Ganadhal Puttaramaiah Chethankumara1, Kakanahalli Nagaraj1* and Venkatarangaiah Krishna2
and Venkatarangaiah Krishna2
1Department of PG Studies and Research in Applied Zoology, Kuvempu University, Shivamogga, Karnataka, India - 577 451.
2Department of PG Studies and Research in Biotechnology, Kuvempu University, Shivamogga, Karnataka, India - 577 451
Corresponding Author Email: knagarajv@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2158
Abstract
The stem bark and leaves of A. semecarpifolia (Lauraceae) have been employed by traditional healers in Western Ghats region of Karnataka, India to treat human breast cancer. The present study was initiated to explore the cytotoxic properties of A. semecarpifolia. The secondary metabolites were extracted from stem bark and leaves. The stem bark methanol extract (SBME) and leaf methanol extract (LME) were subjected to liquid-liquid partition chromatography, followed by evaluating the presence of phytochemical constituents in liquid fractions and their cytotoxic potential against MCF-7 and L6 cells by MTT assay. The qualitative phytochemical screening of theliquid fractions revealed the presence of different secondary metabolites. The quantitative analysis revealed that the liquid fractions were rich in alkaloids, flavonoids and phenolic compounds. Stem bark methanol fraction (SBMF) and leaf methanol fraction(LMF) showed potential cytotoxicity on MCF-7 cells with an IC50 of 47.11±3.53µg/ml and 48.62±2.40µg/ml respectively. Whereas, stem bark chloroform fraction(SBCF) and leaf chloroform fraction(LCF) showed moderate activity on MCF-7 cells. Vinblastine sulphate was used as a reference standard and it showed potent cytotoxic activity against MCF-7 cells with an IC50 of 24.03±2.12µg/ml. Even though Vinblastine is a potentchemotherapeutic drug it affected the viability of normal cells. In comparison with Vinblastine, the liquid fractions showed very less toxicity on normal cells. Hence, the present study suggested that A. semecarpifolia stem bark and leaves are the potent cytotoxic agents against MCF-7 cells.
Keywords
Alkaloids; Alseodaphne semecarpifolia; Cell lines; Cytotoxicity; Flavonoids
Download this article as:| Copy the following to cite this article: Chethankumara G. P, Nagaraj K, Krishna V. In vitro Cytotoxic Potential of Alkaloid and Flavonoid Rich Fractions of Alseodaphne semecarpifolia Against MCF-7 Cells. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Chethankumara G. P, Nagaraj K, Krishna V. In vitro Cytotoxic Potential of Alkaloid and Flavonoid Rich Fractions of Alseodaphne semecarpifolia Against MCF-7 Cells. Biomed Pharmacol J 2021;14(2).Available from: https://bit.ly/3nTZKmK |
Introduction
Ethnomedicine is as old as man’s history and medicinal plants are being used in ethnomedicine to treat number of ailments. They play a key role in the management of world health1. The traditional practices of herbal formulations are the integral part of various medicinal systems before the development of modern science. These herbal remedies are the major sources of traditional medicinal practices for thousands of years2.
In the discovery of herbal drugs Indian traditional medicinal plants will be of immense interest for all the ethnopharmacologists who are looking for novel therapeutic approaches to various health problems filled all over the world3. It becomes appropriate to search for safe and potent herbal medicines which are already in use by traditional practitioners in order to strengthen their efficacy and safety levels through scientific validation. The biological activities of medicinal plants are also recognized in pharmaceutical research as the major resources of phytomedicines4.
The phytochemical molecules derived from plants have biological activity in humans by having some protective properties against certain disease conditions5,6. Hence, there is a great demand to screen bioactive compounds from medicinal plants as a basis for further biomedical investigations. With advanced phytochemical techniques, many active molecules have been screened and developed as potent drugs in modern medicinal systems. The most important secondary metabolites are alkaloids, flavonoids, phenolics and tannin compounds7,8. These secondary metabolites and their altered forms are responsible forvarious therapeutic activities against cancer and other deadly diseases threatening humans all over the world9.
