Ahmed Algazeery1 , Ashraf S. El-Sayed2
, Ashraf S. El-Sayed2 , Fatma M El-Deeb
, Fatma M El-Deeb 3 and Nomier MA4
3 and Nomier MA4
1Zoology Department, Faculty of Science, Zagazig University, 44519, Egypt
2Botany and Microbiology Department, Faculty of Science, Zagazig University , 44519, Egypt
3Faculty of Science- Al-Azhar University, Egypt
4Department of Forensic Medicine and Toxicology, Faculty of Medicine- Zagazig University, 44519, Egypt
Corresponding Author Email:asalgazeery@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2216
Abstract
Despite the remarkable progress in selecting the chemotherapeutic drugs, most are expensive and associated with many adverse effects targeting both cancer and normal cells. The using of polyphenols as natural materials for chemoprevention is considered a promising approach in reducing the tumor proliferation.This study aims to investigate whether a difference between the use of green tea and its component Epigallocatechin-gallate (EGCG) in treatment and protection against tumor. Sixty female Swiss albino mice weighted 20–22 g divided into 6 groups (n=10).The tumor suppression of green tea and EGCG was mirrored by evaluating their antioxidant and the anti-inflammatory effect on tumor markers and DNA integrity.Our results showed that the administration of EGCG showed a significant elevation of both antioxidants and anti-inflammatory markers in serum of EAC-bearing animals and revealed its high curative power to protect than treat tumor growth. Moreover, genomic DNA fragmentations assay present EGCG as a modulatory agent in keeping genome integrity.The administration of green tea and its major constituent EGCG showed a significant a potent protective role in suppressing tumor proliferation than its use in treatment due to its antioxidant and anti-inflammatory effect and maintaining the integrity of underlying genomic DNA that make it a strong barrier which arrest the process of oncogensis.
Keywords
Cell Proliferation;DNA Fragmentation; EGCG; Oxidative Stress; Tumor
Download this article as:| Copy the following to cite this article: Algazeery A, El-Sayed A. S, El-Deeb F. M, Nomier M. A. Differential Modulatory Effect of Epigallocatechin-3-Gallate in Suppression of Tumor Proliferation. Biomed Pharmacol J 2021;14(3) |
| Copy the following to cite this URL: Algazeery A, El-Sayed A. S, El-Deeb F. M, Nomier M. A. Differential Modulatory Effect of Epigallocatechin-3-Gallate in Suppression of Tumor Proliferation. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3n4glWH |
Introduction
Cancer is still the much–feared disease that threat human health. Its victims are still frightened and depressed. It considered as an end-product of multiple physiological and genomic instability which disturb the machineries regulating cells proliferation 1. In addition, the elevation of oxidative stress could stimulate pro-inflammatory cytokines and inhibit cellular antioxidant capacity and this damage continues attach biological molecules including DNA 2. Most of chemotherapeutic drugs still have adverse effects. The naturally existing compounds from plants known as phytochemicals are not only serve as interest resources for novel drugs but also revealed the importance of the diet and its impact, not only on body health but also in prevention of diseases development such as cancer. So, the cellular and its underlying genomic stabilities could be restored by nutritional treatment or protection with herbs which has no side effects3.
The first barrier of defense against oxidative stress involves two main enzymes superoxide dismutase (SOD) and glutathione peroxidase (GSH), which are antioxidants usually measured in the realization of the natural compounds antioxidant activity 4, 5. SOD enzyme modifies the highly reactive superoxide radicals in hydrogen peroxide (H2O2) and molecular oxygen and makes the first antioxidant defense in an oxidative stress condition 6. Similarly, GSH preserves the polyunsaturated cell membranes 5,7.
Several studies were reported that the antioxidant properties of fruits and vegetables can modulate various biological processes including growth and cell proliferation. The use of phytochemical in cancer therapy could understood via regulating cell proliferation, increasing antioxidant status, regulation of the immune system, inactivation of carcinogens, induction of cell cycle arrest and apoptosis [8].Green tea obtained from the Camellia sinensisplant, the major constituents of green tea are polyphenols comprising phenolic acids and catechins those belong to the free iron scavengers, flavonoids. Many botanical flavonoids possess strong antioxidant activities 9, 10.
