Varun Kumar Singh1 , Koushiki Bhattacharjee2
, Koushiki Bhattacharjee2 and Padmapriya Jaiprakash2
and Padmapriya Jaiprakash2
Department of Pathology, Melaka Manipal Medical College, Manipal campus, Manipal Academy of Higher Education, Manipal, Karnataka, India,
Department of Pathology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
Corresponding Author E-mail: koushiki.bhattacharjee13@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2206
Abstract
Introduction: Superficial palpable swellings with helmintic infection as an underlying etiology is usually an accidental finding in the surgically excised specimens. Somatic nematodes and cestodes are the commonly implicated organisms, and the zoonotic nematodes show an emerging trend. The present study aims to reappraise the histopathological findings of helminthic etiology in superficial swellings which were clinically suspected to be of neoplastic/non neoplastic nature. Materials and methods: Thirty six cases of palpable superficial nodules with infective etiology over a period of five years were reviewed. 19/36 were of helminthic etiology were included in the present study. Pertinent demographic and clinical data were retrieved from the medical archives. Results: Amongst the 19 cases, 8 were males and 11 females. Chest wall (4/19), and eyelids (3/19) were the most common sites involved. The size ranged from 0.8-15 cm in greatest dimension. Presence of histiocytes (13/19), granulomas (11/19), eosinophils (10/19), and giant cells (9/19) were the most consistent histological findings. 14 cases had discernible parasite morphology with diagnosis of filarial worms (7/19), Dirofilaria (3/19), cysticercosis (4/19), and hydatid cyst (1/19). Four cases had dead and calcified parasites with no discernible morphology. Conclusion: Granulomatous inflammation and tissue eosinophilia are strong indicators of a parasitic etiology. Subcutaneous and intramuscular filariasis, cysticercosis and hydatid cyst are well documented etoiologies whereas Dirofilariasis is an emerging zoonotic infection with worldwide case reports. Imaging techniques and fine needle aspiration can point towards the diagnosis; however in the absence of characteristic features, histopathology can be relied upon to diagnose a helminthicetiology.
Keywords
Cysticercosis; Dirofilaria; Filaria; Granulomas; Zoonoses
Download this article as:| Copy the following to cite this article: Singh V. K, Bhattacharjee K, Jaiprakash P. Clinicopathological Study of Subcutaneous Heminthicnodules : Unusual Sites and Diagnostic Dilemmas. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Singh V. K, Bhattacharjee K, Jaiprakash P. Clinicopathological Study of Subcutaneous Heminthicnodules : Unusual Sites and Diagnostic Dilemmas. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3i23v7l |
Introduction
Superficial skin/ subcutaneous nodules can have varied neoplastic or non-neoplastic aetiologies. These usually include tumors, skin appendage lesions, tumor-like lesions, inflammatory and infective lesions1. A helminthic infection as an underlying etiology is often an accidental finding in the surgically excised specimens of these swellings. Due to increased international travel, immigration, climate changes, and iatrogenic immunosuppression, uncommon infections like fungal, protozoan, helminthic and ectoparasites are now reported with increasing frequency2. Somatic nematodes and cestodes are the commonly implicated amongst the helminthic agents, and the zoonotic nematodes show an emerging trend with increasing number of case reports inliterature3.
Wuchereriabancrofti, Brugiamalayi, and cysticercosis is responsible for most of the cases in India4,5,6. Recently there has been an increase in subcutaneous nodules caused by Dirofilaria seen in various countries which was considered to be a zoonotic infection earlier7.
The present study aims to evaluate and highlight the aetiological agents and the tissue diagnostic features of superficial lesions of helminthic etiology.
Materials and Methods
This was a single institution, retrospective, observational study undertaken in the department of pathology of a tertiary care center over a period of five years (2012-2017). Thirty-six cases of surgically excised palpable superficial nodules unsuspecting a parasitic etiology were reviewed. Out of the 36, 19 cases diagnosed as having helminthic etiology were included in the study. Electronic archives and test requisition forms were used to collect pertinent demographic and clinical data including the age, sex, clinical diagnosis, site and size of the lesions. Hematoxylin and eosin (H&E) stained slides of the cases were retrieved, and histopathological findings werereviewed.
