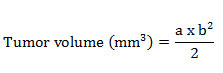Selase Ativui1* , Cynthia A. Danquah1
, Cynthia A. Danquah1 , Newman Osafo1
, Newman Osafo1 and Sammy Ka-Chungu2
and Sammy Ka-Chungu2
1Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, PMB, Kumasi, Ghana
2Department of Pathology, KomfoAnokye Teaching Hospital, P.O. Box 1932, Kumasi, Ghana.
Corresponding Author E-mail: montecelacy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2195
Abstract
Natural products and their bioactive constituents have been investigated for centuries and recognized as a source of valuable therapeutic candidates in the development of contemporary anticancer drugs. Triple negative breast cancers are a sub type of malignant cells formed in the breast tissue caused by uncontrolled and abnormal division. Palmatine, a naturally occurring alkaloid extracted from several medicinal plants in West Africa,has not been extensively investigated for its anti-breast cancer properties especially in triple-negative mammary carcinoma. The 4T1 triple-negative breast cancer cells were transplanted orthotopically into the mammary fat pad of the female balb/c mice. Tumor volume,tumor weight, histology and immunohistochemical analysis were carried out.After 28 days, palmatine (1, 5 and 10 mg/kg) in a dose-dependent manner decreased tumor volume (190.80 ±19.14, 25.40 ± 2.82, 14.20 ± 1.85), reduced tumor weight (1.035± 0.04, 0.8027± 0.01, 0.5090±0.04), inhibited tumor growth (31%, 46%, 66%) and protected against morphological dysplasia induced by the carcinoma (3.50 ± 0.29, 2.25 ± 0.25, 1.75 ± 0.25)respectively. Also, palmatine increased the activity of the tumor protein p53, cyclin-dependent kinase inhibitor 1 (p21) and mouse double minute 2(Mdm2) compared to the untreated carcinoma bearingmice. Overall, palmatine protected against triple negative mammary carcinoma and can be a valuable anticancer compound to treat breast diseases.
Keywords
Anti-Breast Cancer; Natural Compounds; Palmatine; Triplenegative
Download this article as:| Copy the following to cite this article: Ativui S, Danquah C. A, Osafo N, Ka-Chungu S. Palmatine Modulates Triple Negative Mammary Carcinoma by Regulating the Endogenous Function of P53, P21 and Mdm2. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Ativui S, Danquah C. A, Osafo N, Ka-Chungu S. Palmatine Modulates Triple Negative Mammary Carcinoma by Regulating the Endogenous Function of P53, P21 and Mdm2. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3w1uXqv |
Introduction
Breast cancer disease is characterized by the malignant growth of cells in the mammary gland. The triple negative sub types account for 10–20% of breast cancers diagnosed worldwide. This sub type has a negative expression for the estrogen receptor, progesterone receptor and human epidermal growth factor 21,2. Triple negative breast cancers (TNBC) present a great challenge for successful therapy. They are especially difficult to treat due to their aggressive phenotype, disease relapse and metastasis3.
Currently, Anthracycline (doxorubicin) chemotherapy is the main treatment strategy for triplenegative breast cancers 3,4 since they do not respond to hormonal therapies. Doxorubicin,however, is associated with poor tumor selectivity and severe side effects in healthy tissues such as cardiotoxicity5,6. Research to investigate lead compounds to aid the treatment of this diseaseis needed urgently.Consequently, natural compounds have been proposed as a valuable source for discovering new drugs for treating and preventing breast cancer7.
Palmatine, anisoquinoline alkaloid has been extracted from many medicinal plants used in Africa. This compound exhibits various pharmacological properties such as antiviral, antimalarial, antioxidant, anti-inflammatory and anticancer as reported by several studies8–11.The antiproliferative properties of palmatine investigated on several cancerous cells invitro shows much promise and deserve great attention12,13. Nevertheless, to the best of our knowledge,there is little report on the invivo activity of palmatine in triplenegative breast cancer.
In this study, 4T1 triple-negative breast cancer cells derived from the epithelial mammary gland of the female balb/c mice14 were used to investigate the anti-breast cancer properties of palmatine. Orthotopic transplantation of the highly tumorigenic/aggressive 4T1 triplenegative breast cancer cells into the mammary fat pad of Balb/c mice represents the most relevant preclinical model of human triple-negative breast cancer15–17.
