Ahmad Rohi Ghazali1 , Elancheleyen Mahindran1
, Elancheleyen Mahindran1 , Anand Ramalingam1
, Anand Ramalingam1 , Liya Chee1
, Liya Chee1 and Satirah Zainalabidin1*
and Satirah Zainalabidin1*
Department of Biomedical Science, Centerfor Toxicology and Health Risk Studies (CORE), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia.
Corresponding Author E-mail: satirah@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2164
Abstract
Prolonged nicotine exposureescalates the onset and development of cardiovascular diseasesin both active and passive smokers via cardiac injury. Pterostilbene, a resveratrol derivative, has been shown to exhibit high anti-inflammatory,antioxidant and antitumor properties. Nevertheless, its role as a cardioprotective agent in a nicotine-induced rat model is still scarce. Therefore, our study was aimed to investigatethe effects of co-administered pterostilbene against nicotine-induced cardiac injury rat model.Twenty-six male Sprague-Dawley rats were randomly allotted and treated with nicotine (0.6 mg/kg)orin-combination with pterostilbene (10 mg/kg) for 28 consecutive days. Non-invasive tail cuff blood pressure measurements were taken atday-0, day-14 and day-28. Rat hearts were harvested at study endpoint and thechanges in cardiac function parameters and oxidative stress markers were evaluated. The findings have shown that pterostilbene co-administration significantly (P<0.05) reduced the blood pressure and ameliorated nicotine-induced cardiac systolic dysfunction by improving the left ventricular developed pressure (LVDP). In addition, pterostilbene also significantly (P <0.05)attenuatedthe thiobarbituric acid reactive substances (TBARS) level, indicative of protection against nicotine-induced cardiac oxidative stress. In summary, our findings suggest that pterostilbene has the potential to be developed as a natural alternative in protecting the cardiac injuryinduced by nicotine. However further studies are warranted to investigate its efficacy and the underlying mechanism in cardioprotection.
Keywords
Antioxidant; Cardiac dysfunction; Nicotine; Oxidative stress; Pterostilbene
Download this article as:| Copy the following to cite this article: Ghazali A. R. Mahindran E. Ramalingam A. Chee L, Zainalabidin S. Protective Effects of Pterostilbene Against Cardiac Oxidative Stress and Dysfunctionin Nicotine-Induced Cardiac Injury Rat Model. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Ghazali A. R. Mahindran E. Ramalingam A. Chee L, Zainalabidin S. Protective Effects of Pterostilbene Against Cardiac Oxidative Stress and Dysfunctionin Nicotine-Induced Cardiac Injury Rat Model. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3Aa5Ghf |
Introduction
Smoking is one of themain risk factorscontributing to chronic diseases, such as cardiovascular diseases and cancer. According to World Health Organization in 2020, tobacco use primarily cigarette smoking is one of the biggest public health threats in world history, with more than 8 million deaths reported annually worldwide1.A cigarette is made using tobacco leaves, which usually contain nicotine. Prolonged nicotine exposurecan escalate the onset and development of cardiovascular diseases in both active and passive smokers via cardiac injury2.
Nicotine isknown to induce oxidative stress which increases reactive oxygen species (ROS) and therefore lipid peroxidation3. Oxidative stress causes cardiac dysfunction through the amelioration of mitochondrial respiration that attenuates ATP production and necrosis4. Besides that, nicotine triggers sympathetic stimulation which further increase the heart rate and vasoconstriction, thus elevating the peripheral resistance and blood pressure5,6.Subsequently, hypertension leads to left ventricular hypertrophy and cardiac dysfunction7. Several animal studies have shownthat prolonged nicotine as long as 28 consecutive days of administrationwas capable of causing cardiac dysfunction in rat model8,9.
There has been an abundance of studies that investigatesthe potential use of natural products as therapeutic agents against the nicotine-induced cardiac dysfunction. Recently, stilbenes have attracted the interest of the public due to their various beneficial health effects such as anti-inflammatory, anticancer, antioxidant, and anti-dyslipidemia activities10.An example of stilbene is pterostilbene(Figure 1B), which is an analogue of resveratrol (Figure 1A)11. Pterostilbene is a naturally occurring polyphenol compounds that can be found in antioxidant-rich foods like grape wine and blueberries12,13.It has been suggested to have greater bioavailability due to the presence of two methoxy groups (Figure 1B)14.