Cancer is a disease characterized by gradual deterioration and loss of function in the tissues and organs, associated with the lifestyle and environmental factors. Among different types of cancers breast cancer is a prime global health burden and one of the leading causes of deaths in females10.The physical and chemical carcinogens in the environment may induce cell death which may further lead to mutations and cancer causing irreversible damage to DNA. To overcome these risks of cancer, the plant derived anticancer agents play a significant role and many of the modern anticancer drugs are employed in the treatment of various cancers in humans11.
Alseodaphne Semecarpifolia is an evergreen plant commonly known as Nelthare in Kannada12, in Western Ghats region of Shivamogga, Karnataka, India it is well known as ‘Sehunda’13. The Alseodaphne species are the potential source of various biologically active compounds. The secondary metabolites of A. semecarpifolia are known for their various biological activities14-16.The ethno medicinal survey has revealed that the traditional medicinal practitioners in Central Western Ghats of Karnataka, India employed stem bark and leaves of A. semecarpifolia in the treatment of human breast cancer. Even though they are employed in the traditional practices for several years, they are lacking sufficient scientific supports. Hence, the present study was initiated aiming to evaluate the traditional medicinal claims of Alseodaphne semecarpifolia as a potent anticancer agent.
Materials and Methods
Plant sample collection and processing
The stem bark and leaf samples of A. semecarpifolia were collected from Karigudda, Central Western Ghats region, Shivamogga, Karnataka, India. The plant was identified and authenticated by Dr. Y. L. Krishnamurthy., Taxonomist, Department of Applied Botany, Kuvempu University, Shivamogga, Karnataka, India. The voucher specimen (KUBPHS78) is deposited in the herbarium of DBT-BUILDERS project, Kuvempu University for future reference.The collected stem bark and leaves were washed under running tap water to remove the soil and other dust particles followed by washing with distilled water and blotted. They were air dried in the room temperature to remove the water content. Further, the dried samples were powdered using mechanical grinder and stored in air tight container until the extraction.
Crude extraction of secondary metabolites
The powdered stem bark and leaf samples were subjected tosequential soxhletextractionusing solvents of increasing polarity i.e. petroleum ether, chloroform and methanol. The extracts were concentrated using rotary evaporator and the solvents were completely evaporated todryness using water bath and desiccated until further analysis17,18.
Fractionation by partition chromatography
The crude extracts were further fractionated using liquid-liquid partition chromatography.For liquid fractionation 5g of thestem bark and leaf crude methanol extracts were suspended in 100ml of chloroform and centrifuged at 10,000rpm for 10 minutes. The supernatant and residue were collected separately. The fraction collected as a supernatant was considered as chloroform fraction. The fraction collected as a residue was further dissolved in 100ml of methanol and re-suspended with 100ml of hexane in separating funnel. Further it was shaken vigorously for the separation of phytochemical constituents in methanol and hexane. Later the two distinct layers of methanol and hexane were collected separately. The solvents in separated chloroform, methanol and hexane fractions were dried in water bathand desiccated19.
Phytochemical screening of liquid fractions
Qualitative and quantitative phytochemical analysis of stem bark hexane fraction (SBHF), stem bark chloroform fraction (SBCF), stem bark methanol fraction (SBMF), leaf hexane fraction (LHF), leaf chloroform fraction (LCF) and leaf methanol fraction (LMF) was carried out using the standard procedures with some minor modifications20-32. Based on the fractionation yield and presence of phytochemical constituents the chloroform and methanol fractions were selected for cytotoxicity studies.
In vitro cytotoxic activity
Cell culture and maintenance
The MCF-7 (Breast cancer) and L6 (Normal rat myoblast cells) cells were procured from American Type Culture Collection (ATCC). Dulbecco’s Modified Eagle Medium (DMEM) was used to culture the procured stock cells by supplementing with streptomycin, 10% inactivated Fetal Bovine Serum (FBS) and penicillin in a humidified atmosphere of 5% CO2 at 37°C until the cells were confluent.