Evidences presented green tea possessing chemotherapeutic potential,but still a question, whether the administration of green tea and its component Epigallocatechin-gallate (EGCG)are more effective in treatment of existing tumor or rather prevention against tumor formationThus, the main objective of this study was to investigate differential activity of green tea and its active constituent EGCG as a natural therapeutic and preventive agent for tumor proliferation.
Material and Methods
Animals
60 female Swiss albino mice (of age about 8 weeks, 20–22 g body weight) were divided into 6 groups (n=10).The animals were acclimatized for one week before each experiment or one week.
Ethical consideration
This experimental protocol was reviewed and approved by The Institutional Animal Care and Use Committee (ZU-IACUC Committee), Zagazig University and the approval number is ZU-IACUC/1/F/108/2020.
Ehrlich Ascites Carcinoma (EAC)
Ehrlich’s ascites carcinoma (EAC) is an undifferentiated carcinoma, has a high transplantable capability, no-regression, rapid proliferation, short life span, obtained from National cancer institute (Cairo, Egypt). EAC has been used as an experimental model to evaluate the effect of natural plant on tumor cell proliferation11.The tumor was maintained in Swiss mice in the ascitic form, collected by aspiration with a Pasteur pipette, centrifuged for 10 min at 200 xg, and washed twice with phosphate-buffered saline (PBS, pH 7.2). Cell viability was evaluated by trypan blue (0.2%) exclusion test and only cell suspensions with more than 95% viability were used[12].In all experimental protocols, mice were injected intraperitoneally by 2.5 × 106 cells/mouse.
Drugs
Green tea (Camellia sinensis): Green tea was prepared by steeping 2.5 g in 250 mL of boiling water for 5 minutes. After cooling, the mice were administered orally at a daily dose of (250 mg/kg. b.wt.) as 1 % of solution 13.Tea bags (Authentic Green Tea) from Rabea company (Egypt).
EGCG: (-)-EpigallocatechinGallate (EGCG) with purity 99% was purchased from National bio-lab company for Trade, EgyptCat. No.4524. It was freshly prepared by dissolving in water, and then administered orally at a daily dose of 50mg/kg. b. wt. 14.
Sorafenib: Sorafenib (purity 99% it was purchased from National bio-Lab. Company for Trade, Egypt cat no 17307032. It was freshly prepared by dissolved in distal water, then administered orally and daily at a dose of 50 mg/kg. b. wt. 15.
Experimental design
Control group
Animals were received only the normal laboratory diet and tap water.
Ehrlich group
Animals were intraperitoneally injected by 0.2 ml Ehrlich Ascites Carcinoma (2.5 ×106 Ehrlich cells).
Ehrlich+ Sorafenib group
Animals were intraperitoneally injected 0.2 ml Ehrlich Ascites Carcinoma (2.5 ×106 Ehrlich cells). Animal then left for one week and then treated with sorafenib orally (50mg/kg/day) for 5 weeks.
Ehrlich +Green tea group
Animals were intraperitoneally injected by 0.2 ml Ehrlich Ascites Carcinoma (2.5 ×106 Ehrlich cells), and left for one week and then treated with green tea orally (250 mg/kg/day) for 5weeks.
Ehrlich +EGCG group
Animals were intraperitoneally injected by 0.2 ml Ehrlich Ascites Carcinoma (2.5 ×106 Ehrlich cells). Mice were left for one week and then treated with EGCG orally(50mg/kg/day) for 5 weeks.
EGCG group
Animals were treated with EGCG orally (50 mg/kg/day) for 10 days and then intraperitoneally injected once 0.2 ml Ehrlich Ascites Carcinoma (2.5 ×106 Ehrlich cells).
Collection of samples
Blood
At the end of the experiments, animals were fasted overnight, sacrificed and blood samples were collected into plasma tubes and the samples were harvested for determining the activity of GSH, SOD, AFP, IL-8 and IL-8 levels.
The ascetic fluid
After treatment, the ascetic fluid was collected from the peritoneal cavity in graduated falcon tubes. Phosphate buffered saline (PBS) was added to each tube for Ehrlich Ascites Count.
Biochemical analysis
All the biochemical parameters in this study were estimated at National cancer institute (Cairo, Egypt).
