Prerna Tejaswi*, Kumar Devashish and Raj Ranjan Prasad
Department of Pharmacology, MD, DMC, Aryabhatta Knowledge University(AKU), Patna, India
Corresponding Author E-mail: prernatejaswi@yahoo.in
DOI : https://dx.doi.org/10.13005/bpj/2212
Abstract
The coronavirus pandemic is the worst health crisis of our time. There is a massive upsurge of the cases and no specific treatment options of this novel virus (SARS-CoV-2).Due to no time for research and development of a new drug or a vaccine, old and existing broad spectrum antiviral drugs were tried and tested. Favipiravir showed promising results in mild to moderate COVID-19 infection in small studies. The drug is approved with precaution under emergency use, as its safety profile is still not clear. We have considered every aspect of favipiravir and compiled all the latest information about the drug in this review article.
Keywords
COVID-19; Coronavirus; Favipiravir; SARS- CoV-2
Download this article as:| Copy the following to cite this article: Tejaswi P, Devashish K, Prasad R. R. Pharmacological effects of Favipiravir on coronavirus: An update. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Tejaswi P, Devashish K, Prasad R. R. Pharmacological effects of Favipiravir on coronavirus: An update. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3AHM7Nk |
Introduction
The world is facing the worst health crisis of our time. The Coronavirus Disease (COVID -19) has hit us hard. According to the current record, total incidence of COVID- 19 cases till 31-10-2020 was 45,921,794 and mortality was 1,193,912.1 The causative agent of this COVID- 19 infection is a new coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2, (SARS-CoV-2).2 New virus requires new therapeutic options but due to lack of time for research and development of a new drug, there is no choice but to test and treat the disease with existing medicines. Many broad spectrum antiviral drugs are trialled but there is hardly any drug which is found to be potent against coronavirus disease. Favipiravir is one drug which has shown encouraging results in an open label control study done on 80 patients in China.3 There was significant reduction in the duration of viral clearance.3 There are many ongoing randomized and nonrandomized controlled trials being conducted on favipiravirto evaluate its efficacy and safety against SARS-CoV-2.4 Presently, there are 40 clinical trials registered (clinicaltrail.gov) for evaluation of favipiravir in treatment of COVID19.4
The central drugs control organization approved the restricted emergency use of favipiravir tablets for mild to moderate COVID -19 infection based on the little evidence from small studies5. The permission to manufacture and market favipiravir tablets was given to Glenmark Pharmaceuticals by Drugs Controller General of India on 19.06.2020.5
Since the literature available on favipiravir is scant, we aim to fill this knowledge gap through this article.
History and Outbreak of Coronavirus
There are several theories regarding the origin of SARS-CoV-2. According to previous studies SARS-like coronavirus originated in humans from zoonotic reservoirs like bats, Himalayan palm civets and raccoon dogs in China.6-8Live and Wet Markets in Wuhan, China is believed to be the reason for the deadly outbreak of COVID-19 disease.9 Another suspected cause of origin of SARS-CoV-2 is laboratory leak of man manipulated virus.10However, based on current evidence, most credible postulation on origin of SARS-CoV-2 is natural selection of virus in an animal host followed by zoonotic transmission.10Clinical presentation of COVID-19 disease was similar to MERS COV epidemic of 2012, but unlike COVID-19 disease MERS- COV was limited to middle eastern countries only.11,12 In 1965, scientist ‘Tyrell &Bynoe’ found and isolated coronavirus (HCoVs) from respiratory tract of an adult suffering from common cold. The most distinguishing feature of the coronavirus seen under the electron microscope was its spikes, giving it a crown like appearance.11,13,14 Thus Tyrell and group of other virologists named it coronavirus in 1968.11,13,14,15
The disease coronavirus causes in human host and their cellular receptors are summarized in Table 1.15
Table 1: Corona viruses, hosts, diseases, and receptors.15
| Group | Virus | Host | Disease caused | Cellular receptor |
| I | 229E | Human | Respiratory infection | Human APN |
| NL-63 | Human | Respiratory infection | ACE2 | |
| II | OC43 | Human | Respiratory and enteric infection | Neu5,9Ac2-containing moiety |
| HKU1 | Human | Respiratory infection | ||
| SARS-C0V | Human | Severe acute respiratory syndrome | ACE2 |
The first human case of COVID -19 disease was reported in Wuhan, China in December 2019.16 The novel coronavirus causing this outbreak was later named SARS-COV-2.16 The number of cases reported in January 2020 was 9692 and by 31st October, 2020, it was raised to its 45,921,794.1,11 The common clinical manifestations found are of lower respiratory tract like pneumonia, dry cough, fever, dyspnea and diarrhoea.17The confirmed modes of transmission of SARS-CoV-2 infection is from person to person through contact or through larger respiratory droplets and smaller aerosols.17,18 Treatment of COVID-19 is based on the severity of the infection and thus it is classified from asymptomatic to critical cases (Table 2).19 COVID -19 has been classified from asymptomatic to critical disease.