Cell seeding and treatment
MCF-7 and L6 cells were dissociated by using cell dissociating solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The viability of the cells was checked and centrifuged followed by seeding 50,000 cells/well in a 96 well plate. They were allowed to form a monolayer under regular growth conditions followed by treatment with the test samples. The treated and untreated cells were incubated for 24 hours in CO2 incubator at 37°C with 5% CO2, 95% air and 100% relative humidity and these cells were harvested for cytotoxicity studies.
MTT assay
The cytotoxic effect of SBCF, SBMF, LCF and LMF on MCF-7 and L6 cells was determined by MTT assay33. The monolayer cell culture was trypsinized and the cell count was adjusted to 1×105 cells/ml using DMEM containing 10% FBS. 100µl of the diluted cell suspension was loaded onto the respective wells in 96 well plate. After incubating for 24 hours, the supernatant was carefully flicked off when the partial monolayer was observed, followed by washing with DMEM. 100µl of different concentrations of the test samples were added to the partial monolayer and the plate was then incubated at 37°C for 24 hours with 5% CO2 in CO2 incubator. After incubation, the test solutions in the wells were discarded and 100µl of MTT (5mg of MTT in 10ml PBS) solution was added. Again the plate was incubated for 4 hours at 37°C with 5% CO2 in CO2 incubator. After the incubation, the supernatant was removed and 100µl of dimethyl sulfoxide (DMSO) was added and the plate was gently shaken to solubilize the formed formazan. The absorbance was measured at 590nm using a microplate reader. The IC50 values were generated from the dose-response curves and the percentage growth inhibition was calculated.
Statistical analysis
The statistical analysis was performed using GraphPad Prism Software v 5.01 (GraphPad Software Inc., San Diego, CA). The data is presented as mean±SEM of three replicates and it is statistically analyzed using two way analysis of variance (ANOVA) followed by Bonferroni post-test.Thepvalue less than 0.001was considered statistically significant.
Results
Liquid-liquid fractionation yield
The liquid-liquid fractionation yield of stem bark hexane fraction (SBHF), stem bark chloroform fraction (SBCF) and stem bark methanol fraction (SBMF) was 0.05g (1%), 0.40g (8%) and 2.87g (57.4%) respectively. The fractionation yield of leaf hexane fraction (LHF), leaf chloroform fraction (LCF) and leaf methanol fraction (LMF) was 0.11g (2.2%), 0.62g (12.4%) and 2.15g (43%) respectively.
Qualitative phytochemical analysis
The qualitative phytochemical analysis showed that SBHF contains only glycosides and saponins. Alkaloids, glycosides, phenols and terpenoids are the phytochemicals present in SBCF, whereas, SBMF contains alkaloids, flavonoids, glycosides, phenols, saponins and terpenoids. It is noteworthy that flavonoids are present only in SBMF, however, tannins and steroids are absent in all the three stem bark fractions. Similarly, the phytochemical analysis of leaf fractions revealed that LHF contains flavonoids, steroids, glycosides and saponins. LCF showed the presence of alkaloids, steroids, phenols and terpenoids. Whereas, LMF showed to contain alkaloids, flavonoids, steroids, glycosides, phenols, saponins and terpenoids.It is significanct that steroids are present and tannins are absentin all the three leaf fractions. The results of the qualitative phytochemical analysis are presented in Table 1.
Table 1: Qualitative phytochemical analysis of A. semecarpifolia stem bark and leaf liquid fractions.+: Present; -: Absent.
| Sl No. | Phytochemical Constituents | Stem bark liquid fractions | Leaf liquid fractions | ||||
| SBHF | SBCF | SBMF | LHF | LCF | LMF | ||
| 01 | Alkaloids | – | + | + | – | + | + |
| 02 | Flavonoids | – | – | + | + | – | + |
| 03 | Tannins | – | – | – | – | – | – |
| 04 | Steroids | – | – | – | + | + | + |
| 05 | Glycosides | + | + | + | + | – | + |
| 06 | Phenols | – | + | + | – | + | + |
| 07 | Saponins | + | – | + | + | – | + |
| 08 | Terpenoids | – | + | + | – | + | + |
Quantitative phytochemical analysis
The quantitative phytochemical analysis of liquid fractions revealed the presence of alkaloids, flavonoids, tannins, steroids, glycosides, phenolics, saponins and terpenoids in various concentrations of dry weight of the fractions. SBHF showed the presence of only glycosides and saponins with 2.09±0.72µg/mg and 10.37±4.60µg/mg concentrations respectively. SBCF is rich in Alkaloids (26.45±6.79µg/mg) followed by phenolics(18.55±2.86µg/mg). Likewise, SBMF is rich in flavonoids (110.19±11.55µg/mg) and alkaloids (82.45±8.25µg/mg) followed by phenolics (50.64±8.66µg/mg) and saponins (47.46±3.39µg/mg). The results are presented in Table 2.