Evaluation of antioxidant effect
The status of oxidative stress was mirrored in serum by measuring levels. Superoxide dismutase activity [16]and reducedglutathione [17] by using Kits obtained from Sigma-Aldrich; Cat. No. 19160and CS0260, respectively and the developed colors were measured at wavelength given on kits.
Evaluation of tumor proliferation markers
To evaluate the tumor proliferation, animals were dissected and the ascitic fluid was collected from the peritoneal cavity, the volume was measured by a graduated centrifuge tube [18]. Tumor Necrosis Factor Alpha (TNF-α) was determined according to the method of Swardfager[19] by using ELISA Kit obtained from Sigma-Aldrich, Cat. No.; RAB0479.
Evaluation of anti-inflammatoryeffect
Serum α-fetoprotein (AFP) was determined according to the method of Maiolini and Masseyeff [20] by using ELISA Kit obtained from Mybiosource, Cat. No.; MBS590043. Detection is based on using Purified AFP antibody to coat Micro Elisa Strip plate wells to make solid-phase antibody, then add AFP and AFP-antibody labeled with HRP, the color change was measured at wavelength 450 nm. Serum interferon-gamma was determined according to the method of Fischer [21] by using ELISA Kit obtained from ThermoFisher scientific Co, Cat. No.; ERIFNG. While interleukin- 6 [22]and interleukin-8 [23] by using ELISA Kit obtained from MybiosourceCat. No.; MBS355410 and MBS9141543, respectively. The kits based on enzyme-linked immune-sorbent assay.
DNA fragmentation by agarose gel electrophoresis
Genomic DNA extraction of Ehrlich Ascites Carcinomas (EAC) cells was extracted according to the protocol of genomic DNA extractionCat. No 51304from animal tissues [24]according to the manufacturer’sinstructions of the QIAamp DNA Mini Kit. The tissue was homogenized in 1 ml lysis buffer [20 mM Tris-Cl (pH 7.5), 0.15 M NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and 25 mM disodium pyrophosphate] at 37°C for 1 h. Then, 0.4 ml of saturated NaCl was added to each set of cell lysates and incubated on ice for 5 min and centrifuged at 3,000 ×g for 30 min. The DNA was precipitated using chilled ethanol, which was separated by centrifugation. Separated DNA was re-suspended in TAE buffer (40 mM Tris-acetate and 1 mM EDTA), and the purity of the extracted DNA was checked by 2% agarose gel of 0.5 ug/ml ethidium bromide in TAE buffer according to Raj [25]. The DNA bands were observed and photographed under a UV trans-illuminator.
Statistical analysis
The obtained data were subjected to one-way analysis by software package SPSS versions 2015 used for interactive or patched, statistical analysis, produced by SPSS Inc., it was acquired by IBM in 2009; data were expressed as mean ± S.E.
Results
Antioxidants effect
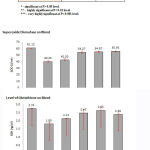
Our results in table (1) and fig. (1) revealed that administration of green tea (250 mg/kg/day) or EGCG (50mg/kg/day) in EAC- bearing mice, in group 4 and 6, respectively, were significantly, increased the antioxidant potential as mirrored by measuring the levels of SOD and GSH in blood when compared with control animals (group 1) and EAC-bearing untreated animals (group 2). This antioxidant property against tumor progression revealed to be more effective when administered prior EAC transplantation (group 6).
Tumor suppressor and anti-inflammatory effect
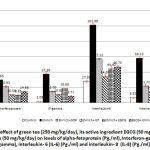
To investigate whether the antioxidant properties of green tea and EGCG would affect tumor proliferation, the count per volume of EAC, levels of tumor markers including tumor necrosis factor-α, luteinizing hormone (LH) and growth hormone (GH) were estimated in blood fig. (2). Our measurements shown that administration of green tea (250 mg/kg/day) or EGCG (50mg/kg/day) in EAC- bearing mice, in group 4 and 6, respectively, were significantly decreased the count of EAC and levels of TNF-α, LH and GH in blood when compared with control animals (group 1) and EAC-bearing untreated animals (group 2). And that tumor formation was suppressed more significantly when green tea or EGCG administered prior EAC transplantation (group 6). On the other hand Results of this study clarified that the anti-tumor activity of green tea and EGCG not only based on their antioxidant properties but also dependent on their anti-inflammatory properties which were evaluated by estimation of a number of inflammatory markers including; α-fetoprotein (AFP), interferon-γ (IF-γ), interleukin-6 (IL-6) and interleukin-8 (IL-8) as in fig. (3). Results revealed that administration of green tea (250 mg/kg/day) or EGCG (50mg/kg/day) in EAC- bearing mice, in group 4 and 6, respectively, were significantly decreased the levels of (AFP), (IF-γ), (IL-6) (IL-8) in blood when compared with control animals (group 1) and EAC-bearing untreated animals (group 2). And that we found their anti-inflammatory effect was increased significantly as noticed by significant decrease in these inflammatory markers when green tea or EGCG administered in (group 6) prior EAC transplantation.