 |
Table 2: The severity of SARS-CoV-2 infection with typical characteristics.19 |
Favipiravir
A favipiravir is structurally 6-fluoro-3hydroxy-2pyrazenecarboxamide and a broad spectrum antiviral drug showing in vitro activity against RNA viruses including influenza virus, respiratory syncytial virus and measles virus20. The chemical structure of favipiravir is shown Figure-1.21
Chemical substitution of pyrazine compound was found to show strong anti-influenza virus activity in vitro and in vivo.21 Favipiravir was discovered as an anti-influenza drug originally.
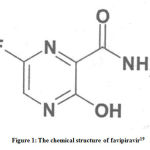 |
Figure 1: The chemical structure of favipiravir21 |
Pharmacodynamics and Mechanism of Action
The mechanism of action of Favipiravir is special as other marketed influenza drugs either inhibit entry or release of the virus . For replication of RNA viruses RNA dependent RNA polymerase (RdRp) is needed as it controls the rate of replication and mutation of the virus to adapt within the host surroundings.22Favipiravir is a prodrug which enters the infected cells through endocytosis and it is transformed to active metabolites favipiravirribofuranosyl triphosphate ( Favipiravir-RTP) through phosphorylation and phosphoribosylation.23-26This active favipiravir –RTP competes with purine nucleoside . It binds to and selectively blocks RdRp which in turn prevents viral transcription and replication.27 The disruption in viral RNA replication leads to increase number and frequency of translation mutations which replaces Guanine (G) by Adenine(A) and Cytosine (C) by Thymine(T) or Uracil (U) , thereby producing devastating mutagenesis in RNA viruses.19,27
Illustrative diagram of intracellular activation and mechanism of action of favipiravir is shown in Figure 2.27
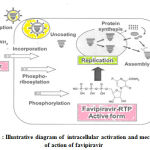 |
Figure 2 : Illustrative diagram of intracellular activation and mechanism of action of favipiravir |
By virtue of its selective inhibition of viral RdRp, favipiravirinhibits viral replications for many viruses asa broad antiviral spectrum.
Phamacokinetics
On oral administration of favipiravir is absorbed well, having bioavailability of 97.6% and plasma protein binding capacity of its 54%.26 The volume of distribution of favipiravir is 15-20 liters.26 Considered oral dose in adults on day 1 at 1600 mg twice, then 600mg twice daily for 4 days followed by 600 mg once daily for 1 day (1600-1day BID+600- 4days BID+ 600-1day once). The estimated AUC on day 1 and day 6 was 446.09 and 553.98 μghr/ml, Cmax was 64.56 and 64.69 μg/ml, tmax was 1.5 hours and t½ of 4.8 ± 1.1 hrs and 5.6 ± 2.3 hrs respectively. Favipiravir is metabolized in liver predominately by Aldehyde Oxidase(AO) and partly by Xanthine Oxidase(XO). It is eliminated by kidney in hydroxylated form26.
Indications of favipiravir
Favipiravir is found to be effective against the following RNA viruses.
