Department of Zoology, Hiralal Mazumdar Memorial College for Women, Kolkata, India.
Corresponding Author E-mail: zoologist.rehan@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2181
Abstract
On 11th March 2020 , the WHO has declared that the Coronavirus 2 ( SARS-CoV-2) or noval coronavirus ( COVID-19) as Global Pandemic in the whole world . Yet there is no vaccine or any kind of special treatment developed even though all the nations are testing several therapeutic molecules. Different herbs that are traditional are also being used with conventional drugs since the COVID-19 outbreak and are showing promising results in for the treatment of the patients. In this paper, we will review on the use of the natural products and its findings on how it is being used for treating and preventing the infection of COVID-19.. Only those reports were included that WHO edited in the situation. The extracts of the various herbal products and the molecules that are purified exert an anti-SARS-CoV-2 action and they inhibit the virus directly since the entry of the virus by replicating them. It is even interesting to find that some of the herb related products are able to block the C-2 receptors that are protease serine TMPRRS2. These are the proteins COVID-19 virus requires to infect the human cells. Additionally it was also seen that the that he natural products we able to inhibit proteins such as papain-like or chymotrypsin-like proteases that were required in the life cycle of the SARS-CoV-2. Hence, it is concluded that the natural products can be used in combination or alone as an alternative for preventing or treating of the novel corona virus infection.
Keywords
ACE2; Corona Virus disease 2019; Medicinal Plants; SARS –CoV-2
Download this article as:| Copy the following to cite this article: Ahmad S. R. Medicinal Plants – Derived Natural Products and Phytochemical Extract as Potential Therapies for Coronavirus : Future Perspective. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Ahmad S. R. Medicinal Plants – Derived Natural Products and Phytochemical Extract as Potential Therapies for Coronavirus : Future Perspective. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3bT7eSd |
Introduction
The infection of novel corona virus (COVID-19) also known as the SARS-Cov-2 made the world declare the infection as a global pandemic which already resulted in large number of deaths in around 216 countries arcos the globe. It was in the city of Wuhan (China) in late December 2019 that the disease appeared because of zoonotic transmission (Mackenzie and Smith, 2020). It was also found out that the SARS-CoV-2 actually showed similarity in the genomic identity, which was as high as 96% with the corona virus that was related to the bats (Zhou et al., 2020). Furthermore, it was also found that the genome of the SARS-Cov-2 and of the Pangolin-Cov were around 91.02% identical. It raised the possibility of the later one being a prompt zoonotic host among the human and the bats (Zhang T. et al., 2020). Even though there are extraordinary efforts being made to tackle the SARS-Cov-2, there have not been any proper, treatment or vaccine developed for the infection (Amanat and Krammer, 2020). It is suggested by some therapeutic approaches that analogs of nucleoside, Remdesivir, drugs that are anti-inflammatory like Lopinavir/Ritonavir can be used for the treatment of COVID-19. Around the globe, there are researchers who are analyzing these drugs or others, and more than 200 clinical trials have been already registered in the government’s clinical trials. However, the efficiency of these drugs in the clinical usefulness to fight against the COVID-19 infection still is not yet clears. Since the very beginning of the outbreak of COVID-19, China has been using traditional herbal medicines. These traditional medicines as matter of fact, showed recovery results around 90% where the total number of people tested were 214 (Hong-Zhi et al., 2020). Moreover, it was also found that some herbal medicines were even effective in preventing SARS-CoV-2 infection from those persons who were healthy and those patients who were having mild symptoms also got relieved by using those medicines (Hong-Zhi et al., 2020). Even in the province of Zhejiang – China, the results that were reported were promising (Xu K. et al., 2020). Traditional medicines in China, namely the Lianhuaqingwen and Shu Feng Jie Du were also recommended since they were quite effective in the fight against the previous infections the H1N1 and the SARS-CoV-1 (Lu, 2020).
Some experts from the Wuhan University’s Zhongang Hospital even added the usage of these traditional medicines for the prevention and treatment of the patients who were exposed to the COVID-19 infection. The usage of many medicinal plants was recommended in many methods to prevent the COVID-19. The aim of this review is on the usage of the traditional herbal medicines and its possibility in the treatment and prevention of COVID-19 (Table 1).
SARS-COV-2
SARS-COV-2 shares the b genus of the order Nidovirales that comes from the Coronaviridae family. SARS-COV-2 has symmetric helical nucleocapsid and is an enveloped single (+) stranded RNA (Khan et al., 2020). The virus has encoding of around 20 distinctive proteins which has four main proteins that are structural (), and other more non-structural proteins such as the RdPd (RNA- dependent RNA polymerase). The main protease of the corona virus (3CLpro), and protease that is papin-like (PLpro) (Chen et al., 2020).
It was found that the angiotens in converting enzyme II (ACE2) was a receptor that played a key role of the SARS-CoV-2 and it allowed the attachment and replication of the virus to the human and bat cells (Walls et al., 2020; Zhou et al., 2020). SARS-CoV-2 by the interaction of motifs that binds receptors of the spike proteins like the RBD (receptor binding domain) and the ACE2 receptors binds itself to the host cells. This interaction triggers the changes that are conformational in the c-terminal of the S2 sub unit, which is the membrane, which is responsible of virus-cell fusion of the spike proteins. The S protein ACE2 which is a complex one is processed by the type 2II cell type of the host proteolytically by the transmembrane serine protese TMPRRS2 that leads to the ACE2 cleavage and hence results in the entry into the cell of the host virally (Jiang et al., 2020; Rabi et al., 2020).
The genomic RNA , after its entry into the host cells and uncrating, gets translated into two types of the polyprotiens (pp 1a and pp lab ) which generates around 15-16 proteins that are non-structural and undergo generation of proteolytic cleavage. The non-structural proteins induce the rearrangement of the membrane o the cell that forms the double membrane vesicles. Whereas the the genomic RNA is then duplicated into the sub genomic RN that in turns results into the amalgam of accessory proteins that are structural (spike, envelope, membrane, and nucleocapsid). In the end, under the ER-Golgi complex that is intermediate, the virions are gathered and later through the secretary, pathways are released (Fung and Liu, 2020).
SARS-CoV-2 is found out to be sharing similarities among the other b genus corona virus such as the NL63 and the SARS-CoV (Fani et al., 2020). However, these viruses need to interact with the ACE2 receptors for entering the host. Hence, there are some contrasts that have been found in the structure as in S proteins length and the region of the receptor binding (Ceccarelli et al., 2020). It is also found out that among the SARS-CoV and the SARS-CoV-2 there is higher homology in the nucleotides, which has been seen in the proteins of both the strains. In reality, both the N and the S2 proteins have around 90-99% similarities in the SARS-CoV and the SARS-CoV-2 viruses (Xu J. et al., 2020).
Role of Natural Products derived by Medicinal Plants against SARS- COV-2
(Runfeng et al. 2020) did a study of a Chinese herbal mixture Lianhuaqingwen (mixture of menthol, mineral called gypsum and 11 other medical species) which has potential in its anti-inflammatory and inhibitory effects against the COVID-19 . In the Chinese tradition , Lianhuaqingwen is generally used for the treatment of fatigue, cough, influenza, pneumonia and even the early stages of the measles (Ding et al., 2017) and it has already been used in USA as the clinical trial in the phase II . It was the Chinese National Health Commission, which recommended this herbal mixture for managing and treating the COVID-19 (Yang Y. et al., 2020). It was in Vero E6 that the anti COVID-19 activity was assessed and these cells were used as cytopathic inhabitation effect and for reduction in the plaque assays. In a dose-dependent manner, these herbal medicines, with the IC50 of 411.2 µg/ml were used to inhibit SARS-CoV-2 replication.
