Afiat Berbudi1,2* , Ali Akbar Fadlillah3
, Ali Akbar Fadlillah3 , Merry Afni4
, Merry Afni4 and Nur Atik5
and Nur Atik5
1Department of Biomedical Sciences, Parasitology Division, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
2Faculty of Medicine, Universitas Pasundan, Bandung, Indonesia.
3Undergraduate Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
4Infectious Disease Research Center, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
5Department of Biomedical Sciences, Cell Biology Division, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
Corresponding Author E-mail : a.berbudi@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2165
Abstract
Patients suffering from skin wounds often have their life heavily affected economically and socially. In general, wound healing consists of four different sequentially and overlapping phases. Interrupting those phases results in extending the healing time. Turmeric (Curcuma longa) is widely used by Indonesians to treat wounds, with curcumin as an active substance, which is proven to have various effects on improving wound healing. Therefore, this study aims to determine how the topical application of turmeric gel on affects the acceleration of wound closure. Analytical laboratory experiments were conducted on 24 male Swiss-Webster mice. Subjects were divided into four groups according to where the excision wounds were made on the back, and observed on days 0, 3, 6, 9, 12, and 15 post wound induction. The wound healing acceleration was observed after 3 (p = 0.0003), 6 (p = 0.0085), and 9 (p = 0.0023) days in C. longa-treated mice compared to the control group. However, there was no significant difference found on days 12 and 15 after wounding. In addition, the area under the curve analysis shows significant difference between the C. longa-treated group compared to the control, but not with the group treated with neither curcumin nor nano curcumin gel. In conclusion, topical application of 3% C. longa extract gel improves excised skin healing.
Keywords
Curcuma Longa Gel; Curcumin Gel; Excision Wound; Nano Curcumin Gel; Wound Healing Acceleration
Download this article as:| Copy the following to cite this article: Berbudi A, Fadlillah A. A, Afni M, Atik N. Effect of Curcuma longa Crude Extract, Curcumin and Nano Curcumin-based Gel Topical Administration on Excised Skin Wound Healing in Swiss-Webster Mice. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Berbudi A, Fadlillah A. A, Afni M, Atik N. Effect of Curcuma longa Crude Extract, Curcumin and Nano Curcumin-based Gel Topical Administration on Excised Skin Wound Healing in Swiss-Webster Mice. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3jI66Wl |
Introduction
Skin wounds have enormous social and economic implications, but the significance of wound research seems to be overlooked because studies have mostly focused on the disease that caused them. Patients with surgical and traumatic wounds, abrasion, and superficial burns are characterised by the occurrence of acute wound.1 Wound healing involves four distinct overlapping phases that must occur at a specific and precise times. Several diseases such Diabetes causes abnormal wound healing because of complex mechanisms that eventually lead to chronic wounds.2 Functional impairment due to chronic wounds causes a decrease in quality of life and a high financial burden for wound care and treatment.3 Therefore, it is necessary to study the factors that induce chronic wounds as well as any potential accelerator of wound healing time.
Plants and their compounds have potential for wound management and treatment. Medicinal plants are not only cheap and affordable, but also believed to be safe for use with rarely encountered hypersensitive reactions.4 Curcuma longa or turmeric is a biopharmaceutical plant highly produced in Indonesia. Locals have been using this plant as ingredient in traditional foods and herbs to resolve tissue inflammation due to its anti-inflammatory, anti-infectious, and antioxidant active substance content. Curcuma longa has undoubtedly the potential to act as a wound healing agent. 5,6
On the other hand, its active compound, curcumin has been developed in a new nano size form that shows improvement on bioavailability after consumed.7 We therefore conducted a study of excised skin wound healing using C. longa administration to examine the impact on wound healing.
Materials and Methods
Experimental in vivo laboratory analytical research was conducted on 48 male mice (Mus musculus) strain Swiss-Webster aged 8-10 weeks and weighing 22–26 g. The mice were obtained from the Pharmacology Laboratory, Faculty of Medicine, Universitas Padjajaran.
Production of Topical Gel
Blank gel was produced using pure gel that was free of any active ingredients. Curcuma longa extract gel was made by combining 96% v/v ethanol extract of C. longa with gel (0.3%). The gels were developed in the Pharmacognosy Laboratory at Padjadjaran University’s Faculty of Pharmacy. Curcumin (0.25% w/v) was mixed into a traditional emulsion with GMO (16 w/v) and Chemophor RH40 to make nanocurcumin gel. The curcumin nanoemulsion was then processed into a gel. This gel was produced in the Semisolid Laboratory of the Faculty of Pharmacy at the Bandung Institute of Technology (ITB). For this experiment, each gel contained 3% C. longa extract, 3% curcumin, and 3% nanocurcumin respectively.