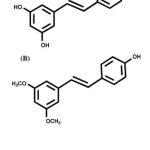 |
Figure 1: (A) Chemical structure of resveratrol (B) Chemical structure of pterostilbene |
Pterostilbene has previously been shown to reduce cardiac oxidative stress in several types of cardiovascular diseases, including myocardial infarction15, diabetic heart disease16,17 and acute doxorubicin-induced cardiotoxicity18. Nevertheless, its role as cardioprotective agent in a nicotine-induced rat model has not yet been explored. Hence, this study was carried out to examine the effects of co-administered pterostilbene on the blood pressure, cardiac function as well ascardiac oxidative stress in rat model of prolonged nicotine administration.
Materials and Methods
Ethics of Animal Experimentation: All experiments reported in adherence with the UKM Animal Ethics Committee (UKMAEC)guidelines (Approval number: FSK/2019/SATIRAH/25-SEPT./1041-OCT.-2019-OCT.-2020).Adultmale Sprague-Dawley rats (200-250g) were acquired from UKM Laboratory Animal Resource Unit (LARU), Faculty of Medicine. All rats underwent acclimatizationand housed in the standard laboratory conditions (ambient 25 ± 3°C, 12 h day/night cycle). The rats were fed with standard rodent pellet and tap water ad libtium.
Study design: After one week of acclimatization, 26 rats were randomly assignedinto three groups (n=8/9): control, nicotine (NIC) and pterostilbene+nicotine (PTS+NIC). Rats from NIC group and PTS+NIC group received 0.6 mg/kg of nicotine (i.p.) (TokyoChemical Industry, Japan) dissolved in normal saline as previously described9. Rats from PTS+NIC group received 10 mg/kg of pterostilbene (J&K Scientific Ltd., China) dissolved in 10% DMSO (i.p.)19, and after 5 minutes, nicotine was administered. As pterostilbene was administered in 10 % DMSO, NIC group rats were also given 10 % DMSO (i.p.). Vehicle control rats were given normal saline and 10 % DMSO vehicle (i.p.). All animals were treatedfor 28 consecutive days prior to sacrifice and tissues collection. Body weight, food and water consumption were also recorded daily during the experimental period.
Blood pressure measurement: Blood pressure measurements were takenat day-0, day-14 and day-28on conscious rats by using CODATM non-invasive blood pressure system (Kent Scientific, USA)20. All rats were accustomed to the CODATMblood pressure system for three consecutive days prior to blood pressurebaselines for the purpose of animal acclimatization to the procedure. The variables measured on non-invasive tail cuff apparatus were systolic blood pressure (SBP), diastolic blood pressure (DBP) as well as mean arterial pressure (MAP).
Langendorff heart perfusion ex vivo
On the 29th day, rat hearts were isolated for the use of Langendorff heart perfusion to study the cardiac function. Before that, heparin (500 unit/kg, i.p.)were injected into the rats to prevent blood agglutination, and followed by KTX (1ml/kg,i.p.) for anaesthesia21. After which the rats have become unconscious and lost their pedal reflex activity, the rats’ hearts were rapidly excised by performing thoracic surgery and taken out to beimmersed in ice-cold Krebs-Henseleit buffersolutionbefore immediately cannulating them to Langendorff isolated heart system (ADInstruments, Australia)via aorta22. The rat heartsunderwent retrograde perfusion at constant pressure mode of~40–60 mmHg, with Krebs-Henseleit buffer solution (in mM: NaCl 118.0; KCl 3.2; MgSO4 1.2; NaHCO3 25.0; KH2PO4 1.18; CaCl2 2.5; glucose 11.1, pH 7.4), which was constantlysupplied with 95 % O2 and 5 % CO2and maintained at 37 °C21. Perfusion pressure and coronary flow (CF) changes were continuously monitored using flow and pressure transducers. In order to measure pressure changes inside the left ventricle, a small and thinlatex balloon filled with waterwhich was attachedto pressure transducer (MLT844, ADInstrument, Australia) was placed into left ventricle (LV) by inserting it viathe bicuspid valve, allowing isovolumic contraction. Rat hearts were left to stabilize for 20 minutesunder continuousflow.Hearts that showed poor function (e.g., CF <10 ml/minandheart rate <70 beats/min) during equilibration were excluded from the study22.After the stabilization period, data of left ventricular developed pressure (LVDP), left ventricular maximumcontraction rate(LV +dP/dtmax) and relaxation rate (LV –dP/dtmax) as well as the isovolumic relaxation time constant (Tau) were collectedusing PowerLab data acquisition system and evaluatedwith LabChart 8.0 (ADInstrument, Australia).The amount of perfusate that flow out from coronary collected in one minute was recorded as the rate of coronary flow9.