Moreover, in the dose dependant manner, these herbal mixtures were also able to reduce in the production of cytokines (Runfeng et al., 2020) that were pro-inflammatory (TNF-a, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10). The use of these mixtures was interesting since the huge generation of the cytokines were showing lethal complications in treating of the COVID-19. Out of the 61 molecules that were studied, seven of them (Wang et al., 2016) reported positive results in for the COVID-19 treatment. It was also reported (Wang et al., 2020) that it was possible to use ritonavir/lopanavir in a combination with the Shefung Jiedu (traditional medicine in China) capsules and rabidly for COVID-19 treatments. It was also found that the conditions of three out of four patients improved quit significantly and were found COVID-19 negative. I the northeast Chongqing province of China another study was made with 132 patients of COVID-19 treating 92% of them with the traditional Chinese medicines. It concluded that the most effective therapeutic approach was using traditional medicines in the combination of Kaletra (Wan et al., 2020). Lung et al. (2020) in his demonstration showed for the treatment of of COVID-19 the aflavin could be used in the silico approaches. It was noticeable that in the catalytic pockets of the COVID-19 RN polymerase that are RN-dependant, theaflavin showed affinities that were promising. However, it is noteworthy to point out that the availability of theaflavin could put a brake in the use since they are not available in large amounts and the skeletons of theaflavin resists the process of degradation which is done by the micro biota (Pereira-Caro et al., 2017). It has also even been concluded after studies made under the single cohort that the herbal medicines have efficiency if used as herbal formula in fighting against SARS and H1N1 viruses in a preventive approach in those areas where the population is high risk (old population, medical staff and their family members, people living in the outbreak areas of COVID-19). A list of 6 herbal species were found that were being used most frequently namely Atractylodes lancea (Thunb.) DC , Atractylodes macrocephala (Koidz), Astragalus mongholicus ( Bunge), Glycyrrhiza glabra L., Lonicera japonica (Thunb) and Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., These species come from the traditional medicines of the Chinese, which is known as Yupingfeng powder (Luo et al., 2020). It was also on the other hand found out that extract of ethanol from the stem of the Sambucus javanica subsp. chinensis (Lindl.) Fukuoka plant had effects of Nl63 with IC50 that ranges from 1.17 (virus yield) to 15.75 µg/ml (virus attachment) showed promising results in treating anti- human corona virus. The ethanol that is extracted reduced the yield of virus, the formation of plaque and its attachment significantly. Moreover, there were three more phenolic acids namely the caffeic, gallic acid and the chlorogenic acid that majorly shows NL63 inhabitation, replication and the attachment of the virus. Among this three of the phenolic acid, caffeic acid was the most potent (Weng et al., 2019). The characterization of the phenolic acid is by their ability to metabolize which is done by the micro biota, which enhances in their bioavailability and its potential to be antiviral can be increased with the help of chain length of alkyl (Kumar and Goel, 2019). Nevertheless, it is controversial about the use of the phenolic acid as they have low absorption and are instable in their alkaline and in their neutral media that limits its usage in the form that is pure. Hence, it could be said that the utility of the phenolic acid components clinically is yet debatable as their mechanism of delivery and bioavailability and the doses that are efficient is yet to be studied further to evaluate in the in vivo models.
Wen et al. (2011) in order to study, anti-SARS-CoV effect, evaluated around 200 traditional Chinese herb extracts using assay that are cell based. Six among them () were inhibit in the replication and growth of the SARS-CoV significantly. The ranges of IC50 were found to be ranging from 25 to 200 mg/ml. It was also discovered by using the FRET assay that the demonstration studied of the extract that is got from the Dioscorea polystachya Turcz tuber and the Cibotium barometz (L.) J. Sm rhizomes created inhabitation which was quite remarking of the SARS-CoV-3CL protease by the IC50 which was around 39 and 44 mg /ml, respectively. Due to the significance of it as a major protein for the genome replication of the SARS-CoV, the helices of the virus, remains a target for the novel antiviral drug.
There were 64 natural molecules that were evaluated from 15 medicinal plants in relation to the inhibitory actions of the helicase of the SARS-CoV, scutellarein and Myricetin and it inhibited the activity of the virus.
At 10 µM, myricetin (IC50 = 2.71 ± 0.19 µM) and scutellarein (IC50 = 0.86 ± 0.48 µM) it was possible to inhibit as much as 90% of the SARS-CoV helicase activity of the ATPase. Hence, according to this study it was seen that the scutellarein and the Myricetin was an excellent solution, which looked promising as a drug, which could be anti-SARS (Yu et al., 2012).
The proteins that are essential for the life cycle of the SARS-CoV-2 are suited as a target that is promising for anti viral drugs. Hence, any products or molecules that can perform to inhibit the proteins of the virus can be used as preventive drugs for SARS-CoV-2 infections (Table 1).
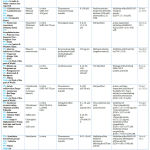 |
Table 1: Natural Product derived by Medicinal Plant used as Anti-COVID -19 |
Natural Products of Medicinal Plants as ACE2- Blockers
The genome of the SARS-CoV-2 penetrates into the host cells using the spike protein that it binds to the receptors of the host (Sigrist et al., 2020). With the use of the critical site of ACE2 structure and the analysis of the phylogenetic structure, it was predicted that the different kinds of animals such as the pigeon, cat and sheep were the immediate significant host of the SARS-CoV-2 (Qiu et al., 2020). It was also demonstrated that the SARS-CoV-2 virus was using the ACE2 receptor in order to penetrate the human cell (Hoffmann et al. (2020) Furthermore, it was reported that for the treatment of the SARS-CoV-2, one of the promising therapeutic approach was using the TMPRRS2 inhibitors. ACE2 and the S proteins can both be cleave by the transmembrane serine protease namely TMPRSS2.
It was suggested in the study that the ACE 2 receptor might turn out to be a major key that is used as a bridge by the SARS-CoV-2 for the transmission into the humans. It was confirmed by the study of Chen et al. (2020) that even though the SARS-CoV and SARS-CoV-2 has similarities in the glycoprotein structure of the spikes as much as 72 %, the interaction rate of SARS-CoV-2 was exhibited higher with the ACE2. It is believed that the inhibitors of the ACE2 alter directly the binding of the sites and block infection from the SRS-CoV-2. In the same way Wrapp et al. (2020) also was able to conclude that the spike of the SARS-CoV-2 had greater affinity with the ACE2 receptors than that of the SARS-CoV. Again Adedeji et al. (2013) was able to demonstrate the mechanism on the ways to block early tie with the ACE2 inhibitors that was efficiently used in the novel anti-SARS drug. Moreover, it was also found out that those patients who were suffering from diabetes and hypertension; even after the use of the inhibitors ACE2, they had higher chance of getting the COVID-19 infection (Guan et al., 2019; Yang X. et al., 2020; Zhang J. J. et al., 2020). The use if inhibitors ACE2 along with other inhibitors such as angiotensin II type – I blockers and receptor led in the reregulation of the ACE2 and it identifies the immediate need for alternates of the ACE2 blockers (Fang et al., 2020). Hence, we can say that products that are derived for the medicinal or natural products are selectively able to block the receptors ACE2 without the process of inhibiting the activity of the enzymes and may result in the useful treatment and prevention of the SARS-CoV-2 infection. The infection that is spreading among the humans without the expressive increase in the ACE2 of the patients and resulting in the increase in the risk of spreading COVID-19.
As there are similarities that are present between ACE 2 and ACE sequence (Guy et al., 2005), the inhibitory effects of those molecules that are present focusing the ACE may have the same kind of effect for the ACE2 and might even lead in the reduction of the entry of the virus. Nonetheless, for the proper evaluation of this hypothesis more studies should be done. Patten et al. (2016) reviewed the effects on ACE2 of the usage in the natural herbal plants. It was reported by them that there were 141 species of medicinal plants that were from around 73 families.