Table 2: Quantitative phytochemical analysis of A. semecarpifolia stem bark liquid fractions.
| Sl
No. |
Phytochemical constituents | SBHF (µg/mg) | SBCF (µg/mg) | SBMF (µg/mg) |
| 01 | Total Alkaloids | – | 16.45±6.79 | 82.45±8.25 |
| 02 | Total Flavonoids | – | – | 110.19±11.55 |
| 03 | Total Tannins | – | – | – |
| 04 | Total Steroids | – | – | – |
| 05 | Total Glycosides | 2.09±0.72 | 0.48±1.22 | 5.32±1.01 |
| 06 | Total Phenolics | – | 18.55±2.86 | 50.64±8.66 |
| 07 | Total Saponins | 10.37±4.60 | – | 47.46±3.39 |
| 08 | Total Terpenoids | – | 4.09±3.09 | 12.69±6.70 |
LHF contains flavonoids, steroids, glycosides and saponins in very minute quantities, whereas, LCF is rich inalkaloids (28.50±6.84µg/mg) and phenolics (15.50±7.92µg/mg). However, LMF is very rich in alkaloids (108.65±5.06µg/mg) and flavonoids (90.73±7.90µg/mg) followed bysaponins (70.30±7.67µg/mg) and phenolics (66.46±4.06µg/mg). The results are presented in Table 3.
Table 3: Quantitative phytochemical analysis of A. semecarpifolia leaf liquid fractions.
| Sl
No. |
Phytochemical constituents | LHF (µg/mg) | LCF (µg/mg) | LMF
(µg/mg) |
| 01 | Total Alkaloids | – | 28.50±6.84 | 108.65±5.06 |
| 02 | Total Flavonoids | 2.61±3.40 | – | 90.73±7.90 |
| 03 | Total Tannins | – | – | – |
| 04 | Total Steroids | 1.48±3.48 | 4.21±8.03 | 19.10±10.04 |
| 05 | Total Glycosides | 0.66±0.91 | – | 8.65±7.75 |
| 06 | Total Phenolics | – | 15.50±7.92 | 66.46±4.06 |
| 07 | Total Saponins | 4.62±1.71 | – | 70.30±7.67 |
| 08 | Total Terpenoids | – | 2.09±4.17 | 13.30±5.85 |
Cytotoxic effects of stem bark and leaf liquid fractions
The cytotoxic activity of SBCF, SBMF, LCF and LMF was observed in a dose dependent manner. SBMF and LMF showed significant cytotoxic activity on MCF-7 cells with an IC50 of 47.11±3.53µg/ml and 48.62±2.40µg/ml respectively. However, SBCF and LCF showed moderate activity on MCF-7 cells with an IC50 of 116.20±2.47µg/ml and 103.80±10.74µg/ml respectively (Fig.1).
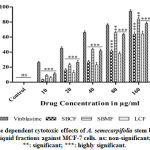 |
Figure 1: Dose dependent cytotoxic effects of A. semecarpifolia stem bark and leaf liquid fractions against MCF-7 cells. ns: non-significant; ***: significant; ***: highly significant. |
The cytotoxic effect of SBCF, SBMF, LCF and LMF was compared with the cytotoxic properties of standard anticancer drug Vinblastine. Vinblastine exhibited significant effect on MCF-7 cells with an IC50 of 24.03±2.12µg/ml. Even though Vinblastine exhibited significant effect against MCF-7 cells, it also affected the viability of normal L6 cells with an IC50 of 88.52±3.56µg/ml, but SBCF, SBMF, LCF and LMF were very least toxic on L6 cells and their IC50 was unable to determine due to their lesser toxicity (Fig. 2).