This studyfurther investigated the activity of palmatine in preventing triplenegative mammary carcinoma throughthe activation of endogenous tumor suppressor genes. Inactivation of tumor suppressor genes such asTumor protein p53 greatly contributes to breast cancer formation and progression. Several clinical studies have reported deletion/mutationof the p53 gene in 80% of TNBC patients. This mutationprevents the recessive function of p53 and impedes its ability to activate other transcriptional genes to promote disease regression18–20. Restoring the endogenous function of p53 is critical in tumor regression and treating triple negative breast cancers.
Materials and Methods
Chemicals and reagents
Palmatine (ChemShuttle, Trust Way, USA), Doxorubicin, Trypan blue, Phosphate buffered salineCarmine stain,Aluminum potassium sulfate(Sigma Aldrich, St. Louis,USA), Roswell Park Memorial Institute (RPMI) 1640medium,Trypsin/EDTA (Pan Biotech, Aidenbach, Germany), Fetal bovine serum (FBS), Penicillin-Streptomycin(Capricorn Scientific, Ebsdorfergrund, Germany),L-glutamine(Pan Biotech, Aidenbach, Germany) Antibodies for p53, p21 and Mdm2(Sigma Aldrich, St. Louis, USA).
Cell culture
The mouse 4T1 triple-negative breast cancer cell line (ATCC CRL-2539) was purchased from AddexBio (San Diego, CA 92117, USA).The 4T1 breast cancer cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% of fetal bovine serum (FBS), penicillin 100 U/mL, streptomycin 100 μg/mL, and L-glutamine 2 mM. The cells were maintained at 37°C in a CO2 5% humidified atmosphere.Before experimenting, the trypan blue assay was performed to determine the viability of the cells.
Animals
Female Balb/c mice 20-30g, 6-8 weeks were obtained from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, kept in the Animal House of the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi.The mice were housed in stainless steel cages with delicate wood shavings as bedding, fed with pellet diet (GAFCO, Tema) with water given adlibitum. The room was kept at a temperature of 24 – 28 oC, relative humidity 60 – 70%, and 12 h light-dark cycle. All protocols used in this study were done in compliance with Animal Welfare Regulations and established guidelines on the use and care of Laboratory animals 21. The animals were acclimatized for a week before the experiment.
Orthotopic transplantation of 4T1 breast cancer cells
Female Balb/c mice were anesthetized, 4T1 breast cancer cells (4× 105) were suspended in 100 μL of serum-free media and injected into the 4th inguinal mammary fat pad to inducecarcinogenesis. The mice were divided into the following groups;
Group1- Normal saline (1 ml/kg) i.p.
Group 2- 4T1 breast cancer cells (4× 105) only.
Group 3-5- Palmatine (1, 5 and 10 mg/kg) i.p.
Group 6- Doxorubicin (5 mg/kg) i.p. per week. Treatment of animals began 48 hours after induction of carcinogenesis and continued for 28 days 22. At the end of the experiment, the mice were euthanized by cervical dislocation and analytical procedures were performed.