All of these medicinal species had 49 such compounds that were purified and natural and had potential in the inhabitation of ACE receptors. Furthermore, it was also found out that there were medicinal plants from more 16 families and 16 species that were found to be blocking the 1A type receptors in the process of in vitro. Sharifi et al. (2013) in the process of in vitro were able to discover four kinds of medicinal plants in Iran that had the potential to inhibit the activity of the ACE receptors more than 80%. These Iranian medicinal plants were namely Quercus infectoria G. Olivier, Onopordum acanthium L, Crataegus laevigata (Poir.) DC., and Berberis integerrima Bunge. It was seen that the G. Olivier was showing around 94% inhabitation of the receptors ACE at 330 mg/ml. This higher rate of inhabitation in this medicinal plant was subjected to the higher rate of phenolic components present in them that made it possible to become an antioxidant. Even though the significant inhibition of the ACE receptors and its activities that were quite antioxidant the extracts of the Q. Infectoria, the usefulness of the herb was tarnished as it had tannins condensed into which interfered in the inhabitation process of the ACE receptors.
While on the contrary, the remaining three Iranian medicinal plants were shown effective as they had significant activities inhibitory of the ACE receptors and since they did not have tannins in them, they enhanced the potential of the antioxidants present in the herbs (Sharifi et al., 2013). It is possible that these medicinal herbs can result in being sources of antiviral molecules. The infection of the virus is generally attended by the stress in the oxidative and it in return enhances the replication of the virus. The species of antioxidants helps in reducing the speed of the spread of virus by targeting those different pathways that signals stress-related oxidative (Fedoreyev et al., 2018). Walls et al. (2020) in his demonstration was able to show that the SARS-CoV and the SARS-CoV-2 had affinities that were particularly similar to the receptor ACE2. Other studies too were able to show that the herbal families of the Chinese traditions were also inhibiting the ACE2 and SARS-CoV-2 interaction quite significantly. Some of the medicinal herbs that were quite significant in their inhibitory activities were Nelumbonaceae, Lauraceae, Magnoliaceae, Oleaceae, Labiatae and Polygonaceae.
The attribution of these herbs were that they produced a protein that is namely emoting in higher levels in Polygonum and Rheum genuses (one, 3, 8-trihydroxy-6-methylanthraquinone) (Figure 2). The presence of emodin aids in blocking the protein ACE2 in its interaction with the SARS-CoV-2 virus in a manner that is dependent on dose with a 200 µM of IC 50 (Ho et al., 2007).
Natural Products Targeting the TMPRSS2
The transmemebrane that is of type II serine-proteins RSS2 as the one the aids in cleaving the spike proteins of S type in the ACE2 receptors and of the MERS and SARS-CoV (Iwata-Yoshikawa et al., 2019). Hoffmann et al. (2020) in his recent demonstration that it is also usable to use the TMPRSS2 for the priming of the S spike protein in the host cells of SARS-CoV-2 apart from the ACE2 receptors. The complex that is formed after there is an interaction that is made between the host ACE2 cells and the spike S protein of the SARS-CoV-2 can be cleave by the interjection of the TMPRSS which might be able to facilitate in the viral entry (Rabi et al., 2020).
Matsuyama et al. (2020) was able to find that the SRS-CoV-2 were highly susceptible towards its expression in the cell if the TMPRSS2 were involved. As it is found that the ACE2 receptors conditions the binding of the SARS-CoV-2 , and since it is possible to cleave the ACE2 receptors with the use of TMPRSS2, it would be able to down regulate the expression of the TMPRSS2 into the cells of the human. This signifies an important means of therapeutic approach to prevent the infection of the SARS-CoV-2 (Schlagenhauf et al., 2020). It was also revealed in various studies that the TMPRSS2 could be down regulating by using several means of natural remedies. In one of those studies, kaempferol was seen to disable the TMPRSS2 expression as high as 79% at 15mM (Da et al., 2019). Similarly, it was also seen that sulforphane, through its Nrf2 translocation was able to de regulate TMPRSS2 (Gibbs et al., 2009; Meyer and Jaspers, 2015). Mamouni et al. (2018) was able to find that if there is made a standard formulation of flavonoid that included kaempferol, luteolin and quercetin in significance towards the suppression of the TMPRSS2 expression.
It was believed that even though the flavonoids had biological effects that are diverse, it was in the demonstration of the study that found at smaller concentration, these three flavonoids had immense synergy. Regardless it was still unclear about its efficiency and the effectiveness of these flavonoids in the patients of the COVID-19. Furthermore, there were various factors that were related to the patients that may cap the formulation and components usefulness for clinical purposes (Fuzimoto and Isidoro, 2020). It is also demonstrated that the TMPRSS2 activity can also be exhibited effectively by the use of the cryptotanshinone at 0.5 µM (Xu et al. 2012).
Natural Products Targeting the PAPAIN-LIKE PROTEINASE (PLPRO)
One of the non-structural proteins in the genome of the SARS-CoV-2 that is encoded is the PLpro. It is a significant protease, which helps in the replication of the virus as it aids in the cleavage of the PP1A and the PP1AB, which is the polyprotiens of the virus and turns them into the proteins that are effectors (Jiang et al., 2020). Furthermore, it is also found that the PLpro acts as one of the antagonist in the immunity of the host (Yuan et al., 2015; Li et al., 2016).
In reality, the main aim of the PLpro was by the production of the interferon that would lead in the IRF3 blocking of the phosphorylation, the translocation of the nuclear, demonization and by the pathways signaling do NF-Kb and its degradation preventing (Wong et al., 2016). It was also seen in receptors like the Toll 3 and in the pathways of the retinoic acid-inducible gene about the effects. Furthermore, the studies also demonstrated that the inhibitor the PLpro in the SARS-CoV-2 inhibits through inactivation the pathways of TLR7 and the pathways signal ling of the TRAF3/6-TBK1- IRF3/NF-kB/AP1 (Yuan et al., 2015).
Arya et al. (2020) in their recent studies in the in silico process did a screening of one of the FDA approved drug and in it the PLpro showed significant potential. It was shown in their demonstration that there were 16 such drugs approved by the FDA ( Amitriptyline, Benzylpenicillin, Biltricide , Chloroquine, Chlorothiazide, Cinacalcet , Ethoheptazine, Formoterol, Levamisole, Naphazoline, Pethidine, Procainamide, Terbinafine, Tetrahydrozoline and Ticlopidine ) that significantly exhibited the affinity to bind of the PLpro of SARS-CoV-2 and it was suggested that these were possibly effective in the agents which were anti-SARS-CoV2.
In the similar way, one of the drug that was aversively alcoholic namely Disulfiram was demonstrated as an inhibitor that was competitive of the SARS-Cov PLpro (Lin et al., 2018).
Cinnamic Amides from Tribulus terrestris
There were numerous components that were able in the inhibitory effects of the PLpro were found and seemed promising. Song et al. (2014) in his demonstration was able to reveal that there were six kinds of cinnamic amides (Terrestriamide, N-trans-Feruloyltryamine, Terrestrimine, N-trans-Caffeoyltryamine, N-trans- Coumaroyltyramine and N-trans-Feruloyloctopamine) that were able to be extracted from the fruit Tribulus terrestris L. and in a dose dependant manner showed that they were able to inhibit the PLpro of the SARS-CoV. It was found that IC50, which is the inhibitory of the PLpro, were found to be Terrestrimine in 15.8-10.1 µM. This showed that the activity of inhibitory of the PLpro of SARS-CoV was best when used with IC50 of 15.8 ± 0.6 µM.
The polar substituent’s such as the alcohole and ketone that was present in the groups of the methylene (C7’and C8’) were involved in the enhancing of the activities of the inhibitory.
