Rakesh V. Mishra1* and Shashikant N. Dhole2
1Department of Pharmaceutics, Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune, Maharashtra, India.
2Department of Pharmaceutics, Modern College of Pharmacy, Moshi, Pune, Maharashtra, India.
Corresponding Author E-mail : mishrarakesh287@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2201
Abstract
Numerous researchers in past have reported the diversified therapeutic effects of Berberine hydrochloride (BERH) for the management of metabolic diseases, however due to poor systemic bioavailability these effects are dose dependant and desired effects were reported at high dose levels. The objective of present investigation is to evaluate and establish the enhancement in pharmacological efficacy of the designed BERH formulation at low oral dose level for the treatment of metabolic diseases constituting metabolic syndrome (MS). In the present investigation, BERH formulation in the dose level of (25 and 50mg/kg/day) was evaluated in cafeteria diet (CD) induced MS model in male Wistar rats for 42 days and compared with available marketed preparation in similar dose level using orlistat as reference drug. Among the studied dose level of BERH formulation the 25 mg/kg/day dose was adequate to produce significant reduction in calorie intake (P < 0.01), body weight, BMI, (P<0.001), organ weight viz. (stomach; P<0.05, liver; P<0.001, heart; P<0.01) and serum biochemical parameters (P<0.001). A significant improvement in lipid peroxidation (P<0.001), catalase (CAT), reduced glutathione (GSH) and superoxide dismutase (SOD) contents (P<0.001) was observed. The histopathological examinations indicated amelioration of liver, heart and pancreas tissues. The current study indicated significant glucose-lowering, hypophagic, anti-obesity, anti-hyperlipidemic and cardio protective activity of the BERH formulation even in much low oral dose level compared to previously reported studies. The observed behavior is attributed due to the enhanced bioavailability of BERH formulation which could be effectively used for metabolic diseases treatment.
Keywords
Berberine Hydrochloride; Bioavailability; Cafeteria Diet; Hyperlipidemic; Metabolic Syndrome; Obesity
Download this article as:| Copy the following to cite this article: Mishra R. V, Dhole S. N. Enhanced Pharmacological Efficacy of Berberine Hydrochloride Loaded Lipid Based Pellets for the Treatment of Metabolic Diseases. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Mishra R. V, Dhole S. N. Enhanced Pharmacological Efficacy of Berberine Hydrochloride Loaded Lipid Based Pellets for the Treatment of Metabolic Diseases. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3wjaLAs |
Introduction
Urbanization, sedentary lifestyles, and changing diets are the characteristic factors of 21st century that leads to the concept of metabolic syndrome (MS). The MS includes clusters of metabolic abnormalities such as obesity, insulin resistance, dyslipidemia and hypertension which are observed together in patients. It is also related with higher possibility of cardiovascular disease and type 2 diabetes mellitus (T2DM) and consequential morbidity and mortality of individuals suffering with MS. Nowadays, MS is the most serious public health concern and clinical challenge which is increasing at an alarming rate worldwide.1 To manage and treat metabolic syndrome various efforts have been made including awareness regarding lifestyle changes to the development of effective and safe medications.2 Despite of this, patient compliance becomes an issue when multiple drugs need to be taken on a long-term basis for the treatment of multifactorial diseases.3 Furthermore considering the adverse effects associated with the available pharmacological treatment for MS, recognization has been growing for drug formulations developed from natural sources. In recent past, phytochemicals have gained much interest for the treatment of metabolic disease constituting MS.4 BERH, an isoquinoline alkaloid, found in different Berberis species plants commonly located in the Eastern hemisphere.5 Numerous researchers in past have reported the the potential therapeutic applications of BERH in the management of multi-factorial diseases such as obesity, T2DM and dyslipidemia constituting MS.6-13 Shen et al.,14 demonstrated that BERH improves insulin resistance by increasing the insulin receptor and activation of insulin signaling pathway. Cok et al.,15 reported that BERH produces antidiabetic effect by enhancing the expression of glucose transporter protein which leads to increase glucose uptake. Kong et al.,9 investigated BERH as effective cholesterol-lowering agent and reported that its lipid lowering activity is due to down regulation of LDL through upregulation expression of low density lipoproteins receptor. Wang et al.,16 showed that BERH reduces blood cholesterol levels by preventing cholesterol absorption and enhancing its excretion. Lee et al.,9 reported that BERH stimulates adenosine monophosphate-activated protein kinase (AMPK) activity could be responsible for its antiobesity effect. Perez et al.,17 showed that BERH decreases triglycerides levels, waist circumference and increases insulin sensitivity which overall reduces risk of MS.