Results
Amongst the 19 cases included in the study, 8 were males, and 11 were females (Range 1-64) with a mean age of 36.36 years. Chest wall (4/19, 21%), and eyelids (3/19, 15.7%) were the most common sites involved. Forehead, neck, and thigh had 2 cases each (2/19, 10.5%), while axilla, abdominal wall, leg, inguinal region, breast, and back had 1 case each(1/19, 5.2%). The size ranged from 0.8 to 15 cm in the greatest dimension.
The clinical suspicion was neoplastic in 7/19 cases. Lymphadenitis was second most common in 4/19 patients followed by an epidermoid cyst in 3/19 patients, one each of sebaceous cyst, dermoid cyst, forehead cyst, cellulitis, and myositis. In none of the cases, an infective etiology was considered as a diagnostic possibility. The demographic details with clinical diagnosis are summarised in Table 1.
Table 1: Demographic and clinical details.
|
Case |
Age
(years) |
Sex |
Site |
Size (cm) |
Clinical
diagnosis |
Histopahology
diagnosis |
||
| 1 | 35 | F | Forehead | 2×1 | Sebaceouscyst | Dirofilariasis | ||
| 2 | 64 | F | Forehead | 2.5×2 | Sebaceouscyst | Filariasis | ||
|
3 |
42 |
M |
Neck |
2.5×1.5 |
Lymphadenitis |
Abcess with
filarial worm |
dead | |
|
4 |
5 |
M |
Right leg |
1.5×1 |
Cellulitis |
Abcess
filariasis |
with | |
| 5 | 40 | M | Chest wall | 4×1.5 | Myositis | Filariasis | ||
|
6 |
25 |
M |
Inguinal region |
2×1.5 |
Lymphadenitis |
Lymphadenitis with
dead filarial worm |
||
| 7 | 38 | F | Eyelid | 0.8×0.5 | ? Tumor | Dirofilariasis | ||
| 8 | 44 | F | Abdominal wall | 4×3.5 | Dermoid cyst | Cysticersosis | ||
| 9 | 23 | M | Chest wall | 1.5×0.6 | ? Neopastic | Cysticersosis | ||
| 10 | 26 | M | Chest wall | 3.5×2.7 | ? Neoplasm | Cysticersosis | ||
|
11 |
52 |
F |
Right thigh |
11×7 |
Soft
tumour |
tissue | Hydatidosis | |
|
12 |
64 |
F |
Right thigh |
15×9 |
Soft
sarcoma |
tissue | Cysticersosis | |
|
13 |
46 |
F |
Left eye |
1.5×1 |
Epidermoid cyst |
Abcess with
filarial worm |
dead | |
| 14 | 52 | F | Chest wall | 1.5×1 | ? Neoplasm | Filariasis | ||
|
15 |
16 |
F |
Neck |
1.5×1 |
Lymphadenitis |
Granulomatous
inflammation with dead filarialworm |
||
|
16 |
49 |
F |
Axilla |
3×3 |
Lymphadenitis |
Granulomatous inflammation
filariasis |
with |
|
| 17 | 1 | M | Eyelid(lower) | 1×1 | Epidermoid cyst | Dirofilaria | ||
| 18 | 33 | M | Back | 2×2 | Lipoma | Filariasis | ||
| 19 | 36 | F | Breast | 1.7×1 | ? Carcinoma | Filariasis | ||
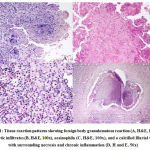
A characterisitic parasite morphology was seen in 15 cases (78.9%), while four cases showed dead or calcified parasites on extensive examination of the tissue. Amongst the other histopathological features, presence of histiocytes (13/19, 68.4%), granulomas (11/19, 57.8%), eosinophilia (10/19, 52.6%) were the top three histopathological findings. These findings were also consistently seen in the cases without a discernable parasite in the sections (Figure 1). Complete findings and the associated agents are summarised in Table 2.
