Ni Putu Ayu Dewi Wijayanti* , Putu Sanna Yustiantara and I Wayan Agus Widiantara
, Putu Sanna Yustiantara and I Wayan Agus Widiantara
Department of Pharmacy, Faculty of Mathematics and Natural Science, University of Udayana, Jimbaran, Indonesia
Corrresponding Author E-mail: dewi_wijayanti@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2208
Abstract
The mangosteen fruit rind fraction positively contains flavonoids and polyphenols which can act as an antioxidant anti-inflammatory. The use of mangosteen rind fraction in topical preparations requires a good delivery system to support percutaneous penetration, one of which is nanoemulsion. The topical use of nanoemulsion has low viscosity and to increase the penetration of active compound, therefore it needs to be formulated into nanoemulgel preparations. In formulating nanoemulgel preparations, the optimum concentration of gelling agents is needed to obtain a nanoemulgel that meets the physical and chemical characteristics of the gel preparation. Therefore we need an optimization of glyceryl polyacrylate gelling agent in order to obtain a nanoemulgel with good characteristics. Nanoemulgel for mangosteen rind fraction was made in four formulas with gelling agent concentrations of glyceryl polyacrylate as much as 1% (F1), 2% (F2), 3% (F3), and 4% (F4). The nanoemulgel has been evaluated for its physical and chemical characteristics including organoleptic test, homogeneity, adhesion, viscosity, dispersibility, and pH. The active substance release test is carried out on a gel that meets the overall requirements for physical and chemical properties. The analysis showed that the F1 produced a nanoemulgel that met the physical and chemical characteristics of the semisolid preparations. Organoleptic of F1 isi semisolid form with yellow color and typically garcinia fruit rind fraction odor, homogen mixture, 1 ± 0.02s adhesion, 8340 ± 555 viscosity, 6.87 ± 0.39 cm2 spreadability, and 6.39 ± 0.01 pH. The results of the release of active substances showed that the F1 formula was able to release 49.9% of active substances in 10 minutes and within 480 minutes was able to release 96.54% of active substances with a total flux of 5.36 µg / cm2 minutes which fulfill the release requirement.Based on these results, it can be concluded that glyceryl polyacrylate with a concentration of 1% can produce good nanoemulgel.
Keywords
Glyceryl Polyacrylate; Mangosteen; Nanoemulgel; Nanoemulsion; Release Test
Download this article as:| Copy the following to cite this article: Wijayanti N. P. A. D, Yustiantara P. S, Widiantara I. W. A. Optimization of Glyceryl Polyacrylate in Nanoemulgel of Mangosteen (Garcinia mangostana L.) Rind Fraction. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Wijayanti N. P. A. D, Yustiantara P. S, Widiantara I. W. A. Optimization of Glyceryl Polyacrylate in Nanoemulgel of Mangosteen (Garcinia mangostana L.) Rind Fraction. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3hqAy5U |
Introduction
Mangosteen is a tropical tree that grows in Southeast Asia. In Ayurvedic medicine the mangosteen use agains inflammation, diarrhea, cholera, and dysentery. The Southeast Asians people use mangosteen to treath the skin infections and wound. The mangosteen rind fraction was positive for polyphenols and flavonoids [10]. The mangosteen rind fraction has antioxidant and anti-inflammatory activity. The use of mangosteen rind fraction directly in topical dosage formulations requires a good delivery system to support percutaneous absorption and penetration. The delivery system that can be used is the nanoparticle system, namely the nanoemulgel.
Nanoemulgel is a nanoemulsion formation in the hydrogel. Nanoemultion can form by the addition of a nanoemulsion system into the hydrogel matrix [5]. The preparation in the form of nanoparticles has better stability compared to conventional emulsions. It can maintain the stability of the preparation against aggregation, separation against gravity and oxidation [16] [22].