Although hypoglycemic effects, lipid lowering effects, and reduction in weight gain have been demonstrated in various preclinical and clinical studies, however these effects are dose dependant and the oral dose of BERH is still high.18-21 The BERH oral dose level reported in various studies demonstrated significant effects ranged from 100 to 500mg/kg per day (animal models) and 0.5 g twice/thrice a day (humans). In some studies the efficacy of BERH was reported in animal models at lower dose when it is administrated intraperitoneally (i.p.), but i.p. injection would not be a suitable route of administration for human use.20 The high dose regime is due to BERH poor systemic bioavailability (< 1%) which limits its clinical use.20-24 Moreover, high dose of BERH may also leads to deleterious side effects. Improving the oral bioavailability of BERH through formulation innovation can enhance its desired pharmacological effects with reduced oral dose.21
In our previous research work we have reported lipid type floating pellet system for bioavailability improvement of BERH.25 The optimized formulation of BERH lipid based pellets consist of gelucire 50/13 in the ratio of 1:2 (drug: gelucire 50/13), NaHCO3: HPMC K4M in the ratio of 1:0.5 (20% w/w) and gelucire 43/01 (27.5% w/w). The designed BERH formulation showed significant enhancement in bioavailability compared to marketed preparation.25 As an extension of previous research work, the current study aims to evaluate and establish the efficacy of the BERH formulation in low dose level for the treatment of metabolic diseases constituting MS. In the present investigation BERH formulation evaluated in cafeteria diet (CD) induced metabolic syndrome model in male Wistar rats and compared with available marketed preparation to establish the enhancement in efficacy. Orlistat was used as the standard drug.
Materials and Methods
Material
BERH obtained from Yucca Enterprises, Mumbai, India. Standard drug Orlistat was obtained from Wockhardt Limited, Aurangabad, India. All the other chemicals and biochemical kits used in the present work were procured from Pathozyme Diagnostics, Mumbai, India.
Cafeteria Diet Induced Metabolic Syndrome Model
The male Wistar rats (180–200 g) were obtained from National Institute of Bio-science, Pune, Maharashtra, India. The animals were housed under controlled humidity and temperature and condition with light and dark cycle each of 12 h. The animals were given free access to water and food. After one-week of acclimatization period, animals were employed for study. The research protocol was permitted by Institutional Animal Ethical Committee (DYPIPSR/IAEC/19/P-11). CD induced metabolic syndrome model in rats is selected as they more resembles to human ultra-processed diet compared to commercial high sugar/fat rodent diets.26,27 The CD composed of condensed milk (8g), bread (8 g), biscuits (6 g), chocolate (3 g), cheese (8 g), dried coconut (6 g) and potato chips (10 g). These diets were provided to the individual rats of group II to VII during the 42 days of the experiment. The CD calorie value indicated in (Table 1).
Table 1: Cafeteria Diet Calorie Value.
| Ingredients | Calorie Value (Kcal/100g) |
| Condensed Milk | 69 |
| Bread | 247 |
| Chocolate | 230 |
| Biscuits | 453 |
| Dried Coconut | 185 |
| Cheese | 402 |
| Boiled Potato | 75 |
Experiment Protocol
The study includes 42 rats divided into seven groups of 06 rats each. The BERH pellet formulation is compared with marketed preparation in similar dose level using orlistat as standard reference drug. The drugs were administered through per oral (p.o.) route using oral gavage once a day for a period of 42 days.