DNA Fragmentation Assay
Isolated DNA from EAC-bearing mice (group 2) showed complete degradation into oligonucleotide fragments forming a clear ladder of apoptosis when separated by agarose gel electrophoresis as shown in fig. (4). While, DNA extracted from treated animals in groups 3,4 and 5 with sorafenib, green tea and EGCG, respectively showed a pattern significantly fragmented as compared to control DNA. The genomic DNA of animals, treated with EGCG prior to EAC transplantation, was highly intact and similar to the normal pattern (fig. 4).
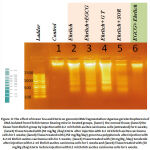 |
Figure 4: The effect of Green Tea and EGCG on genomic DNA fragmentation: Agarose gel electrophoresis of DNA isolated from Ehrlich tumor bearing mice in treated groups. |
Discussion
The processes of tumorgenesis, and even the chemotherapy are associated with release of a number of elevated reactive oxygen species (ROS) and (RNS) in an affected area which activates signal transduction cascades and changes transcription factors and mediates the biological reactions of cell stress. The generated metabolites connected to oxidative stress cause damage to biological molecules including protein, lipids and nucleic acids. The degradation of genomic DNA into nucleosomal fragments was considered the hall marks of mutated unstable cells[26]and by time it becomes tumor 27, 28. The use of anticancer drugs is no longer the best solution to overcome cancer since multiple side effects cannot be ignored as well as its slow-term effect could also lead to its return 29.
Here, the present study designed to investigate whether a preference exist between the use of EGCG as chemotherapeutic and protective agent in suppressing the tumor. Our choice to use Ehrlich Ascites Carcinomas EAC since it proliferates and stimulates the oxidative stress in mice 30, 31 and hens elevates its proliferation32.
Our results obviously confirmed two main results; firstly, the treatment with green tea or EGCG offered chemotherapeutic effect against cell proliferation and that when EGCG administered in EAC- bearing mice, it significantly reduced the volume of tumor and tumor markers in blood such as TNF-α, GH and LH and diminished the inflammatory markers including α-fetoprotein, IL-6 and IL-8 which are considered as tumor marker for hepatic cell carcinomas 33. This effect has been clearly attributed to its antioxidant activities 34, 35 which confirmed its ability to scavenge a wide range of free radicals. Secondly, Supplementation of EGCG prior EAC transplantation, the anti-inflammatory, antioxidant effects were apparently improved and kept the DNA integrity (as shown by DNA fragmentation assay) and this helped in suppressing the tumor proliferation. These results revealed that EGCG can influence tumor suppressors and inhibit cellular proliferation, interfering in this way with the steps of carcinogenesis. The anticancer and anti-inflammatory properties of EGCG may be due to the antioxidant effect of hydroxyl groups which are the main functional constituent of catechins, the family to which epigallo-catechin-gallate EGCG belongs, which can neutralize the reactive oxygen and nitrogen reactive species 36. The integrity of genomic DNA maintained in orally administrated EGCG (50 mg/kg/b.wt.) is proposed to be due to its interfere in initiation, development and progression carcinogenesis by modulating the critical processes of cellular proliferation, differentiation, apoptosis, angiogenesis and metastasis 37. The use of green tea or its component EGCG as a traditional treatment is recommended to increase the benefits as a natural therapeutic and preventive agent for tumor proliferation.
Conclusion
The administration of green tea and its major constituent EGCG showed a significant a potent protective role in suppressing tumor proliferation than its use in treatment due to its antioxidant and anti-inflammatory effect and maintaining the integrity of underlying genomic DNA that make it a strong barrier which arrest the process of oncogenesis.