The gelling agent component is one of the critical factors that can affect the physical and chemical stability of the resulting gel preparation [1]. The gelling agent used is glyceryl polyacrylate. Glyceryl polyacrylate is an ester of glycerin with polyacrylate which can be used as an emollient, surfactant, gelling agent, and emulsifying agent. The matrix gel formation from glyceryl polyacrylate can mix with water and oil so it is very well used as a gelling agent with emulsion active ingredients [11]. The use of glyceryl polyacrylate with an optimal concentration can produce good preparation. A good gel must meet the requirements of the required physical and chemical properties, can release active substances, and be able to penetrate the skin.
The penetration test was carried out for preparations that had met the requirements of the physical and chemical properties of the gel, which was carried out through Franz diffusion cells to obtain the penetration flux of the active substance [4]. The franz diffusion cell is a system that can be used to test the penetration ability of semisolid preparations. Franz diffusion cell concist of a donor compartment and a receptor compartment. The penetration test uses a separation membrane that placed between the donor compartment and the receptor compartment which functions as a simulation of human skin.The purpose of this study was to obtain an optimal nanoemulgel formula for mangosteen rind that fulfills the physical and chemical properties of semisolid preparations and be able to release active substances optimally.
Material and methods
The tools used in this research thermometer, analytical scales (Adam AFP-360L), pH meter (Oakton pH 510 series), Brookfield DV-E viscometer. The materials used in this study according to (Samala and Seridevi, 2016; Sengupta and Chatterjee, 2017) are mangosteen rind nanoemulsion, glycerin (Asland), glyceryl polyacrylate (Asland), aquades (Asland), propylene glycol (Asland), phenoxyethanol. (Asland), caprilyl glycol (Asland).
Preparation of mangosteen fruit rind Nanoemulgel
Glyceryl polyacrylate is added with distilled water and then stirred to form a gel-like mass. Glycerin was added to the distilled water mixture with glyceryl polyacrylate and stirred until it was homogeneous (Mixture I). Phenoxyethanol is first mixed with capryl glycol, and propylene glycol is then added to the mixture I. Nanoemulsion is added gradually while stirring until homogeneous.
Table 1: Nanoemulgel formula for mangosteen rind fraction.
| Material | F1 (%) | F2 (%) | F3 (%) | F4 (%) |
| Mangosteen rind fraction | 12 | 12 | 12 | 12 |
| Glyceryl polyacrylate | 1 | 2 | 3 | 4 |
| Glycerin | 12 | 12 | 12 | 12 |
| Propylene glycol | 10 | 10 | 10 | 10 |
| Phenoxyethanol | 0.7 | 0.7 | 0.7 | 0.7 |
| Caprilyl glycol | 0.3 | 0.3 | 0.3 | 0.3 |
| Aquades | ad 100 | ad 100 | ad 100 | ad 100 |
Note: F is the acronym of formula
Physical and chemical properties of mangosteen rind fraction nanoemulgel
Organoleptic
Organoleptic observations were carried out by directly observing the texture, color, and smell of the nanoemulgel fraction of the mangosteen rind [7].
Homogenity
Homogeneity testing is carried out to produce a homogeneous preparation without any particles or coarse fibers. The test is carried out by smearing the substance on a piece of glass or other suitable transparent material. The presence or absence of coarse particles or fibers was observed [7].
Adhesion
0.25 grams of nanoemulgel placed it in two glass objects, then given a load of 1 kg for 5 minutes. After that, it is lifted and given a load of 80 grams. Time required for two glass objects to separate was calculated. The good adhesion if the time needed to separate the glass objects is more than 1 second [27].
Viscosity
Viscosity measurement is done by placing the sample in a Brookfield viscometer until the spindle is submerged. Set the spindle and speed to be used. Taken 6 speed points, namely 10 rpm, 20 rpm, 30 rpm, 50 rpm, 60 rpm, and 100 rpm [22] [9].
Spreadability
A total of 1 gram of gel preparation is placed carefully on a glass measuring 20 cm x 20 cm. Then covered with mica paper and weighted on it until the weight reaches 125 grams, then measured the diameter formed after 1 minute [10].
pH
PH measurements were carried out by diluting 1 gram of the gel preparation using 10 mL of distilled water. Measured the pH of the diluted solution using a pH meter. The pH meter electrode is immersed in the solution being tested, the pH meter needle is allowed to move until it shows a fixed position. The pH indicated by the pH meter needle was recorded [7].