Tumor data
The mice were palpated for the presence of tumors and tumor size was measured at weekly intervals. The tumor length i.e., largest diameter (a) and the width i.e., shortest dimension (b) perpendicular to (a) of the tumor was measured on days 7,14,21 and 28. The tumor volume was calculated using the formula;
At the end of the experiment, the following data were analyzed:
(A) Tumor volume: the total tumor volume calculated at each palpation over time (B) Tumor growth rate: The change in palpable tumor volume from palpation until sacrificedivided by time. (C) Tumor weight: The tumor weight was recorded at necropsy. (D)
Histopathology
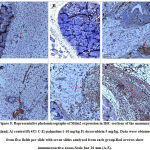
The mammary glands were excised, fixed in 10% neutral-buffered formalin, and embedded in paraffin. Then, 4-µm-thick sections were cut and transferred onto slides. Deparaffinized tissue sections were stained with hematoxylin and eosin (H&E) and examined at 10× magnification. Histology sections from the mammary gland were evaluated for neoplastic morphological changes.A quantitative score of0-4 representing; 0 -Normal morphology, 1-3 – Mild/moderate/severe dysplasia and 4 – Abnormal morphology with invasive carcinoma23
Immunohistochemical detection of p53, p21, and Mdm2 Expression
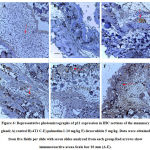
The immunoreactions of p53, p21 and Mdm2were determined in the mammary tissues using their respective mouse polyclonal antibodies. Briefly, 5 µm sized paraffin-embedded tissue sections were de-paraffinized with xylene and endogenous peroxidase activity was quenched with 3% H2O2 in methanol for 30 minutes in the dark. Tissue sections were dehydrated through graded alcohols and subjected to heat-induced epitope antigen retrieval using 10mM sodium citrate. Sections were washed with TBST (Tris Borate Saline Tween-20) and then blocked with 5% BSA (Bovine Serum Albumin) for one hour. Slides were incubated with the respective mouse monoclonal primary antibody diluted with TBS. Slides were then washed for 5 minutes in TBST and incubated for 1 hour with the respective HRP (Horse Radish Peroxidase) conjugated anti-mouse secondary antibody diluted with TBS in a ratio of 1∶200. After washing, slides were incubated with DAB (3,3′-diaminobenzidine tetrahydrochloride) and immediately washed under tap water after color development. Slides were then counterstained with hematoxylin. Slides were mounted with dibutyl phthalate xylene and images under a 10× light microscope. The manifestation of a dark, brown-colored, intracytoplasmic precipitate is indicative of the presence of p53, p21, and Mdm2. The antibody stain intensity of the images obtained wasanalyzed with an IHC Profiler plugin in Image J. The Immunohistochemistry optical density score (IODS) was calculated24.
Statistical Analysis
Experimental data were expressed as Mean ±Standard error of the mean. Analysis of data was done by Graph Prism software version 8.0.1. using One-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparisons. The criteria for significant difference between groups was set at p ≤ 0.05.
Results
Effect of palmatine on mammary carcinoma
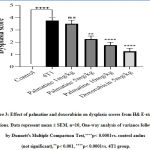
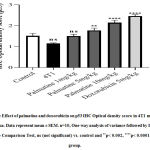
Growth-dependent changes on the mammary gland of carcinoma bearing micewere assessed in the different groups. Tumor volume and weight recorded in the 4T1 group, 28 days post-injection with the 4T1 breast cancer cells was 547.20±18.11 and 1.49 ±0.02respectively (Figure 1 A, C). The growth rate of the tumor from the untreated 4T1 group on day 28 was 5.0 ± 2.24 compared to the control 0.0 ± 0.0 (Figure 1 B).After 28 days,administering palmatine 1, 5 and 10 mg/kg significantly (p < 0.001) in a dose-dependent manner decreased mammary tumor volume 190.80 ±19.14, 25.40 ± 2.82,14.20 ± 1.85, tumor weight 1.035± 0.04, 0.8027± 0.01, 0.5090±0.04 and effectively inhibited tumor growth 31%, 46% and 66% respectively compared tocarcinoma bearing mice(Figure 1 A-D).
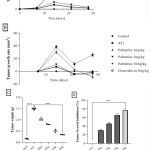 |
Figure 1: Effect of palmatine and doxorubicin on 4T1 mammary carcinoma A) tumor volume B) tumor growth rate C) tumor weight D) tumor growth inhibition. |
Histopathology
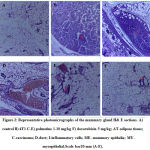
The morphology of the mammary gland from the control group presents a vacuolated appearance of normal acini arranged radially, surrounded by myoepithelial structures with a dysplasia score of 0.0 ± 0.0 (Figure 2 A, 3). Histological analysis established the presence of mammary carcinoma in the hematoxylin and eosin (H&E) stained sections from the 4T1 group. The 4T1 group showed severe mammary carcinoma characterized by solid aggregation of the 4T1 breast cancer cells defined bydilated ductsand lobules filled with cancerous cells with a deterioration of the conjunctive tissue with numerous degrees of cellular and nuclear pleomorphism consistent with malignant epithelial proliferation;a dysplasia score of 3.75 ± 0.25was recorded (Figure 2 B, 3). On the other hand,the histoarchitecture of mammary glands from carcinoma bearing mice treated withpalmatine 1, 5, 10 mg/kg in a dose dependent manner presented a high regression of tumor growth, wide regions of necrosis, little sign of neoplasia, well-separated acini and various areas of tumor cell remnants; dysplasia scores of 3.50± 0.29, 2.25± 0.25, 1.75± 0.25 was recorded respectively(Figure 2 C-F, 3).