Influenza
Favipiravir was discovered during chemical modification of a pyrazine analogue in cells showing anti-influenza virus activity in vitro.27 The drug is selective and powerful blocker of influenza viral RNA polymerase and works well against all strains of influenza virus and its subtypes. Favipiravir is also found to be effective against influenza viruses sensitive or resistant to neuraminidase and M2 inhibitors including Oseltamivir in vitro.27,28
COVID19
Many clinical trials with favipiravir are underway for testing its efficacy and safety on SARS-CoV-2. Few studies with favipiravir are shown in Table 3.29Now the drug is approved in several countries including India. In India, DCGI has allowed restricted emergency use in mild to moderate cases. Thus, currently the drug can only be prescribed after getting informed consent from the patient or their representative for emergency use in prescribed form. Recommended dose on Day 1 is 1800 mg or 9 tablets of 200 mg twice a day followed by 800mg or 4 tablets of 200mg twice a day for maximum 14 days.
Others
Favipiravir has also been promising in the treatment of Ebola and Norovirus infection.23,25
Table 3: Clinical trials involving Favipiravir29
| Study type | Trial outcome and design | Conclusion | Comment |
| Exploratory Randomized, Controlled Trial. 33
|
29 COVID-19 confirmed cases were randomized(1:1:1) to either receive Favipiravir for 14 days or BaloxavirMarboxil (80 mg once a day orally on Day 1and Day 4) or control group
All patients received existing antiviral treatment including lopinavir/ritonavir (400 mg/100 mg, bid, orally) or darunavir/ cobicistat (800 mg/150 mg, qd, orally) and arbidol (200 mg, tid, po.). All of them used in combination with interferon inhalation. On day 14, PCR was undetectable in all control group and 77% and 70 % in Baloxavir and Favipiravirgroups,respectively Furthermore, there was no significant differencebetween all groups in clinical improvement.One patient in the baloxavirmarboxil group, and twopatients in the favipiravir group transferred to ICUwithin seven days after trial initiation. Among all 29patients, there was no death. |
Findings do not support that adding either baloxavir or favipiravir under the trial dosages to the existing standard treatment benefit COVID-19 patients. | Small sample size, non-blinded trial Concurrent use of other antivirals leads to misinterpretation of results |
| Prospective, randomized, controlled, open-label multicenter trial.34 | 236 moderate/severe confirmed COVID-19 casesrandomized; 116 to receive Favipiravir for 10 days and120 to receive Umifenovir (Arbidol) for 10 days and all patients received conventional therapy.
Upon results, clinical improvement at day 7 (primaryend point), did not significantly different between two groups. Whereas, in post-hoc analysis for moderateCOVID-19 patients showed a significant higher clinical improvement in the Arbidol group (62/111, 55.86%) compared to Favipiravir group (70/98, 71.43%). Favipiravir led to shorter latencies to relief for both pyrexia and cough. Whereas, no significant differences were found between both groups in the rate of auxiliary oxygen therapy (AOT) ornon-invasive mechanical ventilation (NMV). |
Favipiravir, compared to Arbidol,
did not significantly improve the clinically recovery rate at Day 7.Favipiravir significantly improved the latency to relief for pyrexia and cough |
Number of severe and critically ill patients were more in favipiravir that undermine the benefit of Arbidol. |
| Open-Label control study.35
|
80 confirmed mild to moderate severity cases ofCOVID-19 were assigned to receive either Favipiravir(n = 35) or Lopinavir /ritonavir (n = 55) (control group)for 14 days and both groups received interferon (IFN)-aby aerosol inhalation. Favipiravir arm showed preferable outcomes compared to control arm, including shorter viral clearance [4 (2.5–9) d vs 11 (8–13) d, P < 0.001] and significant improvement in chest imaging(improvement rate of 91.43% vs 62.22%, P = 0.004). | Favipiravir showed significantly better treatment effects on COVID-19 in terms of disease progression and viral clearance. | Only mild to moderate cases were included in the study. |
| Randomized-controlled open-label interventional phase 3 clinical trial.35 | 100 COVID-19 patients were recruited.50 patients received favipiravir 3200 mg at day 1 followed by 600 mg twice (day 2–day 10). 50 patients received hydroxychloroquine 800 mg at day 1 followed by 200 mg twice (day 2–10) and oral oseltamivir 75 mg/12 h/day for 10 days.PCR negativity for SARS-CoV-2 was 8.1 and 8.3 days in HCQ-arm and favipiravir-arm respectively, difference was not statistically significant (p= 0.7),. Fever and dry cough were reported more frequently in favipiravir arm (36% and 38% respectively) than HCQ –based therapy (24% and 30% respectively) but this was not statistically significant (p > 0.05). In the favipiravir-arm 4 patients (8%) experienced elevated liver transaminases 3–5 times the upper normal limit between Day 7 and Day 14, but improvement was seen within 2 weeks after the end of therapy.In the HCQ-based arm there was one death due to an acute heart failure resulting from myocarditis on Day 8. | Favipiravir was found to be a safe and effective alternative to hydroxychloroquine and could be used safely during home isolation in mild to moderate cases.