Penetration
The penetration test was carried out using the Franz diffusion cell method. The media in the receptor compartment used phosphate buffer with a pH of 7.4. The membrane used is the skin of the Python reticulates. The receptor compartment is filled with phosphate buffer pH 7.4 to the brim and a magnetic stirrer is inserted and the temperature is kept at 32 ° C. The snakeskin is placed between the donor compartment and the receptor compartment with the stratum corneum facing up. 3 grams of gel is put into the assay in the donor compartment and the magnetic stirrer is run at 250 rpm. Samples were taken as much as 2 mL and carried out at minutes 10, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480. The samples taken were then analyzed using UV spectrophotometry to determine the total flavonoid content. [4] [13] [16].
Result
Nanoemulgel of Garcinia mangostanarind fraction
Physical and chemical evaluation
Organileptic
Table 2: Organoleptic test result of Garcinia mangostana rind fraction nanoemulgel
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| Form | Semisolid | Semisolid | Semisolid | Semisolid |
| Colour | Yellow | Yellow | Yellow | Yellow |
| Odor | Typical Garcinia mangostanarind fraction nanoemulgel | Typical Garcinia mangostanarind fraction nanoemulgel | Typical Garcinia mangostanarind fraction nanoemulgel | Typical Garcinia mangostanarind fraction nanoemulgel |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
Homogenity
Table 3: Homogenity test result of Garcinia mangostana rind fraction nanoemulgel
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| Homogeneous | Homogeneous | Homogeneous | Homogeneous | Homogeneous |
| Coarse particle | Free Coarse particle | Free Coarse particle | Free Coarse particle | Free Coarse particle |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
Adhesion
Table 4: Adhesion test result of Garcinia mangostana rind fraction nanoemulgel.
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| Stickiness (s) | 1 ± 0.02 | 1.14 ± 0.04 | 1.26 ± 0.05 | 1.49 ± 0.03 |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
Viscosity
Table 5: Viscosity test result of Garcinia mangostana rind fraction nanoemulgel
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| Viscosity (cPs) | 8340 ± 555 | 20160 ± 681 | 22000 ± 257 | 25800 ± 817 |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
Spreadability
Table 6: Spreadability test result of Garcinia mangostana rind fraction nanoemulgel
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| Spreadability (cm2) | 6.87 ± 0.39 | 4.74 ± 0.84 | 3.35 ± 0.31 | 3.04 ± 0.32 |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
pH
Table 7: pH test result of Garcinia mangostana rind fraction nanoemulgel
| Test | Formula | |||
| F1 | F2 | F3 | F4 | |
| pH | 6.39± 0.01 | 6.36 ± 0.01 | 6.24 ± 0.01 | 6.13 ± 0.02 |
Note: (F1) consentration of glyceryl polyacrylate 1%; (F2) consentration of glyceryl polyacrylate 2%; (F3) consentration of glyceryl polyacrylate 3%; (F4) consentration of glyceryl polyacrylate 4%.
Penetration
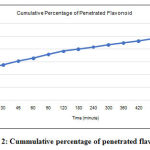 |
Figure 2: Cummulative percentage of penetrated flavonoid. |
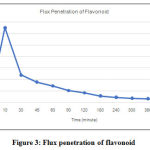 |
Figure 3: Flux penetration of flavonoid. |
Discussion
Organoleptic testing carried out is an observation of shape, color, and smell [20]. The four formulas were made to produce nanoemulgel with a semisolid form, a distinctive odor of mangosteen rind nanoemulsion, and a yellow color such as nanoemulsion. This yellow color is produced by the dye betaxantin and mangostin compounds contained in the mangosteen rind fraction [2] [18].
The homogeneity test was carried out to see the uniformity of the components in the nanoemulgel preparation. Good preparation must be homogeneous and free of particles or coarse fibers so that it does not cause irritation and is evenly distributed when applied [14]. Based on table 3, the entire nanoemulgel formula produces a homogeneous preparation with no particles or coarse fiber. This shows that all formulas meet the requirements of the homogeneity test.