Tissues collection
Heart tissue was collected and weighed after Langendorff analysis. The heart was then excised, and a portion of the left ventricle (LV) was cut for analysis of oxidative stress markers. Hind leg was removed to measure tibia length to normalize heart and other organ weights23. The LV tissue was homogenized in cold 0.01 M Tris-HCl buffer20. Then, supernatant was collected from centrifugation (12,000 rpm, 4°C, 30 minutes) are used for oxidative stress markers analysis.
Assessment of oxidative stress indicators
The indicator of oxidative stress such as TBARS(thiobarbituric acid reactive substances) and GSH were measured using colorimetric assay method. According to Stock and Dormandy (1971), malondialdehyde, an indication of lipid peroxidationwas estimated by concentration of TBARSin LV tissue24.The TBARS concentration in the sample was measured spectrophotometrically at 532mm based on standard curve produced using 1,1,3,4-tetraethoxypropane. Meanwhile, reduced glutathione (GSH)was measured using Ellman assay as previously described25.The GSH level in the LV tissue was measured spectrophotometrically at 415 nmbased on standard curve produced using GSH.
Statistical analysis
The data are portrayed as mean ± standard error of mean (SEM).Graph Pad Prism 8.3 was used as a tool for statistical analysis. Comparisons between groups were performed using one-way or two-way analysis of variance (ANOVA) and subsequentlya Tukey’spost-hoc test unless stated otherwise, where P <0.05 was considered as statistically significant.
Results
Pterostilbene significantly reducedbody weight gain and total water in takein 28 days when compared to control group (both P<0.05; Table 1). In contrary, nicotine administration had no significant effects (P>0.05) on the body weight gain, total food and water intake.
Table 1: Body weight gain, total food and water intake in all experimental groups.
| Parameters | Control | NIC | PTS+NIC |
| Body weight gain (g) | 83.7 ± 9.87 | 70.4 ± 8.21 | 53.7 ± 5.98* |
| Total food intake (g) | 634.3 ± 40.45 | 691.4 ± 66 | 543.4 ± 8.42 |
| Total water intake (ml) | 1668 ± 77.02 | 1526 ± 46.81 | 1362 ± 44.78* |
Note: All values are portrayed as mean ± SEM (n=8-9 per group).
*significant (P<0.05) relative to control group, using one-way ANOVA with Tukey post-hoc test
Table 2 shows the postmortem systemic analysis of rat’s organ weight. After 28 days of treatment, neither administration nicotine nor pterostilbene significantly changed the organ weight of rats. Relative organ weight was also unaltered in each experimental group at end point.
Table 2: Postmortem systemic analysis of rat’s organ weight.
| Parameters | Control | NIC | PTS+NIC |
| Heart weight (mg) | 947.1 ± 35.15 | 899.3 ± 50.96 | 941.9 ± 21.65 |
| Atria (mg) | 41.4 ± 4.35 | 49.4 ± 3.63 | 46.1 ± 3.55 |
| Right ventricle (mg) | 170.2 ± 10.99 | 153.4 ± 13.70 | 175.2 ± 6.20 |
| Left ventricle (mg) | 647.8 ± 19.73 | 611.3 ± 34.58 | 638.1 ± 15.78 |
| Lung weight (mg) | 1546 ± 84.58 | 1349 ± 113.90 | 1339 ± 68.86 |
| Liver weight (mg) | 10196 ± 466.20 | 8928 ± 471.20 | 8760 ± 340.50 |
| Right kidney (mg) | 924.0 ± 32.94 | 873.8 ± 45.07 | 824.6 ± 30.38 |
| Left kidney (mg) | 930.0 ± 39.44 | 895.8 ± 38.26 | 847.7 ± 32.42 |
| Tibia Length (mm) | 40.4 ± 0.71 | 38.5 ± 1.00 | 39.6 ± 0.41 |
| HW: TL (mg/mm) | 23.4 ± 0.71 | 23.3 ± 0.95 | 23.8 ± 0.40 |
| Atria: TL (mg/mm) | 1.0 ± 0.11 | 1.3 ± 0.11 | 1.2 ± 0.10 |
| RV: TL (mg/mm) | 4.2 ± 0.25 | 4.0 ± 0.30 | 4.4 ± 0.13 |
| LV: TL (mg/mm) | 16.0 ± 0.37 | 15.8 ± 0.65 | 16.1 ± 0.27 |
| LW: TL (mg/mm) | 38.4 ± 2.22 | 34.9 ± 2.59 | 33.8 ± 1.51 |
| Liver: TL (mg/mm) | 252.2 ± 11.10 | 231.8 ± 10.22 | 221.1 ± 7.04 |
| LK: TL (mg/mm) | 22.8 ± 0.59 | 22.6 ± 0.83 | 20.8 ± 0.63 |
| RK: TL (mg/mm) | 23.0 ± 0.76 | 23.3 ± 0.75 | 21.4 ± 0.73 |
Note: All values are portrayed as mean ± SEM (n=8-9 per group).