Table 2: Histopathological findings and the associated organism.
| Histopathology | Filariasis | Dirofilariasis | Cysticercosis | Hydatidosis | Dead
calcified worms |
Cases(%) |
| Indentifiable
parasites |
7 | 3 | 4 | 1 | 15 | |
| Histiocytic
infiltrate |
6 | 2 | 2 | 3 | 13 | |
| Granuloma | 4 | 3 | 1 | 3 | 11 | |
| Eosinophils | 4 | 1 | 2 | 4 | 11 | |
| Giant cells | 2 | 2 | 2 | 3 | 9 | |
| Chronic
inflammation |
4 | 1 | 2 | 1 | 2 | 10 |
| Acute
inflammation |
2 | 2 | 1 | 2 | 7 | |
| Necrosis | 2 | 1 | 1 | 3 | 7 |
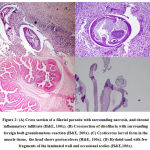
The most common etiological agent seen in the histopathology specimens was filarial worms (7/19, 36.8%) followed by cysticercosis (4/19, 21%), dirofilaria (3/19, 15.7%) and echinococcus (1/19, 5%) (Figure 2). 4/19 cases (21.1%) did not have a discernible parasite morphology, but a calcified remnant which was suspicious of a parasitic etiology.
Discussion
A variety of arthropods, protozoa, and helminths infect the skin and subcutaneous tissues and can be identified by pathologists in cytology and histology preparations. The specific organisms depend on patient’s exposure history. Arthropods are the most common parasite seen and include Sarcoptesscabei, Demodex species and Tunga penetrans. Helminths are less often seen, and include round worms (eg, Dirofilaria spp.), tapeworms (eg, Taenia solium, Spirometra spp.), and flukes (eg, Schistosomaspp.)8,9,10.
Filariasis is usually caused by nematodes including Wucheriabancrofti, Brugia spp. And manifests primary as lymphedema of the extremities, genitalia, and breasts. The most common presentations of W. bancrofti infestation are elephantiasis, chronic lymphedema, epididymitis, funiculitis, and lymphadenitis. Subcutaneous swelling is an extremely uncommon presentation of bancroftian filariasis even in endemic areas11. In contrast to W. bancrofti, Loa loa commonly presents as a subcutaneous nodule. The cycle starts when a female Chrysops takes microfilariae from the blood of an infected individual during a blood meal. Then the microfilariae mature toward infective larvae (L3), which become infective and can be transmitted to another human during the next blood meal. In humans, filarial worms will develop to adult stage and then can produce microfilariae, which can be transmitted to the next individual during another blood meal. The microfilariae have a diurnal periodicity, appearing in the peripheral blood in the day time, and reach their maximum at around midday (11:00 am to 1:00 pm)12 The diagnosis of filariasis is by a demonstration of microfilaria in stained or unstained blood films, circulating filarial antigen detection and demonstration of organism in histopathological sections13. Other histological features seen with filarisis are foreign body giant cells, forming tubercle-like nodules. While intact microfilariae are not generally seen, there may be fragments of the parasite in the granulomata. The eosinophilic debris, in the form of Splendore-Hoeppli material can been seen surrounding the parasite14. The inflammatory reaction develops in nodular fashion around fragmented and necrotic worms. Epithelioid cells and foreign body giant cells appear subsequently. In the present study seven cases with filariasia was seen. Tissue histiocytic infiltrate (6/7 cases), granulomas (4/7 cases), andeosinophilia (4/7 cases) were the most consistent findings. Amongst the four cases with calcified worms, tissue eosinophila (4/4 cases), histiocytes, granulunomas and foreign body giant cells (3/7 cases each) were the most consistent features, pointing to the fact that in the presence of such features an effort should be made to locate an etiology by taking more tissuesections.
