diabetic activity in the Zebrafish model. In the Acute toxicity study, the Zebrafishes were grouped into 6 groups and dosed with 6.25, 12.5, 25, 50, and 100mg/L of MEHG and observed at 0, 24, 48, 72, and 96 hours’ intervals. Foranti-diabetic activity analysis diabetes was induced using streptozotocin (STZ). The Zebrafish were divided into six groups- control group, positive control, diabetic Zebrafish with three doses of MEHG, and standard control (treated with metformin). Results: Acute toxicity studyshowed no significant behavioral changes and LC50 was determined as 100mg/L. In the diabetic study, test groups when compared to the control group showed: a significant reduction in both fasting and postprandial blood glucose levels and significant changes in the regeneration of pancreatic β-cells, and reduced vacuolization in the islets of Langerhans. Images of the regenerating caudal fins taken at 24, 48 and 72-hours post-amputation displayed significant limb regeneration in MEHG treated fish compared to the control group. Conclusion: These results prove that MEHG in STZ- induced diabetic Zebrafish possess potent anti-diabetic action by ameliorating blood glucose regulation, promoting pancreatic cell regeneration,minimizing long-term diabetic complications bypreventing the emergence of metabolic memory but no behavioral changes.
Keywords
Acute toxicity study; Anti-diabetic activity; Halimeda gracilis; Streptozotocin; Zebrafish model
Download this article as:| Copy the following to cite this article: Anadh J. V. S, Nayaka S. R, Usha N. S, Subha V, Manimekalai K, Syed M. Acute Toxicity Study and Antidiabetic Activity of Marine Alga-Halimeda Gracilis Chooranam (HGC) In Freshwater Zebrafish Model. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Anadh J. V. S, Nayaka S. R, Usha N. S, Subha V, Manimekalai K, Syed M. Acute Toxicity Study and Antidiabetic Activity of Marine Alga-Halimeda Gracilis Chooranam (HGC) In Freshwater Zebrafish Model. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3g8PTqv |
Introduction
DiabetesMellitus isone of the heterogeneous metabolic disorder causing both microvascular ¯o vascular complications which is characterized byhyperglycaemia due toalterations in the storage & mobilization of metabolic compounds, including catabolism and anabolism of carbohydrates, lipids & proteins, resulting from defects in insulin synthesis & secretion, resistance to action or both1.
The long-term effects of diabetes lead to the development of specific complications like retinopathy, nephropathy, neuropathy, cardiovascular diseases, and co-morbidities 2.Hence, it is vitalto diagnose the undiagnosed people with diabetes or pre-diabetic stateand impart proper care for them as earlyas possible. Regular medications are utilized to treat diabetes by enhancing insulin sensitivity, increasing insulin production,and reducingblood glucose levels. There is always a drawback in drug treatment,in regulating the blood glucose levels due to certain adverse effects like GIT disturbance, tiredness, weight gain., etc.
In modern medicine, no satisfactory effective therapy is yet available. Marine algae (or) seaweed compounds have contributed to the global search for novel medicinal agents. In the current time, a noticeable number of novel metabolites with potent pharmacological properties have been distinguished from the marine organism whichis one of the abundantsource of structurally diverse natural products 3-5. These algae are the most essential part of the diet of many eastern countries, and their use as food is well documented. Marine algaehave a wide variety of compounds which have promising health benefits, and some have exhibited chelating property on heavy metals 6. They are anabundant natural resource for many bioactive substances like polyunsaturated fatty acids, sterols, proteins, polysaccharides, antioxidants, colours, and trace elements in a focus a lot higher than in earthly plants 7 and offer a wide range of secondary metabolites, which shows different pharmacological actions like anti-cancer, antimicrobial, antifungal, anti-inflammatory, anti-oxidants, anti-fouling, and anti-diabetic activity, etc. 8-9.
Hence, in this study, we have tested the acute toxicity and antidiabetic activity of Halimeda gracilis as various studies on other macro algae have potentially proven to have anti-diabetic action in animal models & patients and it’s being used in alternate medicines to treat diabetes mellitus.