Abbreviations: LV, left ventricle; RV, right ventricle; HW, heart weight; LW, lung weight; LK, left kidney; RK, right kidney; TL, tibia length.
At day-28, nicotine group exhibited no significant differences (P>0.05) in all the blood pressure parameter when compared to the control rats(Figure2A-C). However, the SBP, DBPand MAPwere significant decreased (P<0.05)in PTS+NIC group as compared to the nicotine group (Figure 2A-C).
#P<0.05, ##P <0.01 for NIC vs. PTS+NIC group
*P<0.1 for control vs. PTS+NIC group
Cardiac function was determined ex vivo using Langendorff apparatus. Nicotine group tended to increase the heart rate compared to the vehicle control groups (P=0.3817; Figure 3A). However, the heart rate in PTS+NICvs. nicotine group was significantly reduced (P<0.01) (Figure 3A). As shown in Figure 3B, coronary flow for all experimental groups was not altered in the perfused hearts . On Langendorff analysis, nicotine ratsshowed a tendency of deterioration in cardiac systolic function parameters which are left ventricular developed pressure (LVDP) (P=0.0987) and maximal contraction rate (LV +dP/dtmax) (P=0.2701) as well as impairment of diastolic function such as maximal relaxation rate of the left ventricle (LV–dP/dtmax) (P=0.0788) and isovolumic relaxation time constant (Tau) (P=0.2674) (Figure 3C-F).Co-administration pterostilbene attenuated the nicotine-induced cardiac systolic dysfunction significantly (P<0.05) in LVDP but LV +dP/dtmax only exhibited increasing trend compared to the nicotine group (Figure 3C-D). Meanwhile, cardiac diastolic function parameters such as LV –dP/dtmax and Tau in PTS+NIC group tended to improve (P=0.1175 and P=0.1159 respectively) compared to nicotine group (Figure 3E-F).
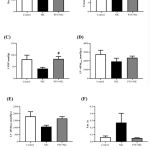 |
Figure 3: Cardiac function parameters such as(A) heart rate, (B) coronary flow, (C) LVDP, (D) LV +dP/dtmax, (E) LV –dP/dtmax and (F) Tau of experimental rats by group(n=4-7/group). |
All values are portrayed as mean ± SEM,one-way ANOVAwith Tukey post-hoc test was used. #P<0.05, ##P <0.01 for NIC vs. PTS+NIC group
Abbreviations: LVDP, left ventricular developed pressure; LV +dP/dtmax, maximal rate of left ventricular contraction; LV–dP/dtmax, maximal rate of left ventricular relaxation; Tau, isovolumic relaxation time constant
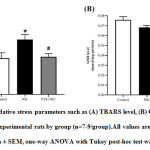
Figure 4 shows the analysis of oxidative stress markers in LV tissues of experimental rats by group.After 28 days,TBARS level was significantly increased in nicotine-administered rats (P<0.05;Figure 4A). Pterostilbene supplementation significantly attenuated the increment in TBARS level (P <0.05; Figure 4A). GSH level was shown statistically non-significant reduction in nicotine group (P=0.1845; Figure 4B). Whereas PTS+NIC group was shown no significant differencesin GSH level compared to nicotine group (P>0.05; Figure 4B).
*P<0.05 for control vs. NIC group
#P<0.05 for NIC vs. PTS+NIC group
Discussion
In this study, we have shownpreliminary findingsthat pterostilbene supplementation attenuates nicotine-induced cardiac dysfunction andoxidative stress. Based on our previous pilot study, we have shown that nicotine administration at the dose of 0.6 mg/kg intraperitoneally for consecutive 28 days was capable of causing cardiac dysfunction in rats9. Pterostilbene dosage was chosen based on previous studies where the dose chosen can reduce the cardiac oxidative stress in animal model of acute doxorubicin-induced cardiotoxicity18.