Immunohistochemical Detection of p53 Expression
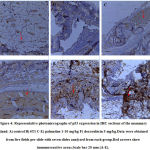
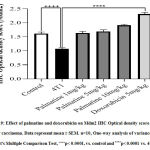
The expression of p53 indicated by a brown stain was evaluated by a semi-quantitative method using the Immunohistochemistry Optical density score (IODS).Tissue sections from mice treated with palmatine at doses 5 mg/kg and 10 mg/kg showed positive immunoreactivityin the cytoplasm for the p53 tumor protein; IODS 1.77 ± 0.12, 2.15 ± 0.12 respectively (Figure 4D- E, 5)compared to the low expression in the untreated 4T1 group; IODS 1.15± 0.03 (Figure 4A- B, 5).
Immunohistochemical Detection of p21 Expression
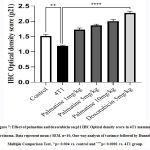
Tissue sections from animals treated with palmatine at doses 1 mg/kg, 5 mg/kg and 10 mg/kgshowed positive immunoreactivity in the cytoplasm for the p21 protein (brown stain); IODS 1.72± 0.06, 1.86± 0.04 and 2.0± 0.07respectively(Figure 6C- E, 7) compared to the low expression inthe untreated 4T1 group; IODS 1.19± 0.03 (Figure 6A – B, 7).
Immunohistochemical Detection of Mdm2 Expression
Tissue sections from mice treated with palmatine at doses 1 mg/kg, 5 mg/kg and 10 mg/kgshowed positive immunoreactivity in the cytoplasm for the Mdm2 protein (brown stain); IODS 1.63± 0.06, 1.69± 0.06 and 1.91± 0.03 respectively(Figure 8C- E, 9) compared to the low expression in the untreated 4T1 group; IODS 1.07± 0.03 (Figure 8A – B, 9).
Discussion
The mouse 4T1 triple negative mammary carcinoma model has several characteristics that make it an effective model for the preclinical evaluation of new anti-cancer therapies. The 4T1 breast cancer cells mimic human triple negative breast cancerin the lack of the expression of estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 (HER2). The 4T1 breast cancer cells are easily transplanted into the mammary fat pad allowing for easy quantification of tumor growth and progression25,26.
The present study was carried out to investigate the protective activity of palmatine in triple-negative mammary carcinoma. The intraperitoneally administered dose of palmatine used in this study wasobtained from effective pharmaceutical concentrations from published reports27–29.
Consistently, there were no symptoms of intoxication, weight loss and anorexia with the doses of palmatine administered.
Following transplantation of the 4T1 breast cancercells, carcinomawas formed. This involves alterations in themicro and macro-environment of the breast cancer cells imparting a selective advantage to the cancerous cells which promotes increased cell proliferation and tumor growth30,31. Such changes often inactivate components of several signal transaction pathways. This issupported by the evidence that a majority of human cancers display persistent inactivation of one or more pathways32–34.Palmatine administration effectively demonstrated a dosedependent reduction in tumor burdenin carcinoma bearing mice suggesting a dose-relatedbeneficialand protectiveinhibition of mammary carcinoma.
Elevated levels of tumor suppressor genes have been associated with the prevention of malignant growth of cancerous cells35. Tumor protein p53 through several mechanisms plays a critical role incell cycle regulation, DNA repair, inhibition of angiogenesis and apoptosis. The p53 gene has been described as “the guardian of the genome”referring to its function in preserving stability by preventing genome mutation. Anticancer drugs that modulate the p53 tumor suppressor pathway are important mediators for better cancer prognosis36,37.
The endogenous tumor protein p53 when synthesized in cells has a short half-life. Nonetheless, the tumor protein p53 when activated by anticancer agents in response to cellular stress signals such as DNA damage, hypoxia, nitric oxide signaling, and oncogene activation result in an increased half-life and transcriptional activity. There was a statistically significant increase in the levels of the p53 protein when carcinoma bearing mice were treated with palmatine. This confirms published reports which state that the 4T1 mammary carcinoma is deficient in the p53 protein and harbor genes that inactivate the p53 protein38. Thus,changes in the level of p53 may be a result of administering a therapeutically effective compound.