Methods
Collection of Halimeda Gracilis
The fresh marine green alga Halimedagraciliswas collected from Rameswaram coastal area, Tamil Nadu, India, and was carried to the lab in plastic bags with seawater. The alga was processed for a thorough wash with seawater & then tap water to remove epiphytes, salts, and other extraneous materials.The seaweeds were
identified and authenticated as Halimedagracilis (Halimedagracilis Harvey ex. J Agarah 1887) by Dr. S.Bragdeeswaran, Associate Professor, Centre of Advanced Study (CAS) in Marine biology, Annamalai University, Parangipettai, Tamil Nadu, India& also prepared herbarium & museum specimens for the repository.The sample was thenshade driedat 37°C and ground to a fine powder. The powder was then stored in the refrigerator for further use 10.
Preparation of extracts
The dried Halimedagraciliswas made into a coarse powder in a mechanical grinder and it was subjected to maceration at 24-25°C in 95% methanol for 72 hours. The methanolic extract was derived after the process of distillation, evaporation, and drying under reduced pressure as per the standard procedure11.
Acute toxicity study
Zebra fish were housed in a home tank at the population density of 2 fish per litre. The aquarium was filled with dechlorinated purified tap water and reverse osmosis water in the ratio of 1:2. The water was analysed and adjusted for the optimum pH, conductivity, salinity, and hardness. The aquarium was equipped with an aquarium filter and aerator units (aquarium aerator and pump). The fish was quarantined for a minimum period of 12 days in the laboratory test room before starting the experiment and the fish was fed with commercial fish food pellets two times per day. The temperature was maintained in the range of 27±2˚C. The fish was provided with a photo period of 12 hours of artificial light and 12 hours of darkness throughout the experiment. They were acclimatized in the test tanks for 4 days before the study and the feeding was stopped 24 hours before the initiation of the drug dose.
Procedure
As per OECD guideline 203 [12],the limit test at 100 mg/L of the test compound demonstrated that the LC50 is greater than this concentration. The maximum concentration of the study was fixed as 100mg/L. The spacing factor of the concentration range was 2.The volume of the exposure medium was 2.5 litres per tank (not exceeding the maximum load of 1g of fish per litre). Exposure medium, maintained at the optimum pH, temperature, dissolved oxygen, and 12 hours of photo period was maintained throughout the study.
Grouping and dosing
As the test compound was sparingly soluble in water, the stock solution was prepared by dissolving the 500 mg of the test compound in 1ml of methanol. The stock solution is aliquoted into different tubes based on the desired concentrations as mentioned (Table 1). The desired concentrations of exposure medium were prepared by adding the aliquoted quantity of the stock solution in the aquarium habitat water of known quantity. During the test period of 96 hours, all experimental fish were treated with MEHGC except normal control fish and observed for mortality, morbidity, and other behavioural changes in Zebrafish.
Table 1: Acute toxicity study groups and doses.
| Group | Total number of fish | Concentration | The volume of test sample per tank(µL) |
| 1 | 8 | 100mg/L | 500 |
| 2 | 8 | 50mg/L | 250 |
| 3 | 8 | 25mg/L | 125 |
| 4 | 8 | 12.5mg/L | 62.5 |
| 5 | 8 | 6.25mg/L | 31.3 |
| Control | 8 | NULL | 0 |
Anti-diabetic activity of MEHGC:
Induction of diabetes in Zebrafish
All experimental fish received STZ (0.35 mg/g body weight, i.p.) single dose for 5 groups for 19 days, except normal control based on its body weight (Figure 1). The fishes were kept on fasting for 12 hours on 20th day and on Day 21, blood samples (1.0 to 2.0 µl/fish) were collected through caudal fin and analysed for blood glucose level as a baseline. Diabetic animals were randomized into 6 groups based on an acceptable range of glucose level ± 20% mean between the groups (Table 2). It was ensured to have a minimum of 8 animals with disease induction in each group at the start of drug treatment 13.