Smoking can cause weight loss as nicotine can stimulate leptin that can suppress appetite26. However, in our study, nicotine treatment had no effects on body weight gain and food intake. Our findings are in consistence with our previousresearch which have demonstrated that nicotine (0.6 mg/kg/day) did not affect the body weight increment27. On the other hand,a study employing a higher dose of nicotine (2mg/kg)in the same duration of 28 days was found to significantly reduce the body weight gain28. The discrepancy is probably due to the low dose of nicotine used in our study that did not alter the rat’s food intake as well as body weight gain. Rats treated with pterostilbeneshowed a remarkablereduction in body weight gainin comparison ofthe control group. Our resultisconsistent with the past studywhich reported that pterostilbene was able to decrease the body weight in fructose-diet diabetes rats16. Our results suggest that reduction in body weight gain may be due to the anti-obesity effect of pterostilbene29-31.
Nicotine is known to activate the sympathetic nervous system through nicotinic acetylcholine (nAChR) receptors and stimulates catecholamines production which could increase the blood pressure and heart rate5. In our present study, analysis of blood pressurerevealed that SBP, DBP and MAPin nicotineratstended to increase through the 28-day induction. It could be due to the activation of compensatory mechanism involving baroreflex to counteract the increase in blood pressure due to the induction of nicotine and this mechanism was supported by Oakes et al.32. On the other hand, the SBP, DBP and MAP in PTS+NIC group decreased as compared to the NIC group. The mechanisms underlying was unexplored in our study.The potential mechanisms involved maybe due to the activation of endothelium nitric oxide synthase (eNOS) phosphorylation by pterostilbene through P13K/Akt pathway,which then stimulatingnitric oxide production in vascular endothelial cells, thereby loweringthe blood pressure33. Future work should be warranted to determine the precise mechanism to further elucidate the role of pterostilbene in blood pressure lowering effect. Next,pterostilbene (given at high dose of 125 mg/kg twice a day) was reported in a clinical study to cause a low blood pressure and weight loss effect in hypercholesterolemia patients34.A clinical study demonstrated that weight loss can decrease blood pressure in a cohort of overweight patients35.Apart from the vasodilation effect of pterostilbene, the significant reduction in blood pressure as shown in PTS+NIC group could also be partially attributable to the decreased in body weight gain observed in PTS+NIC group.Next, the heart rate of nicotine-induced rats has shown an insignificant elevation. Pterostilbene has been manifested to reducethe heart rate in rats subjectedto nicotine. Our finding was consistent with previous studies16,36suggesting negative chronotropic effects of pterostilbene but its mechanisms involved are not yet fully understood.
Cardiac function was measured using the Langendorff apparatus where the perfusion pressure was calibrated to be similar among the experimental groups. Coronary flow was almost similar in all groups, suggesting that there was no vasodilation and ischemia22,37. Prior to the start of systolic dysfunction,one of the initial indications of cardiac dysfunction is the left ventricle (LV) diastolic dysfunction38. According to our results, LV diastolic dysfunction induced by nicotine was tended as indicated by increasedrelaxation time (Tau) together withdecreased ventricular relaxation rate (LV –dP/dtmax)in Langendorff-perfused rat hearts. These alterations indicate that the impaired LV relaxation wasprobably due to stiffness of the ventricular wall39. On the other hand, the tendency of reduction in LV-developed pressure (LVDP) and the LV +dP/dtmax further indicated the failing of LV contraction by nicotine reduced the function of the heart potassium (K+) channel in vitro study40. However, co-administration of pterostilbene suggested that the cardiac dysfunction progression was attenuated,similar to the previous studies in which pterostilbene was able to improve heart function by modulation of calcium (Ca2+) handling proteins15,41.Among the mechanisms of myocardium protection of pterostilbene against nicotine was the role of pterostilbene as an antioxidant. At the same time, a decrease in blood pressure in pterostilbene co-administered treatment with nicotine was also believed to reduce the cardiac dysfunction.The cardioprotective mechanism of pterostilbene may also be due to the blood pressure lowering effect of pterostilbene as depicted in our study which was believed to reduce the nicotine-induced cardiac dysfunction, since hypertension itself was a major key factor in cardiac dysfunction42. The reduction of cardiac dysfunction may not be significant due to low dose of pterostilbene as shown in an animal study using pterostilbene dose of 5 mg/kg per day for 60 days16.