|
Only mild to moderate patients were included in the study. |
Drug Interactions
As there is no specific potent drug for COVID-19 Disease, multiple therapies is unavoidable considering the complications, severity and pre-existing co-morbidities like cardiovascular disease, diabetes, bronchial asthma, hypertension and so on. Therefore, potential drug-drug interactions should be considered as drug is new in the market. So far few confirmed and significant drug interactions are known. Cytochrome P-450(CYP) does not metabolize favipiravir.30 Pyrazinamide when co-administered with favipiravir increases blood uric acid level as reabsorption of uric acid in renal tubules is increased.30Favipiravir with repaglinide increases the blood level of repaglinide by inhibition of CYPZC8.30When favipiravir is used along with theophylline, blood concentration of favipiravir is raised due to the interaction of theophylline with xanthine oxidase (XO).30 Efficacy of favipiravir and sulindac is reduced when co-administered with favipiravir due to inhibition of aldehyde oxidase (AO).30
Contraindications of favipiravir
Pregnancy
Favipiravir is found to be teratogenic in animal studies. Early embryonic death was seen in rats and teratogenicity was detected in mouse, rat, rabbit and monkey at doses similar or lower than clinical dose.30
Fertility
Histopathological changes in testis of rats and young dogs was observed after exposure to favipiravir.30 In rats, changes on the testis and sperm along with low fertility were reported. Thus, in general low fertility may cause due to changes in testis and sperm in male and markable anestrus in females at high doses.30
Severe Renal and hepatic impairment
Since favipiravir is heavily metabolized in liver and excreted in urine, it is contraindicated in severe hepatic and renal failure 30.
Gout and hyperuricemia
Favipiravir has shown to increaseblood uric acid levels which is reversed on drug discontinuation.31
Undesirable effects and adverse drug reactions
Favipiravir observational study in Japan conducted in 2,158 patients and subsequently reported the adverse effects on 24.65% of the subjects. The most prominent adverse events was hyperuricemia in 15.52 % of individuals followed by impaired liver function test(7.37%), rash (1.44%), diarrhoea (0.74%) and acute renal injury with elevated blood creatinine level was found in 0.74% of the patients treated with favipiravir32. Common adverse reactions of Favipiravir are summarized in Table 430.