Wound Induction
Before the experiment, the mice were acclimatised for seven days and weighed. They were housed in regular cages with free access to food and water.
Following the adaptation phase, the body weight was measured again, and elimination was carried out in accordance with the exclusion criteria.
Before wounding, the hair on the back of each mouse was shaved and sterilized. An isofluran inhalation was used to provide anaesthesia before two excision wounds were created. Alcohol 70% was used for aseptic and antiseptic procedures on the wound area. Skin excision was performed using a 6 mm punch biopsy (GlaxoSmithKline GmbH & Co. KG, Germany) to ensure homogenous depth.8,9
Intervention
The experimental animals were divided into four groups, including a control (n = 6) treated with blank gel daily, and three treatment groups treated with turmeric extract gel (n = 6), curcumin gel (n = 6), and nanocurcumin gel (n = 6) topically on the wound surface every day.
Wound Area Analysis
The wound area was observed to determine the effect of turmeric gel on the percentage of skin coverage of the excision wound. Observations were made on the percentage of mice skin wound closure in each treatment group on days 3, 6, 9, 12 and 15 after wound induction. The area of the wound was measured using the Image J application (open source), while initial wound closure was defined as 0% closure.8,9
![]()
Statistical Analysis
Statistical analysis and graph processing were performed using Graph Pad Prism 8. Normality and homogeneity test of wound closure percentage was carried out using Saphira-Wilk and Levene, respectively. The data with normal and abnormal distribution were analysed using one way-ANOVA and Kruskal-Wallis, respectively, to compare the results of the treatments.
Ethical Clearance
The experiment in this study has followed ethical standards in animals, and has received a statement of ethical eligibility from the Research Ethics Committee of the Faculty of Medicine, Universitas Padjajaran with number 705/UN6.C.10/PN/ 2017.
Results
Administration of Topical Curcuma Longa Gel Accelerated Wound Closure on Days 3, 6, and 9 Post Wound Induction
The wound closure percentage shows that administration of topical gel accelerated wound closure on days 3, 6, and 9 post wound induction (p = 0.0003, p = 0.0085 and p = 0.0023, respectively). However, on days 12 and 15 post wound induction, there is no significant differences among the groups (Figure 1).
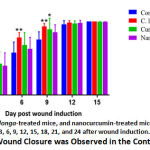 |
Figure 1: Wound Closure was Observed in the Control Group. |
As shown in Figure 1, topical administration of C. longa gel increases the speed of wound closure on days 3, 6, and 9 after induction. The Area Under the Curve (AUC) analysis (Figures 2 and 3) shows that there was a significant difference in the speed of wound closure in the four groups (p < 0.05), more specifically, only the group given C. longa gel has a significant difference compared to the control group (p < 0.05). This is demonstrated by the higher mean AUC of 347.7 in the process of wound closure compared to 289.6 of the control group (Figure 3). Thus, topical C. longa administration significantly accelerates the closure of the excision skin wounds.
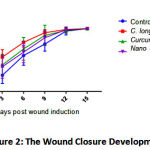 |
Figure 2: The Wound Closure Development %. |
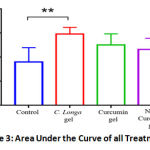 |
Figure 3: Area Under the Curve of all Treatments. |
In the observation, Curcumin gel only showed an increasing wound closure effect on day 9 (p < 0.05), whereas the nanocurcumin treated group did not improve wound closure. Although there is evidence of wound healing acceleration (Figure 3), no significant differences in the AUC of the curcumin gel and nanocurcumin gel groups and the control group were found. Macroscopic features of each group are presented in Figure 4.