Human subcutaneous dirofilariasis is a rare helminthic infection caused by filarial worms of the genus Dirofilaria, which is the natural parasites of dogs, cats, foxes, and wild mammals. Dirofilaria species belongs to the filarial nematodes, causes zoonotic infections in man, occasionally. Subcutaneous dirofilariasis is caused mainly by Dirofilaria repens, which causes subcutaneous nodules in and around the eye15. Human dirofilariasis due to D. repens has increasingly been recognized in India with most cases presenting with ocular menifestations15. Dirofilaria usually presents as subcutaneous nodule, either tender or nontender; occasionally migratory; and may be associated with an abscess. It is commonly found on the eyelids, scrotum, breasts, arms, and legs16. Mode of transmission of dirofilaria is through bite of an infected mosquito of the Culex and Anopheles species. Histologic examination for species identification can be made by analysis of the length and morphology of the parasite; patients do not typically exhibit eosinophilia16. Dirofilariais characterized by a relatively large size, thick cuticle, and prominent musculature with muscle cells extending far into body cavity17. Different Dirofilaria species can be distinguished by their size, thickness of cuticle, and presence or absence of longitudinal ridges17. The presence of thick laminated cuticles,large muscle cells, and wide lateral chords is diagnostic for this parasite. Histopathological sections usually show cross section of the prarasite with surrounding granulomatous tissue reaction with an intense inflammatory cell infiltrate composed of neutrophils, lymphocytes, and plasma cells along with foreign body giant cells18. Despite Dirofilaria being a filarial nematode, the present study describes the cases saperate from those of filariasis as it is an emerging zoonotic parasitic infection and not very well documented in the literature. The present study describes three cases of subcutaneous dirofilariaisis affecting the eyelids. The most consistent tissue reactions were granulomas (3/3 cases), histiocytic infiltration, giant cells, and acute inflammatory infiltrate (2/3 cases each). Scanty tissue eosinophils were seen in onecase.
Human cysticercosis is a potentially deadly infestation and is the consequence of ingestion of eggs of Taenia solium. Cysticercosis is the most common parasitic infestation of the central nervous system, muscle and subcutaneous tissue. About 54% of the patients present with subcutaneous nodules19. Clinical features of cysticerosis shows numerous small papules and nodules, cysts in subcutaneous tissue, skeletal muscles, or mucous membranes, urticaria from leaking cyst fluid19. Histological examination usually shows preserved parasitic morphology with calcospherules. The host tissue reaction ranges from epitheloid to histocytic to lymphocytic proliferation with or without a capsule. The parasite appears in the well formed cyst as usually distorted and often mummified. But the hooklets were relatively preserved up to the late stage20. The present study describes tissue cysticercosis in chest wall in 2 patients and 1 each of abdominal wall and right thigh lesion. The histological findings include inflammation with eosinophilia(2/4 cases), histiocytic granulomas with gaint cells(2/4 cases), necrosis(1/4 cases) and dicernable worms in the microscopic examination(3/4 cases).
Hydatid cyst disease is an endemic parasitic infestation caused by Echinococcusgranulosus and it is an important public health problem in the Mediterranean countries, Middle East, Africa, South America, Asia and Australia21,22. It most commonly affects the liver (60–70%) while lungs are the second most common site (5–27%)21. If parasite passes liver and lungs, it may locate in any organ. Primary subcutaneous hydatid disease means that there is not any primary focus of hydatidosis. The main symptom of subcutaneous hydatosis was mobile painless, slow growing mass and only 30% of the patients complained with pain. Kayaalp C etal22 reported the incidence of subcutaneous hydatid disease as 1.5% among all cases of hydatid disease in endemic areas. Serology is a useful tool particularly for the differential diagnosis of hydatid liver cysts, however, it is usually negative (79%) for subcutaneous hydatid cysts.Microscopically,hydatidcysthas3layers.Innermost(germinallayer)is10-25
microns, contains nuclei, gives rise to brood capsules attached by short stalk in infectious (fertile) cysts, often with daughter cysts. Alsoprotoscolices (attached or separated) with double row of refractile, birefringent, acid fast hooklets 22 – 40 microns and 4 round suckers that comprise “hydatid sand”. Daughter cysts may merge and provide internal septation. The second layer is a laminated membrane beneath germinal layer, which is 1 mm thick, avascular, eosinophilic, refractile and chitinous; strongly positive for Periodic acid Schiff, and Gomori’smethanamine silver. Outer layer is dense fibrovascular tissue with chronic inflammatory cells, variable calcification develops after over a long period of time if the disease goes unnoticed23. The present study described a case of subcutaneous hydatidosis involving the right calf muscles, the tissue reaction showed a pericytic histiocytic infiltrate along with chronic inflammation, the debris on processing showed laminated memebranes, germinal layer and occasionalprotoscoleces.