Several proteins mediate cell cycle regulation by p53, however, p21 is a major therapeutic target for the transcriptional activity of p53. The progression of abnormal cell division into the S phase requires the Cyclin-dependent kinase 2enzymes, which is inhibited by p21. Also, progression into the M phase requires Cell division control 2 which isalso inhibited by p21. The tumor protein p53 increased the expression of p21, an inhibitory protein to induce growth arrest39,40 as such, a significant increase in the expression of the p21 protein was observed with palmatine in a dose-dependent manner.
The Mdm2 is the product of a p53-activated gene. An increase in p53 activity leads to the upregulation of Mdm2. The Mdm2 is also a p53 inducible gene whose protein product binds to p53 and acts as an E-3 ubiquitin ligase that adds ubiquitin to p53 which results in its degradation. This produces an autoregulatory loop where a synthesis of p53 results in the synthesis of Mdm2 which in turn degrades p5341,42. Upregulation of Mdm2 observed for palmatine in a dose-dependent manner is a reliable indication of the endogenous activation of the tumor protein 53 by palmatine.
Conclusion
Palmatine administration effectively inhibits the growth of mammary carcinoma in female balb/c mice evidentfromtumor growth inhibition and the observation of a quasi-normal mammary morphology. These effects of palmatine were associated with the endogenous upregulation of the tumor p53 protein, the translational gene p21 and its negative regulatory gene Mdm2.
Acknowledgment
We would like to thank Mr. Edmund Derry, Mr. Prince Dagadu and Mr. FulgencioGyan at the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST for their technical support.
Conflict of interest
The authors declare no competing interest.
Funding source
This research did not receive any source of funding from agencies in the public, commercial and not-for-profit sectors.
References
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938-1948.
CrossRef - Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16.
CrossRef - Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer Targets Ther. 2016;8:93.
CrossRef - Anjum F, Razvi N, Masood MA. Breast cancer therapy: a mini review. MOJ Drug Des Dev Ther. 2017;1(00006).
CrossRef - Angsutararux P, Luanpitpong S, Issaragrisil S. Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev. 2015;2015.
CrossRef - Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85(9):1219-1226.
CrossRef - Beghyn T, Deprez‐Poulain R, Willand N, Folleas B, Deprez B. Natural compounds: leads or ideas? Bioinspired molecules for drug discovery. Chem Biol Drug Des. 2008;72(1):3-15.
CrossRef - Bhadra K, Maiti M, Kumar GS. DNA-binding cytotoxic alkaloids: comparative study of the energetics of binding of berberine, palmatine, and coralyne. DNA Cell Biol. 2008;27(12):675-685.
CrossRef - Lyamzaev KG, Pustovidko A V, Simonyan RA, et al. Novel mitochondria-targeted antioxidants: plastoquinone conjugated with cationic plant alkaloids berberine and palmatine. Pharm Res. 2011;28(11):2883-2895.
CrossRef - Yan B, Wang D, Dong S, et al. Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol. 2017;45:194-200.
CrossRef - Qin Y, Pang J, Chen W, Zhao Z, Liu L, Jiang Z. Inhibition of DNA topoisomerase I by natural and synthetic mono‐and dimeric protoberberine alkaloids. Chem Biodivers. 2007;4(3):481-487.
CrossRef - Kong W-J, Zhao Y-L, Xiao X-H, Li Z-L, Ren Y-S. Action of palmatine on Tetrahymena thermophila BF5 growth investigated by microcalorimetry. J Hazard Mater. 2009;168(2-3):609-613.
CrossRef - Hambright HG, Batth IS, Xie J, Ghosh R, Kumar AP. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFκB/FLIP. Mol Carcinog. 2015;54(10):1227-1234.
CrossRef - Pulaski BA, Ostrand‐Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2000;39(1):20-22.
CrossRef - Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004;40(6):852-857.
CrossRef - DuPre SA, Redelman D, Hunter Jr KW. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88(5):351-360.
CrossRef - Yang S, Zhang JJ, Huang X-Y. Mouse models for tumor metastasis. In: Rational Drug Design. Springer; 2012:221-228.