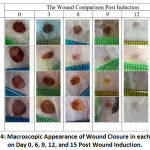 |
Figure 4: Macroscopic Appearance of Wound Closure in each Group on Day 0, 6, 9, 12, and 15 Post Wound Induction. |
Discussion
In general, the four healing phases occur 2-3 weeks post wounding.10 The first phase, coagulation, is required for haemostasis and wound protection.11 The clot and the surrounding wound tissue will release pro-inflammatory cytokines and growth factors. Once bleeding is controlled, inflammatory cells migrate to the wound area (chemotaxis) and initiate an inflammatory phase, which is characterised by sequential infiltration of neutrophils, macrophages, and lymphocytes.12
After arriving at the wound area, polymorph nuclear leucocyte (PMN) phagocytes bacteria and other foreign particles in the wound area. Blood monocytes undergo phenotypic changes when they are in the wound area by becoming M1 macrophages, which are pro-inflammatory. Macrophages cells have the most important role of releasing proteolytic enzymes such as collagenase to clean tissue. After cleaning apoptotic cells, macrophages undergo phenotypic changes into M2 macrophages, which are anti-inflammatory and stimulate keratinocytes, fibroblasts, and angiogenesis needed in tissue regeneration and proliferation.12 Lymphocytes are the last cell type to enter wound blood during the inflammatory phase and produce various interleukins (IL). One of which is IL-1, believed to play a key role in collagenase regulation, collagen repair, and extracellular matrix (ECM). IL-4 and IL-13, which are also produced by lymphocytes, act as inhibitors in M1 activation and trigger M2 activation.13
The proliferation phase is characterised by the proliferation and migration of epithelial cells to a temporary matrix in the wound area. In this phase, granulomatous tissue formation occurs to restore the fibrin matrix and as a new pathway for keratinocyte migration.10,12 Keratinocytes play a role in restoring the protective function of the epithelium. In the final proliferation phase, some of the fibroblasts may differentiate into myofibroblasts, which contract quickly and efficiently to close the wound. The whole healing process stops when the last phase, remodelling, takes place. The interaction of fibroblasts and myofibroblasts produces an extracellular matrix, especially in the form of collagen.10,11 An important feature of the remodelling phase is that there are many new capillary regressions that will restore the density of blood vessels in the wound tissue back to normal and ECM repair with an architecture close to normal tissue.12
A number of studies have been conducted to explore curcumin’s ability to heal cutaneous14–17 and excision wounds.18,19,20,21,22 The role of curcumin in promoting epithelial regeneration, fibroblast proliferation, and vascular density has been explored in various studies.16,22 Curcumin has been shown to increase IL-4 and IL-13, up-regulation of mRNA, and induce M2 polarisation so as to accelerate the proliferation phase of wound healing.23 During the remodelling phase, curcumin has a role in increasing granulomatous tissue formation,14 repair, collagen deposition,19,24 wound contractions, and many positive effects on the proliferation and remodelling phase.18,24,25 However, due to its anti-inflammatory properties, curcumin administration also has a negative effect on the inflammatory phase. Curcumin has an inhibitory function (nuclear factor kappa-light-chain-enhancer of activated B cells) NF-κB and cyclooxygenase-2 (COX-2), inhibits arachidonic acid metabolism, decreases the expression of cytokines IL-1b, IL-6, (tumour necrosis factor alpha) TNF-α, and down-regulation enzymes such as protein kinase C. Experiments on curcumin administration in macrophage cultures showed inhibition of M1 polarisation, TNF-a production, NF-κB phosphorylation, and decreased TLR4 (toll-like receptor 4) protein expression, which is a regulator of macrophage polarisation M1.23,26,27 Macrophage function, as the main regulator of the inflammatory phase, is disrupted due to the effect of curcumin. Considering that the slightest interruption in the overlapping wound healing phase can cause delayed healing, it is necessary to further study curcumin administration during days 3-4 after wound induction when the inflammatory phase of has been completed.28
As commonly known, besides curcumin, there are other active substances in C. longa which have been shown to have a positive effect on the speed of healing wounds. Two other curcuminoids, namely desmethoxycurcumin and bisdemethoxycurcumin, have the same effect as curcumin.2, 26,29 The essential oil of C. longa has strong antibacterial and anti-inflammatory activity in addition to protein, fat, and vitamin, which have an important role in wound healing and regeneration.4,30 This characteristic may be the driving effect of accelerating wound closure compared to curcumin or nanocurcumin gel alone. To better understand the effects of the various substances contained in turmeric, further investigation is thus required.
One of the limitations of this study that should be highlighted is the behaviour of experimental mice, which might have caused the unexpected results. The large number of mice placed in a single cage may affect the topical administration and reduce the effectiveness of the gel. Mice usually scratch, bite, or lick their or other mice’s backs, affecting the gel applied. In addition, the number of experimental animals in this study was relatively small and less homogeneous, which might have cause the large variations of the resulting data.
Conclusion
Our results of wound healing on days 3, 6, 9, 12, and 15 showed a positive effect on the acceleration of wound healing by C. longa gel. Although curcumin gel treatment and its nanoparticle form (nano curcumin) accelerated wound healing, the ability of both gels is still inconclusive and should be tested on a larger sample, at different concentrations and on different test subjects.