Group I: Normal control provided rodent chow diet and 0.5% CMC (NC)
Group II: Fed with Cafeteria Diet (CD)
Group III: Cafeteria diet + Orlistat (45 mg/kg, p.o.) in 0.5% CMC from 8th day (ORL)
Group IV: Cafeteria diet + BERH pellet formulation (25 mg/kg, p.o.) from 8th day (F – 25 mg/kg)
Group V: Cafeteria diet + BERH pellet formulation (50 mg/kg, p.o.) from 8th day (F – 50 mg/kg)
Group VI: Cafeteria diet + BERH marketed preparation (25 mg/kg, p.o.) from 8th day (M – 25 mg/kg)
Group VII: Cafeteria diet + BERH marketed preparation (50mg/kg, p.o.) from 8th day (M – 50 mg/kg)
Effect on Calorie Intake, Body Weight and Body Mass Index
The food ingestion of each group was measured by calculating the difference in weight of preweighed food and the food remain over after 24 h. The daily caloric (kcal) intake was determined by multiplying the food intake by their caloric content and expressed as mean energy intake for a group of six rats in kcal/week. The weights of each rat were taken separately on 0th, 7th and 42nd day. Body mass index (BMI) was determined from formula:
BMI = body weight (g)/ length2 (cm2)
Biochemical Estimation
On 43rd day rats were anesthetized and by retro-orbital method blood was isolated. Serum was immediately collected by centrifuging blood at 4000 rpm for 15 min. The serum levels of glucose,28 total-cholesterol (CH),29 triglycerides (TGs),30 high density protein (HDL), low density protein (LDL),31 Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)32 were estimated using biochemical kits (Pathozyme Diagnostics) on EPOCH, Biotek Instruments, USA (Software Gen 5.3.05).
Effect on Organ Weight and White Adipose Tissue (WAT)
On 43rd day animals were sacrificed; organs viz; liver, stomach, heart and WAT were isolated immediately followed by cleaning (saline phosphate buffer) and weighing. The collected samples were kept at – 70°C till further analysis.
Free Radical Scavenging and Lipid Peroxidation Activity in Cardiac Tissue
Heart portion was crushed and homogenized (10%, w/v) for determination of LPO,33 SOD,34 CAT35 and GSH content using EPOCH, Biotek Instruments, USA (Software Gen 5.3.05).36
Histopathology Study
Histopathology of organs viz; liver, heart and pancreas were studied. Liver, heart and pancreas were fixed in neutral buffered formalin. These tissues were trimmed longitudinally, dehydrated using alcohol of rising grades with cleaning using xylene and implanted in paraffin wax. Further rotary microtome was used to sectioned implanted tissue of 3-4 µm thickness. Hematoxylin & Eosin (H & E) were used to stain all the tissue slides. The tissue slides were observed under microscope (Jeol, Oxford Instrument, United Kingdom) for any histopathological lesions.
Statistical Analysis
Data was represented as the mean ± standard error of the mean (SEM). Results were evaluated by one way/two way ANOVA (analysis of variance) with Bonferroni test using Graph pad prism-7 software. P <0.05 value were considered significant.
Results
Effect on Calorie Intake, Body Weight and BMI
The calorie intake was significantly increased in the CD fed rats by 1.46 fold (P < 0.01) compared to the normal control group. However, the rats received BERH formulation (25mg/kg, 50mg/kg) and orlistat showed a significant decrease in calorie intake per week by 1.35 fold (P < 0.01), 1.40 fold (P < 0.01) and 1.63 fold (P < 0.001) respectively indicated in (Figure 1). The animal group fed with CD for six weeks showed significant rise in body weight and BMI (277.98 ± 3.69 g, 2.65 ± 0.03 g/cm2, P < 0.0001) compared to the normal diet-fed rats (212.20 ± 2.63 g, 1.41 ± 0.04 g/cm2) shown in (Figure 2 & 3). Treatment with BERH formulation in the dose of 25mg/kg and 50mg/kg significantly reduced the increased in body weight and BMI (238.79 ± 2.52 g, 1.96 ± 0.01 g/cm2, P < 0.001) and (231.19 ± 1.65 g, 1.77 ± 0.03 g/cm2, P < 0.001) respectively comparable to orlistat. However, group VI and VII doesn’t showed significant effect on calorie intake, body weight and BMI compared to CD group.