Fine Needle aspiration cytology (FNAC) is a safe, reliable, rapid and cheap method of evaluating swellings. It is well tolerated by patients and usually done as an outpatient procedure. In cases of suspected malignancy, FNAC is a good choice as it prevents spread and in cystic swellings can be therapeutic24. FNAC is currently being used for diagnosis of parasitic infections24. Utility of FNAC in parasitic infections such as cysticerosis, filariasis and hydatid cyst disease has been well documented, but isnotwithour pitfalls25. The cytological specimen is then evaluated and smears are made of the aspirate for further evaluation. Parasitic infections are usually confirmed when there are demonstrable organisms in the smear along with other features of infections. Lymphocytic infiltration, particularly eosinophilic, along with giant cells, fibrous tissue are usually made out in the stained smears.26 In cases where there are no discernible organisms or parts thereof, biopsy with histopathological examination may reveal the diagnosis. Histopathological analysis helps in understanding the interactions between the host and the parasite and aids in the diagnosis where cytopathology does not reveal a conclusive diagnosis27. In some cases like hydatid cyst disease, it is better to an excision then risk spillage of the hydatid fluid which can causereactions9.
Parasitic infections are on an increase in the recent years. Many factors or combinations of factors contributing to disease emergence include ecological changes, such as those due to human activities or to anomalies in climate; travel and immigration, technology and industry; microbial adaptation and change and breakdown of public health measures. The emerging infectious diseases are also attributed to the population growth, ageing population, poverty and malnutrition, environmental pollution, deforestation, crowding, inadequate infrastructure, poor
sanitation and water supply, global warming, development of antimicrobial/insecticide resistance etc. In addition to this the immunocompromised patients, including patients with AIDS, solid organ transplant recipients, and patients on immunosuppressive therapy for disorders, are at high risk for opportunistic parasites27.
Conclusion
Histopathological features like granulomatous inflammation and tissue eosinophilia are strong indicators of a parasitic aetiology. Subcutaneous and intramuscular filariasis, cysticercosis and hydatid cyst are well documented in literature whereas Dirofilariasis is an emerging zoonotic infection in the Indian subcontinent. Imaging techniques and fine needle aspiration can point towards the diagnosis with precision; however in the absence of characteristic features, histopathology can be relied upon to diagnose a helminthic aetiology.
Acknowledgement
The Authors acknowledge the role of the Department of Pathology, KMC Manipal and the support provided by the staff.
Conflict of Interest
There has been no conflict of interest in the present study.
Funding Source
There has been so funding source for this study.
References
- Francesca D. Beaman, Mark J. Kransdorf, Tricia R. Andrews, Murphey, Lynn Arcara, James H. Keeling. Superficial Soft-Tissue Masses: Analysis, Diagnosis, and Differential Considerations. RadioGraphics 2007; 27 (2):509–523.
CrossRef - Nawas ZY,Tong Y,Kollipara R, Peranteau AJ, Woc-Colburn L, Yan AC, Lupi O,Tyring SK. Emerging infectious diseases with cutaneous manifestations: Viral and bacterial infections. J Am Acad Dermatol. 2016 Jul; 75(1):1-16.
CrossRef - Pampiglione S,Rivasi F, Angeli G, Boldorini P, Incensati R.M., Pastormerlo M., M. Pavesi, Ramponi A. Dirofilariasi due to Dirofilaria repens in Italy, an emergent zoonosis: report of 60 new cases, Histopathology 38 (2001)344–354.
CrossRef - Goyal P., Sehgal S., Ghosh S., Mittal D., Kumar A., Singh S. A Cytological Study of Palpable Superficial Nodules of Parasitic Origin: A Study of 41 Cases. Patholog Res 2014; 2014:373472.
CrossRef - Ratnatuna N., Wijesundera M. Histopathological diagnosis of subcutaneous Dirofilaria Repens infection in humans. Southeast Asian J Trop Med Public Health 1999; 30(2); 375-78.
- Norgan AP, Pritt BS. Parasitic Infections of the Skin and Subcutaneous Tissues. Adv 2018 Mar; 25(2):106-123.
CrossRef - Azad K, Arora R, Gupta K, Sharma U. Lymphatic filariasis: Aspiration of adult gravid female worm from a soft tissue swelling. J Cytol 2010; 27:156-7.