CrossRef - Whibley C, Pharoah PDP, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev cancer. 2009;9(2):95-107.
CrossRef - Synnott NC, Murray A, McGowan PM, et al. Mutant p53: a novel target for the treatment of patients with triple‐negative breast cancer? Int J cancer. 2017;140(1):234-246.
CrossRef - Synnott NC, Bauer MR, Madden S, et al. Mutant p53 as a therapeutic target for the treatment of triple-negative breast cancer: preclinical investigation with the anti-p53 drug, PK11007. Cancer Lett. 2018;414:99-106.
CrossRef - Albus U. Guide for the Care and Use of Laboratory Animals (8th edn). 2012.
CrossRef - Zhang Y, Zhang G, Sun X, et al. Establishment of a murine breast tumor model by subcutaneous or orthotopic implantation. Oncol Lett. 2018;15(5):6233-6240.
CrossRef - Ting AY, Kimler BF, Fabian CJ, Petroff BK. Characterization of a preclinical model of simultaneous breast and ovarian cancer progression. Carcinogenesis. 2007;28(1):130-135.
CrossRef - Seyed Jafari SM, Hunger R. IHC Optical Density Score: A New Practical Method for Quantitative Immunohistochemistry Image Analysis. Appl Immunohistochem Mol Morphol. 2017;25(1):e12-e13.
CrossRef - BA P. Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001.
CrossRef - de Souza Garcia CM, de Araújo MR, Lopes MTP, Ferreira M, Cassali GD. Morphological and immunophenotipical characterization of murine mammary carcinoma 4t1. Braz J Vet Pathol. 2014;7(3):158-165.
- Turer CC, Altun G. Effect of palmatine on periodontal tissue destruction in experimental periodontitis rat model. 2017
- Wang L, Wang X, Zhang S-L, et al. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J Nat Med. 2017;71(1):257-264.
CrossRef - Long J, Song J, Zhong L, Liao Y, Liu L, Li X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie. 2019;162:176-184.
CrossRef - Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904-5912.
CrossRef - Weston A, Harris CC. Multistage carcinogenesis. Holland-Frei Cancer Med 6th Ed Hamilt BC Decker. 2003.
- Wang L-H, Wu C-F, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem. 2018;51(6):2647-2693.
CrossRef - Hoon DSB, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23(22):4014-4022.
CrossRef - Osborne C, Wilson P, Tripathy D. Oncogenes and tumor suppressor genes in breast cancer: potential diagnostic and therapeutic applications. Oncologist. 2004;9(4):361-377.
CrossRef - Slattery ML, Herrick JS, Mullany LE, et al. The co‐regulatory networks of tumor suppressor genes, oncogenes, and miRNAs in colorectal cancer. Genes, Chromosom Cancer. 2017;56(11):769-787.
CrossRef - Chumakov PM. Versatile functions of p53 protein in multicellular organisms. Biochem. 2007;72(13):1399-1421.
CrossRef - Zambetti GP. The P53 Tumor Suppressor Pathway and Cancer. Vol 2. Springer Science & Business Media; 2007.
- Yerlikaya A, Okur E, Baykal AT, Acılan C, Boyacı İ, Ulukaya E. A proteomic analysis of p53-independent induction of apoptosis by bortezomib in 4T1 breast cancer cell line. J Proteomics. 2015;113:315-325.
CrossRef - Adimoolam S, Lin CX, Ford JM. The p53-regulated cyclin-dependent kinase inhibitor, p21 (cip1, waf1, sdi1), is not required for global genomic and transcription-coupled nucleotide excision repair of UV-induced DNA photoproducts. J Biol Chem. 2001;276(28):25813-25822.
CrossRef - Benson EK, Mungamuri SK, Attie O, et al. p53-dependent gene repression through p21 is mediated by recruitment of E2F4 repression complexes. Oncogene. 2014;33(30):3959-3969.
CrossRef - Arena G, Riscal R, Linares LK, Le Cam L. MDM2 controls gene expression independently of p53 in both normal and cancer cells. Cell Death Differ. 2018;25(9):1533.
CrossRef - Coffill CR, Lee AP, Siau JW, et al. The p53–Mdm2 interaction and the E3 ligase activity of Mdm2/Mdm4 are conserved from lampreys to humans. Genes Dev. 2016;30(3):281-292.
CrossRef