Good adhesion of nanoemulgel, namely nanoemulgel can adhere more than 1 second [9]. Based on the test results, the four formulas have good adhesion, which is> 1 second. Based on statistical analysis with SPSS, the difference in adhesion of each formula showed insignificant results with a p-value> 0.05, namely 0.210. This means that the glycerin polyacrylate concentration in each formula does not give a significant difference in adhesion.
The viscosity of a preparation can affect the dispersibility parameters and the release of the active substance from nanoemulgel preparations. A good viscosity value for nanoemulgel is in the range of 3,000-10,000 cps [8] [15]. Based on the results of statistical tests, the difference in viscosity of each formula showed significant results with a p-value <0.05. This shows that the glycerin polyacrylate concentration in each formula gives a difference in viscosity. Based on the results of the viscosity test in table 11, nanoemulgel with formula 1 has a viscosity value in the range of 3,000-10,000 cps [8] [15] so that only F1 meets the viscosity test requirements.
The dispersibility test was carried out to determine the speed at which the gel spreads on the skin when applied [21]. Good semisolid preparations have a dispersive power of 5-7 cm2 [9]. The statistical test results of the difference in the spreadability of each formula showed significant results with a p-value <0.05, namely 0.02. This shows that the glycerin polyacrylate concentration in each formula gives a difference in dispersibility. The results of the scattering power in table 8 show that F1 produces a spreadability between 5-7 cm2 so that only formula 1 meets the criteria for the spreadability test.
PH testing on nanoemulgel preparations aims to determine the suitability of pH preparations with skin pH. The required preparation pH is in the range of 4.5-6.5 [12]. The pH of the nanoemulgel produced is in the range 4.5-6.5 so that the entire formula meets the pH test requirements. Based on statistical analysis with SPSS, the difference in pH of each formula showed significant results with a p-value <0.05. This means that the difference in the concentration of polyacrylate glycerin in each formula gives a different pH value.
F1 meet all requirement of goognanoemulgel and continuous to pentration test. Based on Figure 2, the number of flavonoids penetrated at minute 10 reaches 49.9% and at minute 480 reaches 96.54%. Based on research conducted by Mulia et al. (2018) nanoemulgel which is made can release the active substance in the 10 minute as much as 50% and at the 480 minute as much as 95%. When compared with research from Mulia et al. (2018) nanoemulgel is made able to release the active substance from the gel base well.
Based on Figure 3, the maximum penetration flux of the nanoemulgel from the mangosteen rind fraction was found in the 10th minute which reached 13.04 µg / cm2 minutes with a total flux value of 5.36 µg / cm2 minutes. The graph that increases in the first 10 minutes shows the number of active substances penetrated in 10 minutes. Furthermore, there is an insignificant decrease in the graph from the 180 minute indicating that there has been an equilibrium in the concentration of the active substance in the diffusion medium. The value of the cumulative number of penetrated flavonoids and the good penetration flux of active substances is due to the good dispersibility and viscosity characteristics of the nanoemulgel preparation. The dispersion power can affect the diffusion rate across the membrane, with a wide-spreading power it can expand the diffusion area of active substances to increase the diffusion rate of active substances. Viscosity is inversely proportional to the spreadability with the lower the viscosity, the wider the resulting dispersive power so that it can affect the widespread of nanoemulgel and can affect the diffusion rate of active substances [17].
Acknowledgement
We would be thanks to the head of the non-sterile laboratory and phytochemical study program of Pharmacy, Faculty of Mathematics and Natural Sciences, Udayana University.
Conflict of Interest
Authors do not have any conflict of interest.
Funding Source
This study received no external funding.
Reference
- Ardana M, Aeyni V, dan Ibrahim A. Formulation and optimization of hpmc (hidroxy propyl methyl cellulose) gel base with various concentrations. Journal of Tropical Pharmacy and Chemistry, 2015; 3:101-108.
CrossRef - Cosmetic information dossier. New York: Ashland; 2019.
- Astuti KW, Wijayanti NPAD, and Prasetia IGNJA. Development of gel dosage form of ethyl acetate extract of mangosteen rind (Garcinia mangostana L.). Journal of Health Sciences and Medicine UNUD Journals, 2017; 1:28-32.