CrossRef - LaboratoryDiagnosisofInfectionsDuetoBloodandTissueParasites. Clin Infect Dis. 2009; 49(7): 1103-1108.
CrossRef - D’Souza , JakribettuRP., SudharsanaS., AithalaSP. Subcutaneous nodule: A case of Dirofilaria. Int J Appl Basic Med Res. 2013 Jan-Jun; 3(1): 64–65.
CrossRef - Lupi O, Downing C, Lee M, et al. Mucocutaneous manifestations of helminth infections: Nematodes. J Am Acad Dermatol. 2015; 73(6):929-944.
CrossRef - Kollipara R, Peranteau AJ, Nawas ZY, et al. Emerging infectious diseases with cutaneous manifestations Fungal, helminthic, protozoan and ectoparasitic infections. J Am Acad Dermatol. 2016; 75(1):19-30.
CrossRef - Akue J., Eyang-Assengone E., Dieki R. Loa loa infection detection using biomarkers: current perspectives. Res Rep Trop Med. 2018; 9:43–48.
CrossRef - Shenoy RK. Clinical and pathological aspects of filarial lymphedema and its management. Korean J Parasitol.2008;46(3):119–125.
CrossRef - Kayaalp.Hydatidcystoftheliver.L.H.Blumgart,R.J.Belghiti,R.P.DeMatteo, W.C. Chapman, M.W. Büchler, L.E. Hann, M. D’Angleca (Eds.), Surgery of liver biliary tract and pancreas (4th ed), Saunders Elsevier, Philadelphia (2007), 952-970.
- L. Eberhard. Zoonotic filariasis. R.L. Guerrant, D.H. Walker, P.F. Weller (Eds.), Tropical infectious diseases: principles, pathogens, and practice (3rd ed.), Elsevier, New York 2011;750-758.
CrossRef - Permi HS, Veena S, Prasad HK, Kumar YS, Mohan R, Shetty KJ. Subcutaneous human Dirofilariasis due to Dirofilaria repens: Report of two cases. J Glob Infect Dis 2011; 3: 199-201.
CrossRef - Pampiglione, F. Rivasi, G. Canestri-Trotti .Pitfalls and difficulties in histological diagnosis of human dirofilariasis due to Dirofilaria (Nochtiella) repens Diagn Microbiol Infect Dis,1999;34:57-64.
CrossRef - Srinivasamurthy V, Rao M S,Thejaswini M U, Yoganand. Human subcutaneous dirofilariasis. Ann Trop Med Public Health2012;5:349-51.
CrossRef - Chi HS., Chi JG. A histopathological study on human cysticercosis. Korean J Parasitol. 1978 Dec;16(2): 123-133.
CrossRef - Sacchidanand S, Namitha P,Mallikarjuna M, Nataraj H V. Disseminated cutaneous cysticercosis and neurocysticercosis: A rare occurrence. Indian Dermatol Online J 2012; 3:135-7.
CrossRef - Bal, N.E. Kocer, R. Arpaci, A. Ezer, F. Kayaselcuk. Uncommon locations of hydatid cyst Saudi Med J 2008; 29:1004-1008.
- Kayaalp, DiricanA., Aydin C. Primary subcutaneous hydatid cysts: A review of 22 cases. International Journal of Surgery 2011; 9(2):117-121.
CrossRef - Pedrosa I., Saiz A., Arrazola J., Ferreiros J., Pedrosa C. Hydatid Disease: Radiologic and Pathologic Features Complications. RadioGraphics 2000; 20:795–817.
CrossRef - Arora VK, Singh N, Chaturvedi S, Bhatia A. Fine needle aspiration diagnosis of a subcutaneous abscess from enterobius vermicularis infestation: A case report. Acta Cytol. 1997; 41(6):1845-1847.
CrossRef - Yadav YK, Gupta O, Aggarwal R. Cytological diagnosis of parasites presenting as superficial nodular swelling: Report of 35 cases. J Parasit Dis. 2012; 36(1):106-111.
CrossRef - Sołtysiak Z, Rokicki J, Kantyka M. Histopathological diagnosis in parasiticdiseases. Ann Parasitol. 2014; 60(2): 127-131.
CrossRef - Prasad K.J. Emerging and re-emerging parasitic diseases. JIMSA 2010 ; 23(1) :45-50.