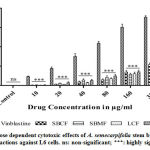 |
Figure 2: Dose dependent cytotoxic effects of A. semecarpifolia stem bark and leaf liquid fractions against L6 cells. ns: non-significant; ***: highly significant. |
Discussion
Medicinal plants are believed to be a potent source of secondary metabolites with significant therapeutic properties. The plant secondary metabolites such as alkaloids, flavonoids, saponins, phenols, tannins, terpenoids, glycosides and steroids are found to be important phytochemical constituents34,35. Most of the Ayurvedic medicines used today are derived from herbal sources. According to World Health Organization (WHO) nearly 80% of the human population all over the world is still relying on herbal remedies for preliminary health care. This is due to their availability, lesser side effects and low cost36,37. Medicinal plants have the ability to synthesize wide variety of secondary metabolites from their each and every part under different stress conditions. This motivates the researchers all over the world to find novel therapeutic drugs from natural sources with potential biological activities38.
Secondary metabolites are not so essential for the normal growth and development of the organisms. Absence of secondary metabolites does not result in immediate death of the organisms, but they have long term impairment on the survivability of the organisms and they often play an important role in protection against certain pathogens and several disease conditions39,40. These secondary metabolites function in conjunction with one another or they may act alone in order to bring desired pharmacological effect41.
In order to derive the factors involved in anticancer properties, phytochemical screening was carried out for stem bark and leaf liquid fractions. The extraction of secondary metabolites was carried out sequentially using solvents of increasing polarity i.e. by selecting solvents of three different polarities such as non-polar, medium polar and polar solvents. Non-polar solvents were used to extract out non-polar compounds, whereas the compounds of intermediate polarity were extracted by using medium polarity solvents and polar solvents were used to extract out polar compounds. The sequential extraction allows the preliminary separation of secondary metabolites of distinct polarities which simplifies the further fractionation.
The fractionation of secondary metabolites was carried out using partition chromatography. The molecules need to be separated by Partition chromatography will interact with two immiscible solvents according to the solubility of the compounds42.The cytotoxicity of the liquid fractions was evaluated by MicrocultureTetrazolium (MTT) assay. Among the different cell viability tests MTT assay is one of the frequently used technique. It is more advantageous, especially its effectiveness and simplicity makes it more suitable to determine in vitro anticancer activities of test drug at the preliminary levels. In this assay the cell viability was determined by using colorimeter43.
In the present study, preliminary phytochemical analysis of A.semecarpifolia stem bark and leaf fractions have showed the presence of different secondary metabolites which are of great importance in the field of drug research. Different active phytochemicals have been found to possess a wide range of activities, which may help in protection against incurable diseases.
The phytochemical analysis of stem bark and leaf liquid fractions revealed that alkaloids, flavonoids and phenolic compounds are the rich components of A. semecarpifolia.This alkaloid, flavonoid and phenolic compounds represent as a most widespread class of bioactive compounds with multiple therapeutic properties44.The anticancer property of medicinal plants is due to the presence of alkaloid, flavonoid and phenolic compounds45-47.In the present investigation it is evident that SBMF and LMF exerted significant dose dependent cytotoxicity against human breast cancer cells. As alkaloid, flavonoid and phenolic compounds are the rich components of SBMF and LMF they might be responsible for their potent activity.The earlier studies have suggested that A. semecarpifolia is a rich source of aporphine alkaloids, isoquinoline alkaloids and phenolic derivatives48-50.Among different subgroups of aporphine alkaloids, benzylisoquinoline alkaloids form the broad subgroup widely distributed in Alseodaphne species51.
The cytotoxic properties of SBMF and LMF were compared with the standard anticancer drug Vinblastine. Although Vinblastine shows apotent activity against cancer cellsit affected the viability of normal cells. In contrast, the cytotoxic effects of SBMF and LMF on normal cells was very least. To be a potent anticancer drug it should be selectively toxic on cancerous cells but not on non-cancerous cells.By considering all these factors the present study has provided the evidence that SBMF and LMF have promising anticancer property, and supported the traditional medicinal claims of A. semecarpifolia as a potent source of anticancer compounds.