Flavonoids from Cullen corylifolium (L.) Medik.
The extract from the seeds of the Cullen corylifolium (L.) Medik are ethanolic and it demonstrated significant effect in the process of inhibitory of the PLpro of the SARS-CoV with the containment of the IC50 at 15 µg/ml. Moreover, there was a list of flavonoid of six number in the extract that was present (corylifol, psoralidin, 40 –O-methylbavachalcone, isobavachalcone, neobavaisoflavone and Bavachinin). These were those extracts, which were very active in the activity of inhibiting the PLpro of the SARS-CoV in a manner of dose dependant. It also contained IC50 in an estimation of 4.2–38.4 µM. The most significant of the inhabitation was performed by the isobavachalcone (IC50 = 7.3 +- 0.8 µM) psoralidin that was present in the extract (IC50 = 4.2 ± 1.0 µM) (Kim et al., 2014).
Flavonoids from Paulowina tomenstosa (Thunb) Steud.
Cho et al. (2013) was able in their studies to find out that there were five more flavonones that were newly geranylated (tomentin E, tomentin D, tomentin C, tomentin B and tomentin A) and were extracted from from the fruit that is known as Paulownia tomentosa (Thunb.) Steud.and it WA found to be ethanolic in nature. Apart for the other seven flavonoids it is believed that were present these were also seen resulting in significantly inhibitory of the PLpro in the SARS-CoV in a manner that is supposedly dose dependant that had IC50 in it that was around 5.0 to 14.4 mM. It was also seen that the flavonoid tomentin E shows large levels of inhibitory activity with the containment of the IC50, which had 5.0 ± 0.06 µM. It was also seen of the molecules that they were having high inhibitory possession since they had three, 4-dihydro-2H-pyran moiety. It was later seen that the flavonoid P. tomentosa was present in mixed inhibitors and was reversible.
Chalcones from Angelica keiskei (Miq.) Koidz
Park et al. (2016) in his in investigation was able to find that there were nine other chalcones that were alkylated and had the potential of inhibition (xanthokeistal A, xanthoangelol G, xanthoangelol B, xanthoangelol E, xanthoangelol D, xanthoangelol F, xanthoangelol, 4-hydroxyderricin and isobavachalcone). He was also able to extract from Angelica keiskei (Miq.) Koidz, some ethanolic extracts that had four coumarins that were potential inhibitors. The flavonoids in this chalcones that were alkylated had contains of IC50 that ranges from 1.2 ± 0.4 to 46.4 ± 7.8 µM and had inhibitory characteristics towards the PLpro of the SARS-CoV in significance that was in a manner of dose dependant. On the contrary, it was even found out that the coumarins that were analyzed were not significantly showing effects of inhabitations of the PLpro of the SARS-CoV. It was even reveled by the kinetic studies that chalcones are somehow not that competitive where as on the other hand, isobavachalcone was a mixed inhibitor. It was even interesting to find out that the component namely xanthoangelol E showed effect of great and enhanced type of effect of inhibitory (= 1.2 ± 0.4 µM), which when compared to the other chalcones that were testes. It was in the used of in silico studies that the activity of inhibitory of xanthoangelol E was stated and was confirmed.
Tanshinones from Salvia miltiorrhiza Bunge
It was even seen that the extracts from the Salvia miltiorrhiza Bunge were ethanolic (30 µg/ml) and was able to inhibit the PLpro of the SARS-CoV as much as 88%. Furthermore, there were tanshinones that were bioactive namely seven of them (rosmariquinone, dihydrotanshinone I, tanshinone I, cryptotanshinone, methyl tanshinonate, tanshinone IIB and tanshinone IIA) which could be identified in the fraction of n-hexane. These tanshinones were seen using the assay of fluorometric, which was evaluated and seen that it had the inhibiting factors toward the PLpro of the SARS-CoV. The molecules from both the components were seen significantly inhibiting activities that were time dependant and had IC50 that ranged from 0.8 to 30 µM. The structure that is present contains dimethyl tetrahydronaphthalen and is related to the potential of enhanced inhibitory. It was also shown that there was an inhibitor which was most potent against the PLpro of SARS-CoV and is called cryptotanshinone and it had IC50 that ranges from 0.8=/= 0.2 µM.
It was even confirmed by the demonstration by the kinetic investigators that there is an inhibitor that is of mixed type namely rosmariquinone for the PLpro of the SARS-CoV and it was used in the alternatives where the tnashinones were not able to compete as inhibitors (Park et al., 2012a).
Diarylheptanoids from Alnus japonica (Thunb.) Steud.
Park et al. (2012b) used for the identification of the diarylheptanoids a fractionation that was guided by activity (rubranoside A, rubranoside B, rubranol, oregonin, hirsutanonol, platyphyllonol-5-xylopyranoside, platyphyllone, hirsutenone and platyphyllenone) that was extracted from the Alnus japonica (Thunb.) Steud and had ethanol as well. It was also done for the evaluation of the assay of the fluorometic and its continuous effect. It was later seen in the results that rubranoside a, rubranoside B, rubranol, oregonin, hirsutanonol and hirsutenone had quite significant activity towards the inhibition of PLpro of the SRS-CoV in a manner that is dose dependant and has IC50 that has ranges from three to 44.5 µM. It was also seen that one of the components had IC 50 that ranged from 4.1 ± 0.3 µM and is namely hirsutenone had great inhibitory potential effect and was not that significant than than of the referred inhibitor known as the curcumin ( 5.7 µM).
It was also studied the the main reason diarylheptanoids was and potentially enhanced in the inhibitory activities as it was due to the presence of groups of cathecol and carbonyl of a,b- unsaturated.
Natural Products Targeting the CHYMOTRYPSIN-LIKE PROTEASE [3CL (PRO)]
One of the proteins of the SARS-CoV-2 virus is kwon as 3CL (pro) and it comes from 16 proteins that are nonstructural. It is believed the 3CL (pro) proteins have a significant in the process of replication, which is known as polyporteins of the virus SARS-CoV-2. Hence, it is considered among the therapeutics as a target, which is potential for the creation of anti-COVID-19 drugs (Zhang L. et al.,2020).
Alkylated Chalcones from Angelica keiskei (Miq.) Koidz
The extracts that are extracted from the Angelica keiskei (Miq.) Koidz, which showed quite some potential against the 3CL (pro) of the SARS-CoV, had coumarins and chalcones that were alkylated and after further investigation it was found that it was using the FRET method which is the fluorescence resonance energy transfer method. It was only in the case of the chalcones that were alkylated apart from the coumarins that showed in a manner of dose dependent that they had effects of inhibitory that were significant and the IC50 ranged from 11.4 ± 1.4 to 129.8 ± 10.3 µM. Furthermore, it was also found that the most potent compound to be the best inhibitor of the 3CL (pro) of the SARS-CoV was, xanthoangelol E . It was also demonstrated in the kinetic studies that both the chalcones that were alkynted showed inhibitions competitively . As it was found out that xanthoangelol E could be sued in the process of inhibiting the PL(pro) of the SARS-CoV too (Park O. K. et al., 2016) this compound is believed to be the most potential in the approaches of therapeutic against COVID-19.