Acknowledgment
We acknowledged all individuals included in our research.
Conflict of interests
No conflict of interest.
Funding Source
No funding source.
References
- Patel, L. R., M. Nykter (2013): “Cancer genome sequencing: understanding malignancy as a disease of the genome, its conformation, and its evolution.” Cancer Lett 340(2): 152-160.
CrossRef - MohdSahardi, N. F. N., Makpol, S. (2019): Ginger (zingiberofficinale roscoe) in the prevention of ageing and degenerative diseases: Review of current evidence. Evidence-Based Complementary and Alternative Medicine, 2019.
CrossRef - Bassam Hassan (2020):Medicinal Plants – Use in Prevention and Treatment of Diseases, chapter: Plants and Cancer Treatment, DOI: 10.5772/intechopen.90568,intechopen.com.
CrossRef - Wang, W., M. X. Xia (2016): “Gene Expression Characteristics and Regulation Mechanisms of Superoxide Dismutase and Its Physiological Roles in Plants under Stress.” Biochemistry (Mosc) 81(5): 465-480.
CrossRef - Xiao, B. H., M. Shi, (2016): “Glutathione Peroxidase Level in Patients with Vitiligo: A Meta-Analysis.” Biomed Res Int 2016: 3029810.
CrossRef - Anju, A., J. Jeswin, (2013): “Molecular cloning, characterization and expression analysis of cytoplasmic Cu/Zn-superoxide dismutase (SOD) from pearl oyster Pinctadafucata.” Fish Shellfish Immunol 34(3): 946-950.
CrossRef - El-Sayed. A.S.A., Ali, G.S. 2020. Aspergillus flavipes is a novel efficient biocontrol agent of Phytophthora parasitica. Biological Control. 140:104072.
CrossRef - Choudhari, A. S., Mandave, P. C., Deshpande, M., Ranjekar, P., &Prakash, O. (2020): Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Frontiers in pharmacology, 10, 1614.
CrossRef - Lepley DM, Li B, Birt DF, et al. (1996): The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 17(11):2367–2375
CrossRef - El-Sayed A.S.A, Abdel-Azeim S, Ibrahim H.M., Yassin M.A., Abdel-Ghany S.E., Esener S.,Ali G.S. 2015: Biochemical stability and molecular dynamic characterization of Aspergillus fumigatus cystathionine γ-lyase in response to various reaction effectors. Enzyme and Microbial Technology 81: 31-46.
CrossRef - Lawal, R. A., Ozaslan, M. D., and Odesanmi, O. S., (2012): CytotCytotoxic an antiproliferative activity of Securidacalongepedunculata aqueous extract on Ehrlich ascites carcinoma cells in Swiss albino miceoxic an antiprdoliferative activity of Securidacalongepedunculata aqueous extract on Ehrlich ascites carcinoma cells in Swiss albino mice.
CrossRef - Warren Strober 2019: Trypan Blue Exclusion Test of Cell Viability, CurrProtoc Immunol.; 111: A3.B.1–A3.B.3.
CrossRef - Adhami, V. M., Malik, A., and Zaman, N. (2007): Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clinical Cancer Research, 13(5), 1611-1619.
CrossRef - Gu, J. W., Makey, K. L., Tucker, K. B., Chinchar, E., Mao, X., Pei, I., …&Miele, L. (2013): EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vascular cell, 5, 9.
CrossRef - El-Sayed, A.S.A., Hassan, A.E.A., Shindia, A.A., Mohamed, S.G., Sitohy, M.Z. (2016) Aspergillus flavipes L-methionine γ-lyase dextran conjugates with enhanced structural proteolytic stability and anticancer efficiency. J.Molec. Catalysis: B-enzymatic, 133:S15-S24.
CrossRef - Nishikimi, M.; Roe, N.A. and Yogi, K. (1972): The occurrence of superoxide anion in the action of reduced phenazinemethosulphate and molecular oxygen. Biochem.Biophys. Res. Commun.,46: 849-854.
CrossRef - Tietze, F. (1969): Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal biochem, 27(3), 502-522.