 |
Figure 1: Inducing STZ -intraperitoneal injection (50µL gas tight syringe). |
Table 2: Dosing for diabetic induced Zebrafish
| S. No | Group | Treatment | Total Fish |
| 1 | Normal Control | Control (Non-Diabetic Fishes) | 10 |
| 2 | Test Drug | STZ + MEHG low dose (200µg/g body weight) | 10 |
| 3 | Test Drug | STZ + MEHG mid-dose(300µg/g body weight) | 10 |
| 4 | Test Drug | STZ + MEHG high dose(500µg/g body weight) | 10 |
| 5 | Positive Control | Streptozotocin (STZ) of 0.35 mg/g body weight | 10 |
| 6 | Standard Drug | STZ + metformin of 0.001 mg/g body weight | 10 |
Dose formulation
The test drug was freshly prepared before administration & the respective doses were given orally for each group based on the bodyweight of the fish for 7 consecutive days 14. (Figure 2).
 |
Figure 2: Drug administered oral. |
Blood Collection
After induction of diabetes on day 21 as baseline values and after treatment on day 28 of the experiment the blood was collected from caudal fin puncture and analysed for blood glucose level in all the test groups13.
Necropsy, organ collection, and pathology
At the end of the experiment, fishes were euthanized with ms-222, for collecting pancreas & intestinal tissue. The collected tissues were fixed in 10% neutral buffered formalin for 48 hours, processed, embedded in paraffin, sectioned on a microtome, and stained with H and E.
Statistical analysis
SPSS was used for statistical analysis and the data expressed as the mean ± standard error (S.E). The differences in blood glucose level (fasting and postprandial) obtained between the diabetic control group, test group, and the positive group was compared by using Student’s t-test and the difference was considered to be statistically significant when the p-value was <0.05.
Results and discussion
Acute toxicity study
The result provides evidence that the treatment of MEHG doesn’t cause mortality up to the maximum concentration, 100 mg/L as there were no morbidity or mortality observed in experimental fish throughout the study.MEHG didn’t cause any remarkable behavioural changes with a different range of concentrations within the period of the test. No abnormal behavioural changes were observed in experimental fish in the entire study, as all the fishes were found to be normal [Table 3-7].
Table 3: Observation at zero hours of dosing
| Observations | Group 1 | Group 2 | Group3 | Group4 | Group5 | Control |
| No. of live fish | 8 | 8 | 8 | 8 | 8 | 8 |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| pH | 7.50 | 7.40 | 7.32 | 7.45 | 7.33 | 7.29 |
| Temperature | 27.5˚C | 27.5˚C | 27.5˚C | 27.5˚C | 27.5˚C | 27.5˚C |
| Behavioral/visible abnormality | Normal | Normal | Normal | Normal | Normal | Normal |
Table 4: Observation at 24th hour of dosing
| Observations | Group 1 | Group 2 | Group3 | Group4 | Group5 | Control |
| No. of live fish | 8 | 8 | 8 | 8 | 8 | 8 |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| pH | 7.57 | 7.35 | 7.33 | 7.62 | 7.60 | 7.30 |
| Temperature | 28˚C | 28˚C | 28˚C | 28˚C | 28˚C | 28˚C |
| Behavioral/visible abnormality | Normal | Normal | Normal | Normal | Normal | Normal |
Table 5: Observation at 48th hours of dosing
| Observations | Group 1 | Group 2 | Group3 | Group4 | Group5 | Control |
| No. of live fish | 8 | 8 | 8 | 8 | 8 | 8 |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| pH | 7.80 | 7.59 | 7.40 | 7.54 | 7.50 | 7.33 |
| Temperature | 26.43˚C | 26.43˚C | 26.43˚C | 26.43˚C | 26.43˚C | 26.43˚C |
| Behavioral/visible abnormality | Normal | Normal | Normal | Normal | Normal | Normal |
Table 6: Observation at 72nd hours of dosing
| Observations | Group 1 | Group 2 | Group3 | Group4 | Group5 | Control |
| No. of live fish | 8 | 8 | 8 | 8 | 8 | 8 |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| pH | 7.80 | 7.61 | 7.50 | 7.17 | 7.38 | 7.18 |
| Temperature | 27.22˚C | 27.22˚C | 27.22˚C | 27.22˚C | 27.22˚C | 27.22˚C |
| Behavioral/Visible abnormality | Normal | Normal | Normal | Normal | Normal | Normal |
Table 7: Observation at 96th hours of dosing
| Observations | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Control |
| No. of live fish | 8 | 8 | 8 | 8 | 8 | 8 |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| pH | 7.67 | 7.46 | 7.39 | 7.18 | 7.21 | 7.18 |
| Temperature | 27.46˚C | 27.46˚C | 27.46˚C | 27.46˚C | 27.46˚C | 27.46˚C |
| Behavioral/Visible abnormality | Normal | Normal | Normal | Normal | Normal | Normal |
Antidiabetic activity of MEHGC
In experiment groups, group 4Zebrafish treated with a high dose of MEHG showed significant reduction [p <0.001] in both the fasting and postprandial blood glucose levels compared to MEHG treated group 2, 3 and untreated group 5 – positive control [ Table 8].