Phlorotannins from Ecklonia cava (Algae)
Park et al. (2013) was able to separate from the brown Alga Ecklonia cava’s ethanolic extract nine kinds of phlorotannins. These pholrotannins was believed to use an assay that was based on cell-free and had inhibitory effects towards the 3CL (pro) of the SARS-CoV. It was seen that eight of these phlorotannins (phlorofucofuroeckol A, dieckol, fucodiphloroethol G, 7- phloroeckol, 2-phloroeckol, dioxinodehydroeckol, eckol and triphloretol A) in a manner of dose dependent showed inhibitory activities that were cleaving against the 3CL(pro) of the SARS-CoV. The IC50 of the phlorotannins were ranging from 2.7 ± 0.6 in the extract dieckol until 164.7 ± 10.8 µM in the extract triphloretol A. Furthermore, it was also seen that six of these phlorotannins (phlorofucofuroeckol A, dieckol, fucodiphloroethol G, 7-phloroeckol, 2-phloroeckol and dioxinodehydroeckol ) were showing activities of cis-cleaving in quite importantly micro molecule in the manner of dose dependent against the 3CL(pro) of the SARS-CoV. The phlorotannin diekol was one of those molecules that were tested (possessing two eckol groups linked by a diphenyl ether) and it showed that the effects of inhibitory was best in the diekcol towards the 3CL(pro) of the SARS-CoV.
The dockings of the molecules and its studies provided the collaborated results and the energy required for binding (11.51 kcal/mol) was the lowest for the 3CL (pro) of the SARS-CoV. It was see that the Diekcol formed bonds they were strong in H form towards the catalytic dyad (His41 and Cys145). However, there are also limitations in the usefulness of these phlorotannins substantially and still validation is needed, as their availability in the biosphere and their metabolism differences is still quite questionable. It is also seen that the micro biota in the gut also had a significant role in the determination go the benefits of the health (Corona et al., 2016). Furthermore, the structure of these phlorotannins is quite complex and are diverse in the linkages in the structures. They even have varied isomers that re conformational for the same weight of the molecule and even lack any kinds of standards that are based in analytics. Additionally there is no clear picture of the relationship that is present among the bioactivity and their structure and it might add other restrictions towards its use in the purposes of clinical usage .
Tanshinones from Salvia miltiorrhiza Bunge
Park O. K. et al. (2012) did an investigation regarding the potential inhibitory activities of the Salvia miltiorrhiza Bunge that was aimed at the 3CL (pro) of the SARS-CoV. It was found by them that there was present the extract of ethanol in the Salvia miltiorrhiza Bunge (30 µg/ml) and it had high results in inhibiting the SARS-CoV-3CL (pro) as high as 60%. Moreover , it was even found out by demonstration that there were present 6 tanshinones (the fraction of the lipophilic) of the plant and it too showed higher inhibitory activities that aimed at the SARS-CoV-3CL(pro) in a manner that was not time dependent but it was dose dependent. The estimated IC50 that was present was around 14.4–89.1 µM. It was also seen that the most significant inhibitor that had an effect is the Dihydrotanshinone I, which has IC50 that ranges from 14.4 ± 0.7 µM. It is also in the regards of the kinetic mechanism that inhibition of the SARS-CoV-3CL (pro) by Salvia miltiorrhiza Bunge tanshinones was found that it was not a competitive inhibitor.
Biflavonoids from Torreya nucifera (L.) Siebold & Zucc.
From the leaves of Torreya nucifera (L.) Siebold & Zucc the extract of ethanol four kinds of bioflavonoid were able to get extracted (sciadopitysin, ginkgetin, bilobetin and amentoflavone) and they were under evaluation for the inhibitory effect of the 3CL(pro) of the SARS-CoV by the use of the FRET methods. It was seen that all of them nearly, the bioflavonoid had IC50 that raged from the value IC50 of 8.3–72.3 µM. had significant inhibitory activities on the SARS-CoV-3CL (pro). It was also found that the diterpenoids that were isolated from the T. nucifera were weaker that the inhibitory effect of the bioflavonoid. It was also seen that the moiety apigenin which at the position c-30 of the flavones are shown as the one which is suggested to have greated effect of inhibitory (Ryu et al., 2010).
Flavonoids
In the expression of the Pichia pastoris GS115 there was an evaluation of seven falvanoids done (ampelopsis, epigallocatechin, gallate, epigallocatechin, gallocatechin gallate, Daidzein, Puerarin and Quercetin) which showed that there had effects that were inhibitory towards the 3CL(pro) of the SARS-CoV and it was able to inhibit around 82, 85 and 91% of the SARS-CoV-3CL(pro) ‘s activities. It was also seen of one of the most competitive inhibitor of the SARS-CoV-3CL(pro) was the gallocatechn gallate which had IC50 that ranged as high as 47 µM and it was also confirmed through studies that it was due to the interaction of the H-bonds and the hydrophobic bonds that the SARS-CoV-3CL(pro) actively formed (Nguyen et al., 2012). However, it is not known how the biological bonds might react how supposedly will the functions of these active molecules might be (Chen et al., 2016). Moreover, it is not studied and evaluated about how strong are the H-bonds and it is also significant to assess the molecules that as testes because in there is an increase in the number of weakened H bonds then their affinity could be increased and that would result in the interaction with the proteins that are off target.
Jo et al. (2020) in his studies was able to evaluate the effects of inhibitory toward the 3CL (pro) of the SARS-CoV of the 64 falvonoids that are using the method FRET. They were able to discover through various demonstration that the inhibitory effects of pectolinarin, rhoifolin and of the herbacetin were greatly higher as they had IC50 that were in the ranges 37.78, 27.45 and 33.17 mM respectively.
Key Considerations
Currently, in a study there were 15 studies that were done in vitro are included. These studies are from countries such as Iran (1/15), China (2/15), Taiwan (3/15) and Korea (9/15). It was also realized that majority of these studies where those which were published between the years which is 2011 to 2018. It was only two of the studies that was form the current year 2020 (Runfeng et al., 2020; Jo et al., 2020) and only one study was taken that was from the year 2019 (Weng et al., 2019).
It was also seen that among all these studies it was only two of them which was done in order to investigate the activity of the antivirus against the SARS-CoV-2 (Runfeng et al., 2020) and the other study was on the NL63 human corona virus (Weng et al., 2019). Apart from these, no other studies were cell based. It was also clear that there were three studies using the extracts of the plant (Runfeng et al., 2020) the mixture of 11 herbs of the Chinese tradition was used in one study. In the extraction of components in the plants, the solvents used for the preparation of the plant were methanol, water and ethanol. Majority of these studies were made in isolation and with the compounds that were natural and were belonging to the classes that were differential in photochemical.
While analyzing these studies in the psychopharmacological researches using the best possible recommendations (Heinrich et al., 2020) there were numerous concerns that was seen and there were concerns that were numerously detected significantly relating to the data and outcomes reports. Under this review, there were a huge amount of traditional and natural herd were reports such as those of kaempferol, phenolic acids and quercetin and they all belonged to the class of the photochemical and are known to be possessing biological activities in large spectrum both in vitro and in vivo.
Hence it was seen that majority of these products resulted in failures. Additionally, there was no inclusion in any of these studies about the consideration of the durability of the compounds, that were “active” in the given extracts and interpretation of the data that was obtained was insufficient.
Additionally, the studies that are presented, all of them, has either some limitations of method or concepts in developing of the project. As a matter of the fact , none of these studies did investigation regarding to the compounds that are isolated and of the herbal extracts considering the ways of proper sourcing them sustainably neither did they register standard names of either the plants that were accepted nor about the compounds. In the very same way, there is biasness also shown in the studies relating to those of the dosage ranges and of the doses, which could be toxic. Contrarily, there was no control measure mentioned in around eight of these studies while the rest of the studies dint even use comparison to justify their positive choice of controls. This is quite a considering factor that results in biasness and limits the study and its methodologies.