CrossRef - Dolai, N., Karmakar, I., and Kumar, R. S., (2012): Evaluation of antitumor activity and in vivo antioxidant status of Anthocephaluscadamba on Ehrlich ascites carcinoma treated mice. Journal of ethnopharmacology, 142(3), 865-870.
CrossRef - Swardfager, W., Lanctôt, K., and Rothenburg, L. (2010): Tumor necrosis factor alpha. Biol Psychiatry, 68, 930-41.
CrossRef - Maiolini R., Masseyeff R. (1975):A sandwich method of enzymoimmunoassay. I. application to rat and human alpha-fetoprotein. Immunol. Meth. 8, 223.
CrossRef
- FischerD G., NovickD, OrchanskyP, RubinsteinM (1988):Two molecular forms of the human interferon-gamma receptor. Ligand binding, internalization, and down-regulation. J Biol Chem Actions, Feb 25;263(6):2632-7.
CrossRef
- Hibi, M., Nakajima, K., & Hirano, T. (1996): IL-6 cytokine family and signal transduction: a model of the cytokine system. Journal of molecular medicine, 74(1), 1-12.
CrossRef - El-Sayed, A.S.A., Laura E. Luff, Salah E. Abdel-Ghany, Gul Shad Ali, Esener, S. (2017) Molecular and spectroscopic characterization of Aspergillus flavipes and Pseudomonas putida L-methionine γ-lyase in vitro. Applied Biochemistry and Biotechnology, 181:1513–1532.
CrossRef - Kuo JH, Jan MS, Jeng J, Chiu HW (2005). Induction of apoptosis in macrophages by air oxidation of dioleoylphosphatidylglycerol, J Control Release, 108: 442–452.
CrossRef
- Gothwal, R.K., Nigam, V.K., Mohan, M.K.,Sasmal, D., Ghosh, P. (2007). Extraction of bulk DNA from Thar Desert soils for optimization of PCR-DGGE based microbial community analysis. Environmental Biotechnology, Vol. 10 No. 3, Issue of July15.
CrossRef
- Nagata, S. (2000): Apoptotic DNA fragmentation. Experimental cell research, 256(1), 12-18.
CrossRef - Khan, A, El-Sayed, A.S.A., Mangravita–Novo, A., Bibi, S., Afzal, Z., Norman, D.J., Ali, G.S (2017): A highly efficient ligation-independent cloning system for CRISPR/Cas9 based genome editing in plants. Plant Methods 13:86 (1-13).
CrossRef - Han K.H. et al. (2016): “Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes.” World J Gastroenterol 22(1): 37-49.
CrossRef - Petrelli, A., and Valabrega, G. (2009): Multitarget drugs: the present and the future of cancer therapy. Expert opinion on pharmacotherapy, 10(4), 589-600.
CrossRef - Gupta, M., Mazumder, U. K., Kumar, R. S. (2004): Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice. ActaPharmacologicaSinica, 25, 1070-1076.
- Zhao, L., Liu, S., Xu, J., Li, W., Duan, G., Wang, H., & Zhou, R. (2017): A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2.
CrossRef - Altun, S., and Ozalpan, A. (2004): Interactive regeneration of liver and growth of Ehrlich ascites tumor in mice. BIOLOGIA-BRATISLAVA-, 59(3), 375-382
- El-Sayed, A.S.A., Iqrar, I., Ali, R., Norman, D., Brennan, M., Ali, G.S. (2018): A glucanolytic Pseudomonas associated with Smilax bona-nox L. displays strong activity against Phytophthora parasitica. Microbiological Research 207: 140-152.
CrossRef - Xu Y, Ho CT, Amin SG, et al. (1992): Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 52(14):3875–3879.
- Shimizu M, Sakai H, Shirakami Y. (2011): Preventive effects of (-)-epigallocatechingallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db Mice. Cancer Prev Res (Phila).4(3):396–403.
CrossRef - Musial, C., Kuban-Jankowska, A., &Gorska-Ponikowska, M. (2020): Beneficial Properties of Green Tea Catechins.International Journal of Molecular Sciences, 21(5), 1744.
CrossRef - Sohail Hussain, Mohammad Ashafaq (2018): Epigallocatechin-3-Gallate (EGCG): mechanisms, perspectives and clinical applications in cervical cancer, J Cancer PrevCurr Res. 9(4):178‒182.
CrossRef