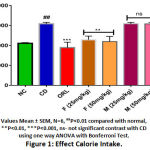 |
Figure 1: Effect Calorie Intake. |
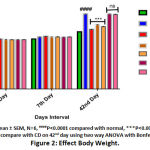 |
Figure 2: Effect Body Weight. |
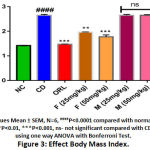 |
Figure 3: Effect Body Mass Index. |
Effect on Serum Biochemical Parameters
The serum levels of glucose, CH, LDL, TGs, ALT and AST was significantly enhanced in the CD fed group compared to the normal diet-fed rats (P<0.0001). On contrary, HDL level was reduced significantly in CD control group (P<0.001). However, BERH formulation significantly reduced the enhanced serum levels of glucose, CH, LDL, TGs, ALT and AST and increased HDL concentration significantly (P<0.001) as indicated in (Table 2 & 3). The BERH marketed preparation in the dose of 50 mg/kg showed effect on serum biochemical parameters but unable to reduce the enhanced glucose level.
Table 2: Effect on Serum CH, TGs, LDL and HDL.
| Groups | Variables | |||
| CH (mg/dl) | TGs (mg/dl) | LDL (mg/dl) | HDL (mg/dl) | |
| Normal | 66.06 ± 1.57 | 63.95 ± 0.85 | 37.94 ± 1.02 | 47.67 ± 1.45 |
| Cafeteria Diet | 110.04 ± 1.31#### | 105.99 ± 1.29#### | 55.89 ± 1.17### | 30.92 ± 0.77### |
| Orlistat | 80.93 ± 1.51*** | 75.43 ± 1.56*** | 39.81 ± 0.95*** | 49.04 ± 1.71*** |
| BERH – F (25 mg/kg) |
95.19 ± 0.61** | 96.12 ± 1.25** | 46.29 ± 1.31** | 42.15 ± 0.92** |
| BERH – F (50 mg/kg) |
77.27 ± 1.03*** | 75.19 ± 0.25*** | 42.16 ± 2.31*** | 48.20 ± 0.96*** |
| BERH – M (25 mg/kg) |
108.39 ± 1.79ns | 107.04 ± 1.65ns | 57.89 ± 2.09ns | 29.46 ± 2.07ns |
| BERH – M (50 mg/kg) |
102. 55 ± 1.56* | 100.27 ± 2.09* | 50.11 ± 1.57* | 37.88 ± 1.96* |
Values Mean ± SEM, n=6, ####P<0.0001, ###P<0.001 compared with normal, ***P<0.001, **P<0.01, *P<0.05, ns- not significant compared with CD group.
Table 3: Effect on Serum Glucose, ALT and AST.
| Groups | Variables | ||
| Glucose (mg/dl) | ALT (u/l) | AST (u/l) | |
| Normal | 68.89 ± 0.45 | 41.36 ± 0.63 | 58.02 ± 0.48 |
| Cafeteria Diet | 107.13 ± 0.92#### | 83.93 ± 0.41#### | 103.52 ± 0.62#### |
| Orlistat | 80.82 ± 1.19*** | 56.92 ± 0.41*** | 62.80 ± 0.69*** |
| BERH – F (25 mg/kg) |
77.96 ± 1.11*** | 66.04 ± 0.77** | 71.92 ± 0.45*** |
| BERH – F (50 mg/kg) |
74.82 ± 1.07**** | 48.89 ± 1.54**** | 65.42 ± 1.67*** |
| BERH – M (25 mg/kg) |
107.88 ± 1.92ns | 82.57 ± 2.14ns | 103.94 ± 2.36ns |
| BERH – M (50 mg/kg) |
106.30 ± 2.62ns | 75.14 ± 1.77* | 92.17 ± 2.05* |
Values Mean ± SEM, n=6, ####P<0.0001, ###P<0.001 compared with normal, ****P<0.0001,***P<0.001, **P<0.01, *P<0.05, ns- not significant compared with CD group.
Effect on Organ and WAT Weight
The CD rat group showed significant increase in the weight of organs (stomach by 2.7 fold; P<0.001, liver 1.91 fold; P<0.0001, heart 2.25 fold; P<0.0001) and WAT by 2.3 fold; P<0.0001 compared to normal control depicted in (Figure 4 & 5). Treatment with BERH formulation significantly reduced the organ weight gain which was induced by cafeteria diet (stomach by 1.28 fold; P<0.05, liver 1.48 fold; P<0.001, heart 1.35 fold; P<0.01) and WAT weight by 1.7 fold; P<0.001 comparable to standard drug. On the other side, group VI and VII doesn’t showed any reduction in the weight gain of organs and WAT.