Conclusion
Several novel cytotoxic compounds are screened from traditional medicinal plants every year to fight against certain cancers. Even though several natural active compounds have unique anticancer properties, they are not used in clinical practices due to limited bioavailability. On the other hand the secondary metabolites derived from natural sources are the potent leads for drug discovery and development.
The choices of modern drugs for the cancer therapies are limited and most of them are accompanied with dose-related toxicities. The development of an effective chemotherapeutic agent is crucial and new strategies need to be put forth which can help for the identification of natural compounds with superior clinical efficacy and lesser toxicity.It is evident from the present study that, Alseodaphne semecarpifolia is a potent anticancer agent against human breast cancer cells.
Acknowledgement
This work was financially supported by DBT, New Delhi, India through DBT-BUILDERS project (Order No. BT/PR9128/INF/ 22/190/ 2013, Dated: 30/06/2015). The authors are thankful toSkandaLifesciences Pvt Ltd, Bangalore, Karnataka, India and department of Applied Zoology, Kuvempu University, Karnataka, India for providing the laboratory facility to carry out this research.
Conflict of Interest
The authors declare no conflicts of interest for this study.
References
- Thakkar K,Parmar V, Patel D andMeshram D. Recent Advances in Herbal Drug Standardization – A Review. J. Adv. Pharm. Res.,2013;4:2130-2138.
- Pandey M. K, Singh G. N, Sharma R and Snehlata. Standardization of YakritPlihantakChurna: An AyurvedicPolyherbal Formulation. Int. J. Pharm. Sci. Res., 2012;3:171-176.
- Devasagayam T. P. A. Introduction to Serial Reviews: Recent Advances in Indian Herbal Drug Research. Clin. Biochem. Nutr., 2007;40:73.
CrossRef - Pillai N. R. Antidiarrheal Activity of Punicagranatum in Experimental Animals. J. Pharmacogn., 1992;30:201-204.
CrossRef - Key T. J, Appleby P and Spencer E. A. Cancer Incidence in British Vegetarians. Br. J. Cancer., 2009;101:192-197.
CrossRef - Richardson M. A, Sanders T and Palmer J. L. Complementary/Alternative Medicine Use in a Comprehensive Cancer Center and the Implications for Oncology. J.Clin.Oncol., 2000;18:2505-2514.
CrossRef - Bekele G andHazare S. T. Isolation and Characterization of Bioactive Compounds from Medicinal Plants of Ethiopia- A Review. Current trends in Biomedical Engeneering and Biosciences.,2017;7:1-4.
CrossRef - Li H. B, Jiang Y and Chen F. Separation Methods Used for Scutellariabaicalensis Active Components. J.Chromatogr. B., 2004;1:277-290.
CrossRef - Thilagavathi T, Arvindganth R, Vidhya D andDhivya R. Preliminary Phytochemical Screening of Different Solvent Mediated Medicinal Plant Extracts Evaluated. Res. J. Pharm.,2015;6:246-248.
CrossRef - Liang C, Pan H, Li H, Zhao Y andFeng Y. In vitro Anticancer Activity and Cytotoxicity Screening of Phytochemical Extracts from Selected Traditional Chinese Medicinal Plants. , 2017;22:543-551.
- Altobelli E andLattanzi A. Breast Cancer in European Union: An Update of Screening Programmes as of March 2014 (Review). Int. J.Oncol., 2014;45:1785-1792.
CrossRef - Charles A, Joseph M andRamani A. V. In-vitro Antioxidant Potential of Alseodaphnesemecarpifolia Leaf Extract. J. Exp. Biol., 2012;2:354-357.
CrossRef - Kumar A. K. M andShivaraju H. P. A Study on Traditional Knowledge and Medicinal Applications of the Endemic Herbal Species in the Western Ghats of Shimoga Region, Karnataka, India. J. Res. Chem. Environ., 2016;6:1-13.
CrossRef - Charles A andRamani A. V. Phytochemical Screening and Antimicrobial Resistance of Alseodaphnesemecarpifolia J. Chem. Pharm. Res., 2011;3:205-211.