Oxidative stress, which occurs as a consequence of an imbalanced ROS production and antioxidant status, is a majormechanism resultingin nicotine-induced cardiac dysfunctionrat model9,43. In the heart, mitochondria and NADPH oxidase (NOX) are the primarysources of ROS production44. Previously, we had shown that prolonged nicotine administration was able to cause myocardial oxidative stress evidenced by the increase of NOX2 gene expression and mitochondria ROS production after 28 days9. Increased mitochondria and NADPH-driven ROS generation can initiate lipid peroxidation in the heart; hence augmented TBARS level in the nicotine rats. Our observation issimilar to several previous studies demonstrated that nicotine administration for duration of 21~28 days could cause the increase level of lipid peroxidation in the heart8,27,45,46. Interestingly, co-administration with pterostilbene significantly attenuated the increase in TBARS level, suggesting the protective action against the cardiac oxidative damage caused by nicotine. The potential mechanisms involved maybe due to the ability of pterostilbene to activate the AMPK/Nrf2/HO-1 signaling pathway which can also increase the expression of antioxidant enzymes and subsequently inhibit oxidative stress16. Moreover, the ability of pterostilbene to activate another signaling pathways which is AMPK/SIRT1/PGC1α may also be among the other mechanisms contributing to the decrease in TBARS level18.
GSH is one of the endogenous antioxidants which plays an important role in scavenging H2O247. Excessive ROS production can deplete the endogenous antioxidant levels. In the present study, nicotine administration for 28 daysconsecutively showed a decreasing trend of the GSH level in the heart27,28.It was likely that sub-chronic administration of nicotine has not yet cause depletion of GSH level as GSH is the second line of defense in antioxidant defense system28. Other endogenous antioxidant such as superoxide dismutase (SOD) could quickly interact with the superoxide generation by nicotine27,48. Future work therefore is warranted to measure the activity of SOD enzymes to verify whether nicotine could inhibit SOD activity. For the PTS+NIC group, no significant changeswere shown in the GSH level as nicotine itself did not affect the GSH level in the rat model. The evidence of cardiac oxidative stress would be more accurate through immunohistochemistry studies that showing an increase in 3-nitrotryosine content in left ventricle9.
Conclusion
Our study demonstrated pterostilbene supplementation could reduce the deterioration of heart function by possibly acting as an antioxidant in the nicotine-induced cardiac injury rat model. Future studies are warranted to further investigate the protective mechanism of pterostilbene from cardiac injury caused by the oxidative stress.
Acknowledgment
We would like to acknowledge Facultyof Pharmacy, UKM for their Langendorff apparatus and would also like to thank Miss Shafreena Shaukat Ali, Mr. RajasegarAnamalleyand Miss NurellyaFaqhiraah Aziz for their technical support.
Conflict of Interest
We declare no conflict of interest in our paper.
Funding Source
We received funding from Universiti Kebangsaan Malaysia (Grant number: UKM-DIP-2018-034).
References
- WHO. “Tobacco.”[Online]. Available: http:// www. who. int/news-room/fact-sheets/detail/tobacco. Accessed May 27. 2020.
- Hu N and Ren J. Nicotine, cigarette smoking and cardiac function: an update. Toxicol Res, 2014; 3(1): 7-10.
CrossRef - Suleyman H, Gumustekin K, Taysi S, Keles S, Oztasan N, Aktas O, et al. Beneficial effects of Hippophae rhamnoides L. on nicotine induced oxidative stress in rat blood compared with vitamin E. Biol Pharm Bull, 2002; 25(9): 1133-1136.
CrossRef - Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, et al. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem, 2006; 281(44): 33789-33801.
CrossRef - Williams R J C. Heart rate and nicotine: A chronic problem. Int Heart Vasc Dis J, 2013; 1(1 (eng)): 18-24.
- Talukder M H, Johnson W M, Varadharaj S, Lian J, Kearns P N, El-Mahdy M A, et al. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol, 2011; 300(1): H388-H396.
CrossRef - Lip G. Hypertensive heart disease: a complex syndrome or a hypertensive cardiomyopathy? Eur Heart J, 2000; 21: 1653-1665.