Table 8: Antidiabetic activity of MEHG in Zebrafish
| Groups | Fasting blood glucose level | Postprandial glucose level | P values |
| 1 – Control | 52.0 ± 5.732 | 80.13 ± 9.311 | <0.001 |
| 2 – STZ + low dose | 249.50 ± 11.123 | 330.13 ± 8.509 | <0.001 |
| 3 – STZ + mid dose | 186.38 ± 15.892 | 290.50 ± 6.162 | <0.001 |
| 4 – STZ + high dose | 99.13 ± 8.459 | 140.13 ± 5.194 | <0.001 |
| 5 – Positive control | 294.75 ± 9.192 | 359.50 ± 9.871 | <0.001 |
| 6 – STZ + metformin | 66.0 ±7.17 | 107.25 ± 10.512 | <0.001 |
Fasting and postprandial glucose levels are expressed as mean ±SEM, P<0.05 when compared to control and group 6.
Histopathological examination
The pancreatic cells in group 1 fishes exhibited normal morphology, whereas, in group 2 and group 3 fishes, vacuolation in the exocrine pancreas was observed. In group 4, most of the fishes exhibited regenerative morphology and exhibited normal pancreatic architecture. Few fishes exhibited vacuolation in the exocrine pancreas. In the untreated group 5, high pancreatic damages were observed like pancreatic degeneration and vacuolation in islets. Group 6 fishes showed normal pancreatic cellular architecture [Figure 3].
Hence from obtained histopathological examination analysis group 4 exhibited almost normal pancreatic cellular morphology compared to MEHG treated group 2, 3 and untreated group 5.
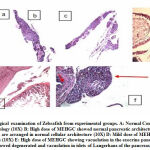 |
Figure 3: Histopathological examination of Zebrafish from experimental groups. |
A: Normal Control pancreas section showing normal morphology (10X)
B: High dose of MEHGC showed normal pancreatic architecture (20X)
C: Metformin showing pancreatic cells are arranged in normal cellular architecture (10X)
D: Mild dose of MEHGC showing vacuolation in the exocrine pancreas (10X)
E: High dose of MEHGC showing vacuolation in the exocrine pancreas (10X)
F: Untreated group showed degenerated and vacuolation in islets of Langerhans of the pancreas (40X)
Therefore, these results revealed that group 4 exhibited better efficacy compared to other treated and untreated groups. However, group 6 exhibited a good ameliorative effect compared to group 4.
Discussion
Seaweeds or marine algae are crude nonflowering plants without true root stem and leaves10. They are the sustainable living sources of food, fodder, and fertilizer in many parts of the world. Macro algae are found to have potentially highly active secondary metabolites with diverse activities in various researches reports which have been screened widely to isolate life-saving drugs or biologically active substances worldwide. Marine algae are a rich source of ingredients such as polyunsaturated acids, β-carotene, and their pigment carotenoids, sulphated polysaccharide, and sterol[15]. They also contain secondary components that have the potential to be developed in various fields such as pharmaceuticals, cosmetics, and other industrial purposes such as biomass, biofuels, bio-oil, biodiesel,etc., and the waste can be used as fertilizer and fodder for animal (or) fish [16].