CrossRef - Charles A, Joseph M andRamani A. V. In-vitro Antioxidant Potential of Alseodaphnesemecarpifolia Leaf Extract. J. Exp. Biol., 2012;2:354-357.
CrossRef - Puttaramaiah C. G, Venkatarangaiah K andKakanahalli N. Screening In vitroAnticancer Activity of AlseodaphnesemecarpifoliaNees Stem Bark Extracts against Some Cancer Cell lines. J., 2019;11:884-888.
CrossRef - Ahmad A, Alkarkhi A. F. M, Hena S andKhim L. H. Extraction, Separation and Identification of Chemical Ingredients of Elephantopus Scaber Using Factorial Design of Experiment. Int. J. Chem., 2009;1:36-49.
CrossRef - Dantu A. S, Shankarguru P, Devi D. R andHari B. N. V. Evaluation of In vitro Anticancer Activity of Hydroalcoholic Extract of Asian J. Pharm. Clin. Res., 2012;5:59-61.
- Oldoni T. L. C, Cabral I. S. R, d’Arce M. A. B. R, Rosalen P. L, Ikegaki M andNascimento A. M. Isolation and Analysis of Bioactive Isoflavonoids and Chalcone From A New Type of Brazilian Propolis. Purif. Technol., 2011;77:208-213.
CrossRef - Harborne J. B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall;2005.
CrossRef - Edeoga H. O, Okwu D. E andMbaebie B. O. Phytochemical Constituents of Some Nigerian Medicinal Plants. J. Biotechnol., 2005;4:685-688.
CrossRef - Zhishen J, Mengcheng T andJianming W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem., 1999;64:555-559.
CrossRef - Senguttuvan J, Paulsamy S andKarthika K. Phytochemical Analysis and Evaluation of Leaf and Root Parts of the Medicinal Herb, Hypochaerisradicata for In vitroAntioxidant Activities. Asian Pac. J. Trop. Biomed., 2014;4:359-367.
CrossRef - Siddhuraju P and Becker K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringaoleifera) Leaves. J. Agric. Food. Chem.,2003;51:2144-2155.
CrossRef - Makkar H. P, Siddhuraju P and Becker K. Methods in Molecular Biology: Plant Secondary Metabolites. Totowa: Human Press; 2007.
CrossRef - Durai M. V, Balamuniappan G, Anandalakshmi R, Geetha S and Kumar N. S. Qualitative and Quantitative Analysis of Phytochemicals in Crude Extract of Big-Leaf Mahogany (Swieteniamacrophylla). J. Herb. Med.,2016;4:88-91.
- Singh R, Verma P. K and Singh G. Total Phenolic, Flavonoids and Tannin contents in Different Extracts of Artemisia absinthium. Intercult. Ethnopharmacol., 2012;1:101-104.
CrossRef - Malik S. K, Ahmad M and Khan F. Qualitative and Quantitative Estimation of Terpenoid Contents in Some Important Plants of Punjab, Pakistan. J. Sci., 2017;69:150-154.
- Indumathi C, Durgadevi G, Nithyavani S andGayathri P. K. Estimation of Terpenoid Content and its Antimicrobial Property in Enicostemmalitorrale. J. ChemTech Res., 2014;6:4264-4267.
- Devanaboyina N, Ramalakshmi N, Satyanarayana, Sudeepthi P, Chakradhar K. H andRaju N. P. K. Preliminary Phytochemical Screening, Quantitative Estimation and Evaluation of Antimicrobial Activity of Alstoniamacrophylla Stem Bark. International Journal of Science Inventions Today.,2013;2:31-39.
- Ajiboye B. O, Ibukun E. O, Edobor G, Ojo A. O andOnikanni S. A. Qualitative and Quantitative Analysis of Phytochemicals in Seneciobiafrae Int. J. Invent. Pharm. Sci., 2013;1:428-432.
- El-olemy M. M, Al-muhtadi F. J, andAffi A. F. A. Experimental Phytochemical: A Laboratory Manual, Saudi Arabia: King Saud University Press;1994.