Acknowledgments
We thank our colleagues from the Laboratory of Pharmacognosy and Pharmacy Natural Ingredients, Pharmacy Faculty, Universitas Padjajaran; and the Laboratory of Semisolid, Pharmacy Faculty of Institut Teknologi Bandung.
Conflict of Interests
Authors declare no conflict of interests.
Funding Source
This study was sponsored and financed by the Universitas Padjadjaran Internal Research Grant for Basic Research (No. 855/UN6.3.1/PL/2017).
References
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human Skin Wounds: A Major Snowballing Threat to Public Health and Economy. Wound Repair Regen 2009; 17: 763-771.
CrossRef - Pawar RS, Toppo FA, Mandloi AS, Shaikh S. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian J Pharmacol. 2015; 47: 160-166.
CrossRef - Graves N, Zheng H. The prevalence and incidence of chronic wounds: A literature review. Wound Pract Res J Aust Wound Manag Assoc. 2014; 22: 4-12.
- Raina R, Parwez S, Verma PK, Pankaj NK. Medicinal Plants and their Role in Wound Healing. Vetscan. 2008; 3: 1-7.
- Ginting C. The Effect of Amount of Turmeric Powder on Quality of Tofu at Room Temperature Storage. 2014;9(2):108–14.
- Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci, 2014; 116: 1-7.
CrossRef - Flora G, Gupta D, Tiwari A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30(4):331-68.
CrossRef - Berbudi A, Hasibah UH, Faried A, Putri AC, Hamda ME. Administration of Curcuma longa Extract Topically does not Accelerate Skin Excision Wound Closure in Alloxan-induced Diabetic Mice Model. Biomed Pharmacol J. 2018;11(1):437–44.
CrossRef - Berbudi A, Anshori H, Andromeda A, Kwarteng A, Putri AC, Atik N. Topical administration of Curcuma longa L. extract gel increases M2 macrophage polarization and collagen density in skin excision. J Appl Pharm Sci. 2021; 11(01): 095-100.
- Kandhare AD, Alam J, Patil MVK, Sinha A, Bodhankar SL. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2016;54(3):419-32.
CrossRef - Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;12;366(9498):1736-43.
CrossRef - Guo S, DiPietro LA. Critical review in oral biology & medicine: Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
CrossRef - Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85.
CrossRef - Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with curcumin incorporated collagen films. 2004;25:1911–7.
CrossRef - Liang G, Yang S, Zhou H, Shao L, Huang K, Xiao J, et al. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur J Med Chem. 2009;44(2):915-9.
CrossRef - Mohanty C, Das M, Sahoo SK. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol Pharm. 2012;9(10):2801–11.
CrossRef - Tummalapalli M, Berthet M, Verrier B, Deopura BL, Alam MS, Gupta B. Composite Wound Dressings of Pectin and Gelatin with Aloe vera and Curcumin as Bioactive Agents. Int J Biol Macromol. 2016;82:104-13.
CrossRef - Jagetia GC, Rajanikant GK. Acceleration of wound repair by curcumin in the excision wound of mice exposed to different doses of fractionated γ radiation. 2012;9(1):76–92.
CrossRef - Joe B, Vijaykumar M, Lokesh BR. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit Rev Food Sci Nutr. 2004;44(2):97-111.
CrossRef - Kloesch B, Becker T, Dietersdorfer E, Kiener H, Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int Immunopharmacol. 2013;15(2):400-5.
CrossRef - Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;15;20(8-9):714-8.
CrossRef - Thangapazham RL, Sharad S, Maheshwari RK. Skin Regenerative Potentials of Curcumin. BioFactors. 2013;39(1):141–9.
CrossRef - Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015 Aug;85:131-9.26.
CrossRef - Qian Z, Dai M, Zheng X, Xu X, Kong X, Li X, et al. Chitosan-alginate sponge: Preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol. 2009;2009:595126.
CrossRef - Durgaprasad S, Reetesh R, Hareesh K, Rajput R. Effect of a topical curcumin preparation (BIOCURCUMAX) on burn wound healing in rats. J Pharm Biomed Sci. 2011; 8(23):1-3.
- Jurenka JS. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma longa: A Review of. Altern Med Rev Med Rev. 2009;1414(22):141–53.
- Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, et al. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell Physiol Biochem. 2015;36(2):631-41.
CrossRef - Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004; 187 (5) Supplement 1: S11-S16.
CrossRef - Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091–112.
CrossRef - Bagchi A. Extraction of Curcumin. J Environ Sci Toxicol Food Technol. 2012;1(3):1–16.
CrossRef