CrossRef - Gumustekin K, Taysi S, Alp H H, Aktas O, Oztasan N, Akcay F, et al. Vitamin E and Hippophea rhamnoides L. extract reduce nicotine‐induced oxidative stress in rat heart. Cell Biochem Funct, 2010; 28(4): 329-333.
CrossRef - Ramalingam A, Budin S B, Fauzi N M, Ritchie R H and Zainalabidin S. Angiotensin II Type I Receptor Antagonism Attenuates Nicotine-Induced Cardiac Remodeling, Dysfunction, and Aggravation of Myocardial Ischemia-Reperfusion Injury in Rats. Front Pharmacol, 2019; 10: 1493.
CrossRef - Tsai H-Y, Ho C-T and Chen Y-K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J Food Drug Anal, 2017; 25(1): 134-147.
CrossRef - Akinwumi B C, Bordun K-A M and Anderson H D. Biological activities of stilbenoids. Int J Mol Sci, 2018; 19(3): 792.
CrossRef - Langcake P and Pryce R. A new class of phytoalexins from grapevines. Experientia, 1977; 33(2): 151-152.
CrossRef - Rimando A M, Kalt W, Magee J B, Dewey J and Ballington J R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem, 2004; 52(15): 4713-4719.
CrossRef - Wang P and Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors, 2018; 44(1): 16-25.
CrossRef - Lacerda D, Ortiz V, Türck P, Campos-Carraro C, Zimmer A, Teixeira R, et al. Stilbenoid pterostilbene complexed with cyclodextrin preserves left ventricular function after myocardial infarction in rats: possible involvement of thiol proteins and modulation of phosphorylated GSK-3β. Free Radic Res, 2018; 52(9): 988-999.
CrossRef - Kosuru R, Kandula V, Rai U, Prakash S, Xia Z and Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther, 2018; 32(2): 147-163.
CrossRef - Kosuru R, Cai Y, Kandula V, Yan D, Wang C, Zheng H, et al. AMPK contributes to cardioprotective effects of pterostilbene against myocardial ischemia-reperfusion injury in diabetic rats by suppressing cardiac oxidative stress and apoptosis. Cell Physiol Biochem, 2018; 46(4): 1381-1397.
CrossRef - Liu D, Ma Z, Xu L, Zhang X, Qiao S and Yuan J. PGC1α activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging (Albany NY), 2019; 11(22): 10061.
CrossRef - Tastekin B, Pelit A, Polat S, Tuli A, Sencar L, Alparslan M M, et al. Therapeutic Potential of Pterostilbene and Resveratrol on Biomechanic, Biochemical, and Histological Parameters in Streptozotocin-Induced Diabetic Rats. Evid Based Complement Alternat Med, 2018; 2018.
CrossRef - Si L Y-N, Ali S A M, Latip J, Fauzi N M, Budin S B and Zainalabidin S. Roselle is cardioprotective in diet-induced obesity rat model with myocardial infarction. Life Sci, 2017; 191: 157-165.
CrossRef - Lim Y-C, Budin S B, Othman F, Latip J and Zainalabidin S. Roselle polyphenols exert potent negative inotropic effects via modulation of intracellular calcium regulatory channels in isolated rat heart. Cardiovasc Toxicol, 2017; 17(3): 251-259.
CrossRef - Bell R M, Mocanu M M and Yellon D M. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol, 2011; 50(6): 940-950.
CrossRef - Huynh K, Kiriazis H, Du X-J, Love J, Jandeleit-Dahm K, Forbes J, et al. Coenzyme Q 10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia, 2012; 55(5): 1544-1553.
CrossRef - Stocks J and Dormandy T. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol, 1971; 20(1): 95-111.
CrossRef - Ellman G L. Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82(1): 70-77.
CrossRef - Audrain‐McGovern J and Benowitz N. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther, 2011; 90(1): 164-168.
CrossRef - Zainalabidin S, Shahidin S and Budin S B. Hibiscus sabdariffa Linn.(Roselle) protects against nicotine-induced heart damage in rats. Sains Malays, 2016; 45(2): 207-214.
- Joukar S, Shahouzehi B, Najafipour H, Gholamhoseinian A and Joukar F. Ameliorative effect of black tea on nicotine induced cardiovascular pathogenesis in rat. EXCLI J, 2012; 11: 309.
- Gomez-Zorita S, Fernández-Quintela A, Lasa A, Aguirre L, Rimando A M and Portillo M P. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J Agric Food Chem, 2014; 62(33): 8371-8378.