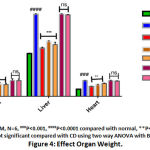 |
Figure 4: Effect Organ Weight. |
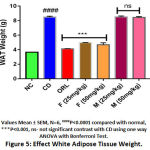 |
Figure 5: Effect White Adipose Tissue Weight. |
Free Radical Scavenging and Lipid Peroxidation Activity in Cardiac Tissue
CD fed rats depicted a significant enhancement in lipid peroxidation value by 2.92 fold; P<0.0001 compared to normal control group. Treatment with BERH formulation (25mg/kg, 50mg/kg) and orlistat significantly reduced lipid peroxidation value by 2.05, 3.04 and 2.71 fold (P<0.0001) respectively in contrast with CD group shown in figure 6. A 1.26 fold; P<0.05 decrease in lipid peroxidation is also observed with the BERH marketed preparation (50 mg/kg) compared to CD group. On the other side the CD fed rats depicted significant reduction in cardiac SOD, CAT and GSH by 1.85, 1.86 and 2.45 fold (P<0.0001) respectively in contrast with normal control group. Furthermore, the rats received BERH formulation (25mg/kg, 50mg/kg) and orlistat showed a significant enhancement in cardiac SOD contents by (1.46; P<0.001, 1.80; P<0.0001, 1.57; P<0.0001 fold), CAT contents by (1.72, 1.65, 1.75 fold; P<0.001), GSH content by (2.13, 2.27, 2.16 fold; P<0.001) respectively contrast with CD group shown in Figure 6. Although the BERH marketed preparation in the studied dose level has not showed significant effect on SOD, CAT and GSH content.
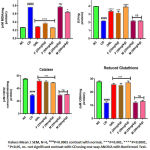 |
Figure 6: Effect Lipid Peroxidation, Superoxide Dismutase, Catalase and Reduced Glutathione. |
Histopathology Study
The histopathological examination of rats from group I to IV was conducted. Microscopic liver evaluations of normal control don’t show any lesion of pathological consequence and showed normal histology, portal triad (large arrow), hepatocytes (small arrow) {H & E, 400X} indicated in Figure 7. CD fed group showed sinusoidal dilation and hepatocellular cytoplasmic vacuolation. Rats treated with standard drug orlistat and BERH formulation (25 mg/kg) showed normal histology, portal triad and hepatocytes. Microscopic examination of pancreas of normal control group showed normal histology, acini (A), inater-calated duct (IcD), interlobular duct (ID), ilets of langerhans (IL) {H & E, 400X} shown in Figure 8. CD fed group showed cytoplasmic vacuolation at ilets of Langerhans (CV). Groups received standard drug orlistat and BERH formulation (25 mg/kg) showed normal histology with absence of cytoplasmic vacuolation. In the histopathological studies, heart sections of normal control group showed normal histology, cardiac myocytes (arrow) {H & E, 400X} depicted in Figure 9. CD fed group showed deposition of fat globules in cardiac myocytes. Groups treated with standard drug orlistat and BERH formulation (25 mg/kg) showed normal architecture of myocytes with no fatty changes.
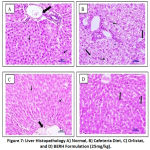 |
Figure 7: Liver Histopathology A) Normal, B) Cafeteria Diet, C) Orlistat, and D) BERH Formulation (25mg/kg). |
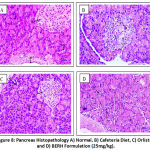 |
Figure 8: Pancreas Histopathology A) Normal, B) Cafeteria Diet, C) Orlistat, and D) BERH Formulation (25mg/kg). |
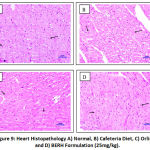 |
Figure 9: Heart Histopathology A) Normal, B) Cafeteria Diet, C) Orlistat, and D) BERH Formulation (25mg/kg). |
Discussion
Initially, pilot study was employed to evaluate the efficacy of the BERH pellet formulation in male Wistar rats and different effective low dose levels were selected as 25 and 50 mg/kg once a day and compared with similar dose level of marketed preparation. The rats received BERH formulation (25 and 50mg/kg) showed significant effects comparable to standard orlistat drug.