In spite of the various potential exhibitions of the natural molecules and of the natural extracts on the plants on the anti-SARS-CoV-2 effects, numerous limitations should be able to be considered. The general idea is that due to the recent outbreak , the final conclusions of these studies needs to validated as these data and information can be still consider as immature and usefulness of these components clinically should be on restriction. In some cases, the species does not even show relation towards the use in between the effect of SARS-CoV-2 and of the traditional ethno pharmacological use. There are other factors as well that is needed to be taken in consideration such as the stages of the disease, the administrative modes, the health of the digestive system of the patients, the dosage that are safe, exposure times, etc are to be evaluated as well for the effects of the herbal medicines against the SARS-CoV-2. Furthermore, there is need to have clarification in the mechanisms and about the targeted pathways of such products that might aid in the enhancement of the clinical usage. The assessment of these natural products along with the validation for the drugs to be antiviral might show promising alternatives to explore.
Conclusion
Even now there natural medicinal plants, that have quite some potential in order to become alternatives in the treatment and prevention of several diseases. In China alone, there has been usage of herbal medicines that are traditional, has been used, and are demonstrating results that are quite positive among the patients of the COVID-19 and in improving the effect on their health. Currently in the review above there has been discussion made on the herbal traditional medicines that show promising usage in order to treat and for the possible prevention of the COVID-19. Even though the evaluation in the above studies is not sufficient and still lacks proper validation in the treatment as any kinds of anti-SARS-CoV-2 effects. There are some products that are available naturally and since they have IC50 that is below the 10 µM. there might be a considering as potential drug for the COVID-19 agents as they demonstrate activities that act as protein blockers in the receptor ACE2 of the SARS-CoV-2.
However, there has been detection of large number of limitations and they are in direct relation towards the action’s specificity that these kinds of products exert. It can be seen through the indication in many studies that the products that are based on the medicinal and herbal plants might provide aid in the fight against the COVID-19. There should be a further study that is required to be carried out in order to properly evaluate these products in the fields of clinical usage against the pandemic infection outbreak of the COVID-19. Mixtures of these natural products after enhanced assessment and validation, might be made with several combinations might become validated as an anti-COVID-19 drug and in future might be constituted as assessment of alternative as preventive and therapeutic approaches.
Acknowledgement
First and foremost, I would to thank Allah Almighty for giving me the strength, knowledge, ability and opportunity to undertake this research work and to preserve and complete it satisfactorily. Without his blessings, this achievement would not have been possible. I take pride in acknowledging the insightful guidance of Dr. Soma Ghosh , Principal , Hiralal Mazumdar Memorail College for Women , Kolkata , West Bengal , India , for sparing her valuable time whenever I approach her and showing me the way ahead .
I would also like to express my gratitude to my entire colleagues of H M M College for Women , Kolkata who have been so helpful and cooperative in giving their support at all times to help me to achieve my goal .
My acknowledgment would be incomplete without thanking the biggest source of my strength, my family and the blessing of my late parents.
Conflict of Interests
The author declare that there exist no commercial or financial relationship that could , in any way , lead to potential conflict of interest .
Funding Source
Author declared that he hasn’t received any financial assistance from any organization for conducting above mentioned work.
References
- Adedeji AO, Severson W, Jonsson C, Singh K, Weiss SR, Sarafianos SG. Novel Inhibitors of Severe Acute Respiratory Syndrome Coronavirus Entry That Act by Three Distinct Mechanisms. Journal of Virology. 2013;87(14):8017-8028. doi:10.1128/jvi.00998-13
CrossRef - Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52(4):583-589. doi:10.1016/j.immuni.2020.03.007
CrossRef - Arya R, Das A, Prashar V, Kumar M. Potential inhibitors against papain-like protease of novel coronavirus (SARS-CoV-2) from FDA approved drugs. 2020. doi:10.26434/chemrxiv.11860011.v2
CrossRef - Basu A, Chakraborty S. Faculty Opinions recommendation of Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature. 2020. doi:10.3410/f.737422643.793575066
CrossRef - Bioavailability of Black Tea Theaflavins: Absorption Metabolism, and Colonic Catabolism. doi:10.1021/acs.jafc.7b01707.s001
CrossRef - Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research. 2020;178:104787. doi:10.1016/j.antiviral.2020.104787
CrossRef - Chen D, Oezguen N, Urvil P, Ferguson C, Dann SM, Savidge TC. Regulation of protein-ligand binding affinity by hydrogen bond pairing. Science Advances. 2016;2(3). doi:10.1126/sciadv.1501240
CrossRef - Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochemical and Biophysical Research Communications. 2020;525(1):135-140. doi:10.1016/j.bbrc.2020.02.071
CrossRef - Chi JH, Kim YH, Sohn DH, Seo GS, Lee SH. Ameliorative effect of Alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomedicine & Pharmacotherapy. 2018;108:1767-1774. doi:10.1016/j.biopha.2018.10.050
CrossRef - Cho JK, Curtis-Long MJ, Lee KH, et al. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorganic & Medicinal Chemistry. 2013;21(11):3051-3057. doi:10.1016/j.bmc.2013.03.027
CrossRef - Cho S-H, Kim H-S, Lee W, et al. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. International Immunopharmacology. 2020;82:106146. doi:10.1016/j.intimp.2019.106146
CrossRef - Chokpaisarn J, Urao N, Voravuthikunchai SP, Koh TJ. Quercus infectoria inhibits Set7/NF-κB inflammatory pathway in macrophages exposed to a diabetic environment. Cytokine. 2017;94:29-36. doi:10.1016/j.cyto.2017.04.005
CrossRef - Clinical and epidemiological characteristics of Coronavirus Disease 2019 (COVID-19) patients. doi:10.37473/fic/10.1101/2020.04.02.20050989
CrossRef - Corona G, Ji Y, Anegboonlap P, et al. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. British Journal of Nutrition. 2016;115(7):1240-1253. doi:10.1017/s0007114516000210
CrossRef - Da J, Xu M, Wang Y, Li W, Lu M, Wang Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Analytical Cellular Pathology. 2019;2019:1-10. doi:10.1155/2019/1907698
CrossRef - Ding Y, Zeng L, Li R, et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complementary and Alternative Medicine. 2017;17(1). doi:10.1186/s12906-017-1585-7
CrossRef - Du H-Z, Hou X-Y, Miao Y-H, Huang B-S, Liu D-H. Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chinese Journal of Natural Medicines. 2020;18(3):206-210. doi:10.1016/s1875-5364(20)30022-4
CrossRef - Du J, Hu Z, Dong W-J, Wang Y, Wu S, Bai Y. Biosynthesis of large-sized silver nanoparticles using Angelica keiskei extract and its antibacterial activity and mechanisms investigation. Microchemical Journal. 2019;147:333-338. doi:10.1016/j.microc.2019.03.046