CrossRef - Nagao K, Jinnouchi T, Kai S and Yanagita T. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J Nutr Biochem, 2017; 43: 151-155.
CrossRef - Moreira C, Rodrigues A, Cordeiro L, Mario É, Rosa D and Botion L. Pterostilbene, a natural analogue of resveratrol, decreases hepatic lipid deposition by increasing triacylglycerol hydrolases activity (1116.6). FASEB J, 2014; 28(1_supplement): 1116.1116.
CrossRef - Oakes J M, Xu J, Morris T M, Fried N D, Pearson C S, Lobell T D, et al. Effects of Chronic Nicotine Inhalation on Systemic and Pulmonary Blood Pressure and Right Ventricular Remodeling in Mice. Hypertens, 2020; 75(5): 1305-1314.
CrossRef - Park S H, Jeong S-O, Chung H-T and Pae H-O. Pterostilbene, an active constituent of blueberries, stimulates nitric oxide production via activation of endothelial nitric oxide synthase in human umbilical vein endothelial cells. Plant Foods Hum Nutr, 2015; 70(3): 263-268.
CrossRef - Riche D M, Riche K D, Blackshear C T, McEwen C L, Sherman J J, Wofford M R, et al. Pterostilbene on metabolic parameters: a randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med, 2014; 2014.
CrossRef - Hinderliter A, Sherwood A, Gullette E C, Babyak M, Waugh R, Georgiades A, et al. Reduction of left ventricular hypertrophy after exercise and weight loss in overweight patients with mild hypertension. Arch Intern Med, 2002; 162(12): 1333-1339.
CrossRef - Nirwane A and Majumdar A. Resveratrol and pterostilbene ameliorate the metabolic derangements associated with smokeless tobacco in estrogen deficient female rats. J Funct Foods, 2016; 23: 261-277.
CrossRef - Sutherland F J and Hearse D J. The isolated blood and perfusion fluid perfused heart. Pharmacol Res, 2000; 41(6): 613-627.
CrossRef - Ellims A H, Iles L M, Ling L-h, Hare J L, Kaye D M and Taylor A J. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson, 2012; 14(1): 1-9.
CrossRef - Røe Å T, Aronsen J M, Skårdal K, Hamdani N, Linke W A, Danielsen H E, et al. Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovasc Res, 2017; 113(10): 1161-1172.
CrossRef - Wang H, Shi H and Wang Z. Nicotine depresses the functions of multiple cardiac potassium channels. Life Sci, 1999; 65(12): PL143-PL149.
CrossRef - Lacerda D, Türck P, Campos-Carraro C, Hickmann A, Ortiz V, Bianchi S, et al. Pterostilbene improves cardiac function in a rat model of right heart failure through modulation of calcium handling proteins and oxidative stress. Appl Physiol Nutr Metab, 2020; 45(9): 987-995.
CrossRef - Watanabe R, Suzuki J-i, Wakayama K, Kumagai H, Ikeda Y, Akazawa H, et al. Angiotensin II receptor blocker irbesartan attenuates cardiac dysfunction induced by myocardial infarction in the presence of renal failure. Hypertens Res, 2016; 39(4): 237-244.
CrossRef - Hu N, Guo R, Han X, Zhu B and Ren J. Cardiac-specific overexpression of metallothionein rescues nicotine-induced cardiac contractile dysfunction and interstitial fibrosis. Toxicol Lett, 2011; 202(1): 8-14.
CrossRef - Niemann B, Rohrbach S, Miller M R, Newby D E, Fuster V and Kovacic J C. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol, 2017; 70(2): 230-251.
CrossRef - Ramalingam A, Budin S B, Lim Y C, Si Y N L and Zainalabidin S. Dietary UKMR-1 roselle supplementation prevents nicotine-induced cardiac injury by inhibiting myocardial oxidative stress. Sains Malays, 2016; 45(7): 1131-1137.
- Şener G, Özer Şehirli A, İpçi Y, Çetinel Ş, Cikler E, Gedik N, et al. Taurine treatment protects against chronic nicotine‐induced oxidative changes. Fundam Clin Pharmacol, 2005; 19(2): 155-164.
CrossRef - Mavangira V and Sordillo L M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res Vet Sci, 2018; 116: 4-14.
CrossRef - Ighodaro O and Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med, 2018; 54(4): 287-293.
CrossRef