Table 4: Adverse reactions observed in Japanese Clinical Studies and the Global Phase III clinical study 32
| Sl. No | reactions | ≥1% | 0.5-≤ 1% | <0.5% |
| 1 | Hepatic | SGOT increased
SGPT increased GPT increased |
Blood ALP increased
Blood billirubin increased
|
|
| 2 | Gastrointestinal | Diarrhoea (4.76%) | Nausea, Vomiting , Abdominal pain | Abdominal discomfort, Duodenal ulcer, Haematochezia, Gastritis |
| 3 | Haematologic | Neutrophil count decreased
WBC count decreased |
WBC count increased,
Reticulocyte count decreased, Monocyte increased |
|
| 4 | Metabolic disorders | Blood uric acid increased (4.79%),
Blood triglyceride increased
|
Glucose in urine present | Blood potassium decreased |
| 5 | Respiratory | Asthma,Oropharangeal pain,Rhititis,Nasopharyngitis | ||
| 6 | Others | Blood CK(CPK) increased, Blood in urine present, Tonsil polyp, Pigmentation, Dysgeusia, Bruise, Blurred vision, eye pain, vertigo,superaventicular extrasystole | ||
| 7 | Hypersensitivity | Rash | Eczema, Pruritus | |
|
· Given dose was lower than the approval dose |
||||
Conclusion
The COVID-19 disease does not seem to disappear anytime soon. Currently, a second wave of this pandemic has hit the European Union Countries. Scientists have predicted massive rise in infections this winter in India. Favipiravir has shown unique account and broad spectrum of antiviral activity against RNA viruses which has driven the researchers to carry out multiple clinical trials on it against the devastating SARS-CoV-2. It was found that favipiravir shortens the time for viral clearance and shows improvement in chest findings. It is considered to be relatively safe for short term use. However, we need more substantial evidence confirming its efficacy and safety. Favipiravir is the drug of choice at present for mild to moderate cases until a new efficient antiviral drug or vaccine is found for COVID-19. Health care providers must monitor and report any unfavourable outcomes related to its use on COVID-19 patients. Large scale studies are required for corroboration of existing data.
As there is continuous upsurge of cases and frequent changes in the treatment protocols physicians should be updated from time to time about new progress in treatment of COVID-19.
Acknowledgement
Authors would like to thank Dr Arvind Kumar for his valuable suggestions and assistance in the preparation of this manuscript.
Conflicts of Interest
None to declare.
Funding source
No funding resource was required for this review.
References
- https://www.worldometers.info/coronavirus/ Retrieved: Nov 03,2020
- Tang D, Comish P, Kang R (2020) The hallmarks of COVID-19 disease. PLoS Pathog 16(5): e1008536. https://doi.org/10.1371/journal.ppat.1008536
CrossRef - Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study [published online ahead of print, 2020 Mar 18]. Engineering (Beijing). 2020;10.1016/j.eng.2020.03.007. doi:10.1016/j.eng.2020.03.007
CrossRef - https://clinicaltrials.gov/ct2/results?cond=Covid19&term=favipiravir&cntry=&state=&city=&dist= Retrieved: Nov 03,2020
- https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NjIxMw== Retrieved: Nov 03,2020
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 Oct 28;310(5748):676-9. doi: 10.1126/science.1118391. Epub 2005 Sep 29. PMID: 16195424.
CrossRef - Graham RL, Baric RS. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84(7):3134-3146. doi:10.1128/JVI.01394-09
CrossRef - Guan Y, Zheng BJ, He YQ, et al. ISOLATION AND CHARACTERIZATION OF VIRUSES RELATED TO THE SARS CORONAVIRUS FROM ANIMALS IN SOUTHERN CHINA. In: Institute of Medicine (US) Forum on Microbial Threats; Knobler S, Mahmoud A, Lemon S, et al., editors. Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. Washington (DC): National Academies Press (US); 2004. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92471/
- Purohit D, Saini M, Pathak N, Verma R, Kaushik D, Katiyar P, Jalwal P, Pandey P. COVID-19 ‘The Pandemic’: An Update on the Present Status of the Outbreak and Possible Treatment Options. Biomed Pharmacol J 2020;13(3).
CrossRef - Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020 Mar 14;9(1):558-570. doi: 10.1080/22221751.2020.1736644. PMID: 32172672; PMCID: PMC7103735.
CrossRef - McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967;57(4):933-940. doi:10.1073/pnas.57.4.933
CrossRef - Witte KH, Tajima M, Easterday BC. Morphologic characteristics and nucleic acid type of transmissible gastroenteritis virus of pigs. Arch Gesamte Virusforsch. 1968;23(1):53-70. doi: 10.1007/BF01242114. PMID: 4300586.
CrossRef - Weiss R., and Navas-Martin, S.(2005) Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 69, 635–664
CrossRef - https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200423-sitrep-94-covid-19.pdf Retrieved: Nov 03,2020
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.