CrossRef - Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine. 2020;8(4). doi:10.1016/s2213-2600(20)30116-8
CrossRef - Fani M, Teimoori A, Ghafari S. Comparison of the COVID-2019 (SARS-CoV-2) pathogenesis with SARS-CoV and MERS-CoV infections. Future Virology. 2020;15(5):317-323. doi:10.2217/fvl-2020-0050
CrossRef - Fedoreyev S, Krylova N, Mishchenko N, et al. Antiviral and Antioxidant Properties of Echinochrome A. Marine Drugs. 2018;16(12):509. doi:10.3390/md16120509
CrossRef - Fung TS, Liu DX. Human Coronavirus: Host-Pathogen Interaction. Annual Review of Microbiology. 2019;73(1):529-557. doi:10.1146/annurev-micro-020518-115759
CrossRef - Fuzimoto AD, Isidoro C. The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds – Additional weapons in the fight against the COVID-19 pandemic? Journal of Traditional and Complementary Medicine. 2020;10(4):405-419. doi:10.1016/j.jtcme.2020.05.003
CrossRef - Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proceedings of the National Academy of Sciences. 2009;106(39):16663-16668. doi:10.1073/pnas.0908908106
CrossRef - Guy J, Lambert D, Warner F, Hooper N, Turner A. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 2005;1751(1):2-8. doi:10.1016/j.bbapap.2004.10.010
CrossRef - Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews. 2018;18(1):241-272. doi:10.1007/s11101-018-9591-z
CrossRef - Heinrich M, Appendino G, Efferth T, et al. Best practice in research – Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology. 2020;246:112230. doi:10.1016/j.jep.2019.112230
CrossRef - Ho T, Wu S, Chen J, Li C, Hsiang C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Research. 2007;74(2):92-101. doi:10.1016/j.antiviral.2006.04.014
CrossRef - Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. Journal of Virology. 2019;93(6). doi:10.1128/jvi.01815-18
CrossRef - Ji P, Chen C, Hu Y, et al. Antiviral Activity of Paulownia tomentosa against Enterovirus 71 of Hand, Foot, and Mouth Disease. Biological and Pharmaceutical Bulletin Biological ^|^ Pharmaceutical Bulletin. 2015;38(1):1-6. doi:10.1248/bpb.b14-00357
CrossRef - Jiang S, Hillyer C, Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends in Immunology. 2020;41(6):545. doi:10.1016/j.it.2020.04.008
CrossRef - Jo S, Kim S, Shin DH, Kim M-S. Inhibition of SARS-CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry. 2019;35(1):145-151. doi:10.1080/14756366.2019.1690480
CrossRef - Kalpana D, Han JH, Park WS, Lee SM, Wahab R, Lee YS. Green biosynthesis of silver nanoparticles using Torreya nucifera and their antibacterial activity. Arabian Journal of Chemistry. 2019;12(7):1722-1732. doi:10.1016/j.arabjc.2014.08.016
CrossRef - Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochemical and Biophysical Research Communications. 2004;323(1):264-268. doi:10.1016/j.bbrc.2004.08.085
CrossRef - Kil Y-S, Pham ST, Seo EK, Jafari M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Archives of Pharmacal Research. 2017;40(6):655-675. doi:10.1007/s12272-017-0892-3
CrossRef - Kim DW, Seo KH, Curtis-Long MJ, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. Journal of Enzyme Inhibition and Medicinal Chemistry. 2013;29(1):59-63. doi:10.3109/14756366.2012.753591
CrossRef - Koo HJ, Lee S, Chang KJ, et al. Hepatic anti-inflammatory effect of hexane extracts of Dioscorea batatas Decne: Possible suppression of toll-like receptor 4-mediated signaling. Biomedicine & Pharmacotherapy. 2017;92:157-167. doi:10.1016/j.biopha.2017.05.036
CrossRef - Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports. 2019;24. doi:10.1016/j.btre.2019.e00370
CrossRef - Lee J-W, Seo K-H, Ryu HW, et al. Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. Journal of Ethnopharmacology. 2018;210:23-30. doi:10.1016/j.jep.2017.08.028
CrossRef - Lee M-J, Nho J-H, Yang B-D, et al. Subchronic toxicity evaluation of ethanol extract of Cassia tora L. seeds in rats. Regulatory Toxicology and Pharmacology. 2019;109:104487. doi:10.1016/j.yrtph.2019.104487
CrossRef - Li S-W, Wang C-Y, Jou Y-J, et al. SARS Coronavirus Papain-Like Protease Inhibits the TLR7 Signaling Pathway through Removing Lys63-Linked Polyubiquitination of TRAF3 and TRAF6. International Journal of Molecular Sciences. 2016;17(5):678. doi:10.3390/ijms17050678
CrossRef - Li Y, Fu X, Duan D, Liu X, Xu J, Gao X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Marine Drugs. 2017;15(2):49. doi:10.3390/md15020049
CrossRef - Liang J, Yan C, Zhang Y, Zhang T, Zheng X, Li H. Rapid discrimination of Salvia miltiorrhiza according to their geographical regions by laser induced breakdown spectroscopy (LIBS) and particle swarm optimization-kernel extreme learning machine (PSO-KELM). Chemometrics and Intelligent Laboratory Systems. 2020;197:103930. doi:10.1016/j.chemolab.2020.103930
CrossRef - Liu R, Su B, Huang F, et al. Identification and analysis of cardiac glycosides in Loranthaceae parasites Taxillus chinensis (DC.) Danser and Scurrula parasitica Linn. and their host Nerium indicum Mill. Journal of Pharmaceutical and Biomedical Analysis. 2019;174:450-459. doi:10.1016/j.jpba.2019.05.071
CrossRef - Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). BioScience Trends. 2020;14(1):69-71. doi:10.5582/bst.2020.01020
CrossRef - Luo H, Tang Q-L, Shang Y-X, et al. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chinese Journal of Integrative Medicine. 2020;26(4):243-250. doi:10.1007/s11655-020-3192-6
CrossRef - Ma JN, Kang SY, Park JH, Park Y-K, Jung HW. Effects of Rhizome Extract of Dioscorea Batatas and Its Active Compound, Allantoin, on the Regulation of Myoblast Differentiation and Mitochondrial Biogenesis in C2C12 Myotubes. 2018. doi:10.20944/preprints201806.0398.v1
CrossRef - Mackenzie JS, Smith DW. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiology Australia. 2020;41(1):45. doi:10.1071/ma20013
CrossRef - Mamouni K, Zhang S, Li X, et al. A Novel Flavonoid Composition Targets Androgen Receptor Signaling and Inhibits Prostate Cancer Growth in Preclinical Models. Neoplasia. 2018;20(8):789-799. doi:10.1016/j.neo.2018.06.003
CrossRef - Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308(12). doi:10.1152/ajplung.00028.2015
CrossRef - Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308(12). doi:10.1152/ajplung.00028.2015
CrossRef - Nasab FK, Khosravi AR. Ethnobotanical study of medicinal plants of Sirjan in Kerman Province, Iran. Journal of Ethnopharmacology. 2014;154(1):190-197. doi:10.1016/j.jep.2014.04.003
CrossRef - Nguyen TTH, Woo H-J, Kang H-K, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnology Letters. 2012;34(5):831-838. doi:10.1007/s10529-011-0845-8
CrossRef - Park EY, Kim EH, Kim MH, Seo YW, Lee JI, Jun HS. Polyphenol-Rich Fraction of Brown AlgaEcklonia cavaCollected from Gijang, Korea, Reduces Obesity and Glucose Levels in High-Fat Diet-Induced Obese Mice. Evidence-Based Complementary and Alternative Medicine. 2012;2012:1-11. doi:10.1155/2012/418912
CrossRef - Park J-Y, Jeong HJ, Kim JH, et al. Diarylheptanoids from Alnus japonica Inhibit Papain-Like Protease of Severe Acute Respiratory Syndrome Coronavirus. Biological and Pharmaceutical Bulletin. 2012;35(11):2036-2042. doi:10.1248/bpb.b12-00623
CrossRef - Park J-Y, Kim JH, Kim YM, et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorganic & Medicinal Chemistry. 2012;20(19):5928-5935. doi:10.1016/j.bmc.2012.07.038
CrossRef - Park J-Y, Kim JH, Kwon JM, et al. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic & Medicinal Chemistry. 2013;21(13):3730-3737. doi:10.1016/j.bmc.2013.04.026