In previously reported studies BERH in the dose of (150, 200 or 300mg/kg/day) has been reported to decrease the calorie intake, body weight and BMI whereas no significant effects observed in dose of (75 or 100 mg/kg/day).18,37,38 In our current study we have observed significant effect of BERH formulation on calorie intake (P < 0.01), body weight and BMI (P < 0.001) at lower dose level (25mg/kg/day). The mechanism reported that BERH stimulates AMP-activated protein kinase activity which is the probable reason for its antiobesity effect.10
A qualitative lipid abnormalities which include an elevation of TGs, LDL, CH, with elevated glucose and lower levels of HDL is characteristics of dyslipidemia and subsequent risk of metabolic diseases.39 The obtained results of present research indicated that treatment with BERH formulation in the studied dose level significantly reduced the increased serum biochemical parameters (P < 0.001). The lipid lowering effect of BERH treatment is consistent with previous reported studies.18,38 However, BERH in past have not been reported to produce significant hypoglycemic effect at low dose level.21 Additionally, in our study we have observed significant effect of BERH formulation on reduction of increased serum glucose level (P < 0.001) in the low dose of 25mg/kg/day. A number of studies have been reported proposing multiple mechanisms behind lipid lowering effects of BERH. The reported studies suggested that BERH upregulates lipolysis gene expression, upregulates liver LDL receptor expression, downregulates lipogenesis gene expression, prevents CH absorption and enhance its excretion.9,16,40 In addition BERH through AMPK signaling pathway enhances glucose transporter protein 1 expression and enhances the sensitivity of insulin receptors collectively improves glucose utility.14,15,41
Obesity, diabetic complications and hyperlipidemia collectively enhance systemic oxidative stress-imbalance among reactive oxygen species, free radicals in tissues and antioxidants.42 Numerous researchers through preclinical and clinical studies reported that BERH in higher dose regime (200, 300 or 500mg/kg/day) inhibits peroxisome proliferator – activated receptor gamma (PPARϒ) in adipocytes to counter act increased adiposity,43 reduces oxidative stress and regulates extra-cellular matrix synthesis and cell proliferation.38, 44-46 However, the results of current investigation reveals that BERH formulation significantly reduced the weight gain of organs (Stomach, Liver, Heart), WAT with significant improvement in lipid peroxidation, SOD, CAT and GSH contents at the studied low dose level.
At the end of study, histopathological studies of organs (liver, heart and pancreas) were conducted. The histological examination reveals that group treated with BERH formulation (25mg/kg/day) indicated normal histology, portal triad, hepatocytes in liver, ilets of langerhans with no cytoplasmic vacuolation in pancreas and normal architecture of cardiac myocytes. The observed results with BERH treatment were consistent with earlier reported studies instead higher doses were required for amelioration of liver, heart and pancreas tissues.18,38
In the present investigation we observe that BERH marketed preparation in similar dose level was unable to produce significant effects on the studied parameters. The observed behavior could be attributed due to the enhancement in bioavailability of the designed BERH formulation which is responsible for the significant glucose-lowering, hypophagic, anti-obesity, anti-hyperlipidemic and cardio protective activity even in low oral dose level of 25 mg/kg/day. Based upon the results of present investigation it would be interesting to evaluate the efficacy of designed BERH formulation in human subjects suffering with metabolic syndrome.
Conclusion
The BERH pellet formulation evaluated in cafeteria diet induced metabolic syndrome model in male Wistar rats indicated significant glucose-lowering, hypophagic, anti-hyperlipidemic and cardio protective activity. Amongst the studied dose level of BERH formulation the 25 mg/kg/day dose was adequate to produce significant metabolic effects comparable to reference drug orlistat. Treatment with BERH formulation in low dose level significantly reduced the increased calorie intake, serum biochemical parameters, body weight, BMI, organs and WAT weight with improvement in lipid peroxidation, CAT, SOD and GSH contents. The histopathological examinations also indicated amelioration of liver, heart and pancreas tissues in much lower dose of BERH formulation. On the other side, the BERH marketed preparation in the similar dose level was unable to produce significant metabolic effects. The observed behavior is attributed due to the enhanced bioavailability of BERH. In future, the reported results of current studies will be important for supporting the extension of these findings to clinical trials and to demonstrate the BERH efficacy for treatment of metabolic diseases and related complications in human subjects.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
The authors are thankful to Dr. S. S. Chitlange for providing kind support to carry out the research work. The authors acknowledge Wockhardt Limited, Aurangabad, India for providing gift sample of orlistat.