In the present study, HGC used in acute toxicity study didn’t show any abnormal behavioural changes, mortality, and morbidity in the Zebrafish even after 96hours at maximum concentration 100mg/L.
The MEHGC showed quite significant antidiabetic activity at higher doses (500µg/g body weight) by reducing blood glucose levels (p<0.001) showing better efficacy in comparison with other doses. It also maintained the normal morphology of pancreatic cells & regenerative property.
We would like to conclude that Halimeda gracilis has better efficacy as antidiabetic, by maintaining the normal morphology of pancreatic cells & also has regenerative property without causing any mortality & morbidity in the acute toxicity test. It can be included in the diet of diabetic patients for the prevention and treatment of their condition.
Acknowledgment
Dr. S.Bragdeeswaran, Associate Professor CAS in Marine biology, Annamalai University, Parangipettai, Tamil Nadu, India for identifying and authenticating the species and preparing the herbarium.
Competing interests
The authors have no competing interests to declare.
Funding Source
Not applicable
Reference
- Piero MN, Nzaro JM &Njagi JM. (2014) Diabetes mellitus – a devastating metabolic disorder. Asian Journal of Biomedical & Pharmaceutical Sciences. 04 (40); 1-7.
- Soumya D, Srilatha B. (2011) Late-stage complications of diabetes and insulin resistance. J DiabetMetabol.; 2: 1-7.
- Wijesekara I, Pangestuti R and Kim SK. (2011) Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae.Carbohydrates Polym. 84:14-21.
CrossRef - Guven KC, Percot A, and Sezit E. (2010) Alkaloids in marine algae.Mar Drugs.8:269-84.
CrossRef - EI-Gamal AA. (2010) Biological importance of marine algae.Saudi Pharm J.18:1-25.
- Nwosu F,Morris J, Victoria AL,Stewart D, and Heather A. (2011) Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae.Food Chemistry. 126:1006-12.
CrossRef - Wang H, Fu Z, and Han C. (2014)The potential applications of marine bioactive against diabetes and obesity.American Journal of Marine Science. 2(1):1-8.
- Abou-Elela, Gehan M, Elnaby AH, Hassan AH, Ibrahim, and Okbah MA. (2009) Marine natural products and their potential applications as anti-infective agents.World ApplSci J.7(7):872-880.
- Mohammed A, Adelaiye AB, Abubakar MS, and Abdurahman EM. (2007) Effects of aqueous extract of Ganodermalucidum on blood glucose levels of normoglycemic and alloxan-induced diabetic Wistar rats.J of Medicinal Plants Res.1(2):34-37.
- Ashwini Kumar P, Soundarapandian P, Jagan K, Anatharaman P, Kannan D, Sanjeev Kumar, et al. (2014) Associated Fauna in cultured seaweed Kappaphycusalvareziiof Vellar Estuary (South East Coast of India). Int J Res Mar Sci.3 Suppl 2:37-43.
- Vasudevarao B, Sravanthi DJ. (2017) GC/MS analysis and In-vitro antioxidant activity of methanol extract of Ulothrixflacca and its main constituent Dimethyl sulfone. IOR J Pharm Biol Sci.12(1):93-104.
- OECD Guidelines for Testing of Chemicals (No.203; Adopted: 17th July 1992) (OLIS-1998.)Organization for Economic Co-operation and Development – Principles of GLP and Compliance Monitoring (as revised in 1997).
- Intine, R.V., Olsen, A.S., Sarras, M.P. (2013)A Zebrafish Model of Diabetes Mellitus and Metabolic Memory. J. Vis. Exp. (72), e50232, doi:10.3791/50232.
CrossRef - Kim, E.-A. et al. (2016)A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in the alloxan-induced hyperglycemia zebrafish model. RSC Adv. 6, 78570–78575.
CrossRef - Priya et al. (2012) Antibacterial activity of some selected seaweeds from the Gulf of Mannar coast, South India. Asian J Pharm Clin Res.5 Suppl 4:89-90.
- Nivedita Sharma et al. (2017) Industrial and biotechnological applications of Algae: A Review. Journal of Advances in plant biology.1(1):1-25.
CrossRef