CrossRef - Park OK, Choi JH, Park JH, et al. Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia. 2012;83(8):1666-1674. doi:10.1016/j.fitote.2012.09.020
CrossRef - Patten GS, Abeywardena MY, Bennett LE. Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Critical Reviews in Food Science and Nutrition. 2013;56(2):181-214. doi:10.1080/10408398.2011.651176
CrossRef - Qiu Y, Zhao Y-B, Wang Q, et al. Predicting the Angiotensin Converting Enzyme 2 (ACE2) Utilizing Capability as the Receptor of SARS-CoV-2. 2020. doi:10.20944/preprints202003.0091.v1
CrossRef - Rabi FA, Zoubi MSA, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens. 2020;9(3):231. doi:10.3390/pathogens9030231
CrossRef - Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacological Research. 2020;156:104761. doi:10.1016/j.phrs.2020.104761
CrossRef - Ryu YB, Jeong HJ, Kim JH, et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorganic & Medicinal Chemistry. 2010;18(22):7940-7947. doi:10.1016/j.bmc.2010.09.035
CrossRef - Sadat-Hosseini M, Farajpour M, Boroomand N, Solaimani-Sardou F. Ethnopharmacological studies of indigenous medicinal plants in the south of Kerman, Iran. Journal of Ethnopharmacology. 2017;199:194-204. doi:10.1016/j.jep.2017.02.006
CrossRef - Schlagenhauf P, Grobusch MP, Maier JD, Gautret P. Repurposing antimalarials and other drugs for COVID-19. Travel Medicine and Infectious Disease. 2020;34:101658. doi:10.1016/j.tmaid.2020.101658
CrossRef - Sharifi N, Souri E, Ziai SA, Amin G, Amanlou M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU Journal of Pharmaceutical Sciences. 2013;21(1). doi:10.1186/2008-2231-21-74
CrossRef - Shi Y, Wang X, Wang N, Li F-F, You Y-L, Wang S-Q. The effect of polysaccharides from Cibotium barometz on enhancing temozolomide–induced glutathione exhausted in human glioblastoma U87 cells, as revealed by 1H NMR metabolomics analysis. International Journal of Biological Macromolecules. 2020;156:471-484. doi:10.1016/j.ijbiomac.2020.03.243
CrossRef - Sigrist CJ, Bridge A, Mercier PL. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Research. 2020;177:104759. doi:10.1016/j.antiviral.2020.104759
CrossRef - Smit C, Peeters MYM, Anker JNVD, Knibbe CAJ. Chloroquine for SARS-CoV-2: Implications of Its Unique Pharmacokinetic and Safety Properties. Clinical Pharmacokinetics. 2020;59(6):659-669. doi:10.1007/s40262-020-00891-1
CrossRef - Song J, Zhang F, Tang S, et al. A Module Analysis Approach to Investigate Molecular Mechanism of TCM Formula: A Trial on Shu-feng-jie-du Formula. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1-14. doi:10.1155/2013/731370
CrossRef - Song YH, Kim DW, Curtis-Long MJ, et al. Papain-Like Protease (PLpro) Inhibitory Effects of Cinnamic Amides from Tribulus terrestris Fruits. Biological and Pharmaceutical Bulletin. 2014;37(6):1021-1028. doi:10.1248/bpb.b14-00026
CrossRef - Tayel AA, El-Sedfy MA, Ibrahim AI, Moussa SH. Application of Quercus infectoria extract as a natural antimicrobial agent for chicken egg decontamination. Revista Argentina de Microbiología. 2018;50(4):391-397. doi:10.1016/j.ram.2017.12.003
CrossRef - Tian C, Chang Y, Liu X, et al. Anti-inflammatory activity in vitro, extractive process and HPLC-MS characterization of total saponins extract from Tribulus terrestris L. fruits. Industrial Crops and Products. 2020;150:112343. doi:10.1016/j.indcrop.2020.112343
CrossRef - Walls AC, Park Y-J, Tortorici MA, Wall A, Mcguire AT, Veesler D. Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. 2020. doi:10.1101/2020.02.19.956581
CrossRef - Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. Journal of Medical Virology. 2020;92(7):797-806. doi:10.1002/jmv.25783
CrossRef - Wang C-H, Zhong Y, Zhang Y, et al. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Molecular BioSystems. 2016;12(2):606-613. doi:10.1039/c5mb00448a
CrossRef - Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. BioScience Trends. 2020;14(1):64-68. doi:10.5582/bst.2020.01030
CrossRef - Wei T, Deng K, Gao Y, et al. SmKSL overexpression combined with elicitor treatment enhances tanshinone production from Salvia miltiorrhiza hairy roots. Biochemical Engineering Journal. 2020;158:107562. doi:10.1016/j.bej.2020.107562
CrossRef - Wen C-C, Shyur L-F, Jan J-T, et al. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. Journal of Traditional and Complementary Medicine. 2011;1(1):41-50. doi:10.1016/s2225-4110(16)30055-4
CrossRef - Weng J-R, Lin C-S, Lai H-C, et al. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Research. 2019;273:197767. doi:10.1016/j.virusres.2019.197767
CrossRef - Weng J-R, Lin C-S, Lai H-C, et al. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Research. 2019;273:197767. doi:10.1016/j.virusres.2019.197767
CrossRef - Wong L-YR, Lui P-Y, Jin D-Y. A molecular arms race between host innate antiviral response and emerging human coronaviruses. Virologica Sinica. 2016;31(1):12-23. doi:10.1007/s12250-015-3683-3
CrossRef - Wrapp D, Wang N, Corbett KS, et al. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. 2020. doi:10.1101/2020.02.11.944462
CrossRef - Wu Q, Yang X-W. The constituents of Cibotium barometz and their permeability in the human Caco-2 monolayer cell model. Journal of Ethnopharmacology. 2009;125(3):417-422. doi:10.1016/j.jep.2009.07.017
CrossRef - Xu D, Lin T-H, Li S, et al. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Letters. 2012;316(1):11-22. doi:10.1016/j.canlet.2011.10.006
CrossRef - Xu J, Zhao S, Teng T, et al. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi:10.3390/v12020244
CrossRef - Yang B, Kim S, Kim J-H, Lim C, Kim H, Cho S. Gentiana scabra Bunge roots alleviates skin lesions of contact dermatitis in mice. Journal of Ethnopharmacology. 2019;233:141-147. doi:10.1016/j.jep.2018.12.046
CrossRef - Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. International Journal of Biological Sciences. 2020;16(10):1708-1717. doi:10.7150/ijbs.45538
CrossRef - Yu M-S, Lee J, Lee JM, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic & Medicinal Chemistry Letters. 2012;22(12):4049-4054. doi:10.1016/j.bmcl.2012.04.081
CrossRef - Yuan L, Chen Z, Song S, et al. p53 Degradation by a Coronavirus Papain-like Protease Suppresses Type I Interferon Signaling. Journal of Biological Chemistry. 2014;290(5):3172-3182. doi:10.1074/jbc.m114.619890
CrossRef - Yun J-W, Kim S-H, Kim Y-S, et al. Enzymatic extract from Ecklonia cava : Acute and subchronic oral toxicity and genotoxicity studies. Regulatory Toxicology and Pharmacology. 2018;92:46-54. doi:10.1016/j.yrtph.2017.10.034
CrossRef - Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730-1741. doi:10.1111/all.14238
CrossRef - Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020. doi:10.1126/science.abb3405
CrossRef - Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology. 2020;30(8):1578. doi:10.1016/j.cub.2020.03.063
CrossRef - Zhang T, Zhu M, Chen X, Bi K. Simultaneous Analysis of Seven Bioactive Compounds inSambucus Chinensis Lindlby HPLC. Analytical Letters. 2010;43(16):2525-2533. doi:10.1080/00032711003731399
CrossRef - Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. doi:10.1038/s41586-020-2012-7
CrossRef - Zhu G, Luo Y, Xu X, Zhang H, Zhu M. Anti-diabetic compounds from the seeds of Psoralea corylifolia. Fitoterapia. 2019;139:104373. doi:10.1016/j.fitote.2019.104373
CrossRef - Čopra-Janićijević A, Čulum D, Vidic D, Tahirović A, Klepo L, Bašić N. Chemical composition and antioxidant activity of the endemic Crataegus microphylla Koch subsp. malyana K. I. Chr. & Janjić from Bosnia. Industrial Crops and Products. 2018;113:75-79. doi:10.1016/j.indcrop.2018.01.016
CrossRef