CrossRef - Chellapa P, Mohamed AT, Keleb EI, Elmahgouni A, Eid AM, Issa YS, et al. Nanoemulsion and nanoemulgel as a topical formulation. International Organization Scientific Research Journal of Pharmacy, 2015; 5:43-47.
- Damayanti H, Wikarsa S, and Jafar G. Nanoemulgel formulation of mangosteen (Garcinia mangostana L.) skin extract. JurnalRisetKefarmasian Indonesia, 2019; 1:166-176.
CrossRef - Depkes RI. Indonesian Pharmachopoeia, 5th ed. Jakarta: Departemen Kesehatan Republik Indonesia; 2014: 1196.
- Eugresya G, Avanti C, and Uly SA. Development of formulas and pH stability tests for facial wash gel containing ethanol extract of kesambi bark. Media Pharmaceutica Indonesia, 2017; 1:181-188.
CrossRef - Garg A, Aggarwal D, Garg S, and Sigla AK. Spreading of semisolid formulation. Pharmaceutical Technology, 2012; 1:1-10.
- Kurniasari NLY. Optimization of the total uptake of mangosteen (Garcinia mangostana L.) rind fractions in the nanoemulsion system using the SNEDDS (self-nanoemulsifying drug delivery system) method. Jimbaran: Udayana University Press; 2019.
- Levi K and Dauskardt RH. Biomechanics of the barrier function of human stratum corneum. Treatment of Dry Skin Syndrome, 2012; 15:233-254.
CrossRef - Martin A, Bustamante P, and Chun AHC. Physical Pharmacy, 4th Ed. London: Lea and Febiger; 1993: 324-361.
CrossRef - Mulia K, Ramadhan RMA, and Krisanti, EA. Formulation and characterization of nanoemulgel mangosteen extract in virgin coconut oil for topical formulation. MATEC Web of Conferences. 2018; 156:1-7
CrossRef - Naibaho OH, Yamlean PVY, and Wiyono W. Effect of ointment base on the formulation of basil leaf extract ointment (Ocimum sanctum L.) on the skin of the back of rabbits made with Staphylococcus aureus infection. PharmaconJurnalIlmiahFarmasi, 2013; 2:27-33.
- Pertiwi RD, Kristanto J, and Praptiwi GA. Antibacterial activity test of gel formulation for thrush from saga leaf extract (Abrusprecatorius Linn.) Against Staphylococcus aureus bacteria. JurnalIlmiahManuntung, 2016; 2:239-247.
CrossRef - Pratiwi L, Fudholi A, Martien R and Pramono S. Physical and chemical stability tests for SNEDDS (self-nanoemulsifying drug delivery system) and ethyl acetate fraction nanoemulsions of mangosteen peel (Garcinia mangostana L.). Traditional Medicine Journal, 2008; 23:84-90.
CrossRef - Sinko P. Martin’s physical pharmacy dan pharmaceutical sciences, 6th Ed, China: Lippincot Williams & Wilkins; 2011.
- Sudarsono G, Wahyuono D, Donatus S, and Purnomo IA. Medicinal plants II. Yogyakarta: Gadjah Mada University Press; 2002.
- Sugihartini N and Wiradhika RY. Gel formulation of ethanol extract of mangosteen peel (Garcinia mangostana L.) as a medication for burns in wistar rats. Indonesian Journal of Medicine and Health, 2017; 8:110-117.
CrossRef - Supomo, Sapri, and Komalasari AN. Antioxidant gel formulation of mangosteen rind extract (Garcinia mangostana L.) based on carbopol. JurnalIlmiahIbnuSina, 2016; 1:50-60.
- Voight R. Bukupengantarteknologifarmasi, Yogyakarta: Gadjah Mada University Press; 1994.
- Yuliasari S, Fardiaz D, Andarwulan N, and Yuliani S. Characteristics of red palm oil nanoemulsion enriched with beta carotene. JurnalLittri, 2011; 20:111-121.
CrossRef