- Alley M. C, Scudiere D. A, Monks A, Hursey M. L, Czerwinski M. J and Fine DL, et al. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a MicrocultureTetrazolium Assay. Cancer. Res., 1988;4:589-601.
- Tiwari P, Kumar B, Kaur M,Kaur G andKaur H. Phytochemical Screening and Extraction, A Review. Int. J.Pharm. Sci., 2011;1:98-106.
- Viji M andMurugesan S. Phytochemical Analysis and Antibacterial Activity of Medicinal Plant Cardiospermumhalicacabum J. Phytol.,2010;2:68-77.
- Malar C. G. R andChellaram C. Phytochemical Screening, Total Flavonoid, Total Terpenoid and Anti-Inflammatory Activity of Aqueous Stem Extract of J. Chem. Pharm. Sci., 2017;10:550-556.
CrossRef - Murugesan D andPonnuswamy R. D. Potential Anti-inflammatory Medicinal Plants – A Review. J. Pharm. Pharm. Sci.,2014;6:43-49.
- Igbinosa O. O, Igbinosa E. O andAiyegoro A. Antimicrobial Activity and Phytochemical Screening of Stem Bark Extracts from Jatropacurcas(Linn). J. Pharm. Pharmacol., 2009;3:58-62.
- Thirumurugan D, Cholarajan A, Suresh S. S, Raja andVijayakumar R. An Introductory Chapter: Secondary Metabolites;2018.
CrossRef - Rehab A, Hussein, Amira A and EI-Anssary. Plant Secondary Metabolites: Key Drivers of the Pharmacological Actions of Medicinal Plants. Pharmacogn. Phytochem., 2018;6:32-36.
CrossRef - Anand U, Herrera N. J, Altemimi A andLakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites., 2019;9:1-13.
CrossRef - Ingle P. K, Deshmukh A. G, Padole D. A, Dudhare M. S, Moharil M. P andKhelurkarV. C. Phytochemicals: Extraction Methods, Identification and Detection of Bioactive Compounds from Plant Extracts. Pharmacogn. Phytochem., 2017;6:32-36.
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assay. J immunolmethods., 1983;65:55-63.
CrossRef - Stevigny C, Bailly C andQuetin-Leclercq J. Cytotoxic and Antitumor Potentialities of Aporphinoid Alkaloids. Curr Med Chem-Anticancer Agents.,2005;5:173-182.
CrossRef - El-Ansari M. A, Ibrahim L. F andSharaf M. Natural Phenolics: A Source of Anticancer Agents. Pharm. J., 2019;18:1-7.
CrossRef - Isah T. Anticancer Alkaloids from Trees: Development Into Drugs. Rev., 2016;10:1-11.
CrossRef - Ahmed S. I, Hayat M. Q, Tahir M, Mansoor Q, Ismail M and Keck K. Pharmacologically Active Flavonoids From The Anticancer, Antioxidant And Antimicrobial Extracts of Cassia angustifolia BMC ComplementaryAltern. Med.,2016;16:460.
CrossRef - Charles A, Stanly L. A, Joseph M andRamani A. V. GC-MS Analysis of Bioactive Components on the Bark Extract of AlseodaphnesemecarpifoliaNees (Lauraceae). Asian J. Plant Sci. Res., 2011;1:25-32.
- Charles A, Joseph M andRamani A. V. Phytochemical Analysis of Alseodaphnesemecarpifolia Leaf Extract by GC-MS. Asian J. Pharm. Clin. Res., 2013;6:89-92.
- Thakur B. K, Anthwal A, Rawat D. S, Rawat B, Rashmi and Rawat M. S. M. A Review on Genus Alseodaphne: Phytochemistry and Pharmacology. Mini-Rev. Org. Chem., 2012;9:433-445.
CrossRef - Mukhtar M. R, Zahari A, Nafiah M. A, Hadi A , Thomas A. H and Hiroko N. F. 3′, 4’-Dihydrostephasubine, A New Bisbenzylisoquinoline from the Bark of Alseodaphnecorneri. Heterocycles.,2009;78:2571-2578.
CrossRef