CrossRef - https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-how-is-covid-19-transmitted?gclid=EAIaIQobChMIl-PjnKDf7AIVCLeWCh2WTwjDEAAYASAAEgKll_D_BwE Retrieved: Nov 03,2020
- Baj J, Karakuła-Juchnowicz H, Teresiński G, et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J Clin Med. 2020;9(6):1753. Published 2020 Jun 5. doi:10.3390/jcm9061753
CrossRef - Jochmans D, van Nieuwkoop S, Smits SL, Neyts J, Fouchier RA, van den Hoogen BG , 2016. Antiviral activity of favipiravir (T-705) against a broad range of paramyxoviruses in vitro and against human metapneumovirus in hamsters. Antimicrob Agents Chemother60:4620–4629. doi:10.1128/AAC.00709-16.
CrossRef - Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005 Mar;49(3):981-6. doi: 10.1128/AAC.49.3.981-986.2005. PMID: 15728892; PMCID: PMC549233.
CrossRef - Teoh SL, Lim YH, Lai NM, Lee SWH. Directly Acting Antivirals for COVID-19: Where Do We Stand?. Front Microbiol. 2020;11:1857. Published 2020 Aug 5. doi:10.3389/fmicb.2020.01857
CrossRef - Wu R, Wang L, Kuo HD, et al. An Update on Current Therapeutic Drugs Treating COVID-19 [published online ahead of print, 2020 May 11]. Curr Pharmacol Rep. 2020;1-15. doi:10.1007/s40495-020-00216-7
CrossRef - Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446-454. doi:10.1016/j.antiviral.2013.09.015
CrossRef - De Clercq E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem Asian J. 2019 Nov 18;14(22):3962-3968. doi: 10.1002/asia.201900841. Epub 2019 Aug 7. PMID: 31389664; PMCID: PMC7159701.
CrossRef - Agam Vora, Mangesh Tiwaskar Chest Physician, Vora Clinic, Mumbai, Maharashtra; 2Physician and Diabetologist, Shilpa Medical Research Centre, Mumbai, Maharashtra Received: 02.07.2020; Accepted: 15.07.2020
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449-463. doi:10.2183/pjab.93.027
CrossRef - Moss RB, Steigbigel RT, Sanders RL, Fang F. Perspective: emerging challenges in the treatment of influenza and parainfluenza in transplant patients. Adv Virol. 2011;2011:910930. doi: 10.1155/2011/910930. Epub 2011 Jul 7. PMID: 22312357; PMCID: PMC3265318.
CrossRef - Jomah S, Asdaq SMB, Al-Yamani MJ. Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. J Infect Public Health. 2020 Sep;13(9):1187-1195. doi: 10.1016/j.jiph.2020.07.013. Epub 2020 Aug 3. PMID: 32773212; PMCID: PMC7396961.
CrossRef - Fabiflu (pakage insert). India: Glenmark Pharmaceuticals Ltd.: 2020
- Mishima E, Anzai N, Miyazaki M, Abe T. Uric Acid Elevation by Favipiravir, an Antiviral Drug. Tohoku J Exp Med. 2020 Jun;251(2):87-90. doi: 10.1620/tjem.251.87. PMID: 32536670.
CrossRef - http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200529.pdf Retrieved: Nov 03,2020
- https://www.businessinsider.in/science/health/news/there-are-five-companies-making-favipiravir-now-and-it-is-good-for-supply-and-prices/articleshow/77394485.cms Retrieved: Nov 03,2020
- https://www.financialexpress.com/lifestyle/health/covid-19-dr-reddys-launches-generic-favipiravir-at-rs-99-tablet-to-offer-free-home-delivery-in-42-cities/2060216/ Retrieved: Nov 03,2020
- Lou Y., Liu L., Qiu Y. Clinical outcomes and plasma concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. medRxiv. 2020
CrossRef - Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J. 2020. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. – DOI
CrossRef - Dabbous, H.M., El-Sayed, M.H., El Assal, G. et al. Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial. Sci Rep 11, 7282 (2021). https://doi.org/10.1038/s41598-021-85227-0
CrossRef