References
- VanWormer JJ, Boucher JL, Sidebottom AC, Sillah A, Knickelbine T. Lifestyle changes and prevention of metabolic syndrome in the heart of New Ulm Project. Prev Med Rep 2017; 6: 242-245.
CrossRef - Mishra RV, Dhole SN. Dipeptidyl peptidase-4 inhibitors: potential for treatment of metabolic syndrome and developed formulation approaches. Asian J Pharm Clin Res 2017; 10 (11): 20- 26.
CrossRef - Sattigeri JA, Sethi S, Davis JA, Ahmed S, Rayasam GV, Jadhav BG, et al. Approaches towards the development of chimeric DPP4/ACE inhibitors for treating metabolic syndrome. Bioorg Med Chem Lett 2017; 27(11): 2313-2318.
CrossRef - Seo JB, Choe SS, Jeong HW, Park SW, Shin HJ, Choi SM, et al. Anti-obesity effects of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp Mol Med 2011; 43(4): 205–215.
CrossRef - Kosalec I, Gregurek B, Kremer D, Zovko M, Sankovic K, Karlovic K. Croatian barberry (Berberis croatica Horvat): a new source of berberine-analysis and antimicrobial activity. World J Microb Biot 2009; 25(1): 145- 150.
CrossRef - Gao S, Basu S, Yang G, Deb A, Hu M. Oral bioavailability challenges of natural products used in cancer chemoprevention. Prog Chem 2013; 25(09): 1553-1574.
- Jantova S, Cipak L, Cernakova M, Kostalova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol 2003; 55(8): 1143–1149.
CrossRef - Kettmann V, Kostalova D, Jantova S, Cernakova M, Drimal J. In vitro cytotoxicity of berberine against HeLa and L1210 cancer cell lines. Pharmazie 2004; 59(7): 548-551.
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 2004; 10(12): 1344-1351.
CrossRef - Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006; 55(8): 2256- 2264.
CrossRef - Sanchez CJ. Increase in action potential duration and inhibition of the delayed rectifier outward current IK by berberine in cat ventricular myocytes. Br J Pharmacol 1996; 117(7): 1427-1434.
CrossRef - Shen N, Li X, Zhou T, Bilala MU, Du N, Hu Y, et al. Shensong Yangxin capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. J Ethnopharmacol 2014; 157: 161-170.
CrossRef - Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos 2004; 32(4): 405–412.
CrossRef - Shen N, Huan Y, Shen ZF. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic beta-cell. Eur J Pharmacol 2012; 694 (1-3): 120- 126.
CrossRef - Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011; 93(7): 1187-1192.
CrossRef - Wang Y, Yi X, Ghanam K, Zhang S, Zhao T, Zhu X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism 2014; 63(9): 1167-1177.
CrossRef - Perez KG, Gonzalez M, Martinez E, Robles J, Espinel M. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2013; 11(5): 366–369.
CrossRef - Hwang K, Ahn J, Kim S, Ha TY. Anti-obesity Effect of Berberine in Mice Fed a High Fat Diet. J Food Sci Nutr 2009; 14: 298-302.
CrossRef - Hua Y, Ehli EA, Kittelsrud J, Ronan P, Munger K, Terry Downey T, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 2012; 19(10): 861– 867.
CrossRef - Wang Y, Zidichouski J. Update on the Benefits and Mechanisms of Action of the Bioactive Vegetal Alkaloid Berberine on Lipid Metabolism and Homeostasis. Cholesterol 2018; 17: 1-18.
CrossRef - Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016; 109: 274- 282.
CrossRef - Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci 2002; 92(12): 2614–2621.
CrossRef - Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol 2002; 91(4): 193-197.
CrossRef - Zhang Y, Cui YL, Gao LN, Jiang HL. Effects of 𝛽-cyclodextrin on the intestinal absorption of berberine hydrochloride, a P-glycoprotein substrate. Int J Biol Macromol 2013; 59(8): 363-371.
CrossRef - Mishra R, Dhole S. Lipid-based floating multiparticulate delivery system for bioavailability enhancement of berberine hydrochloride. J Appl Pharm Sci 2019; 9(11): 36- 47.
CrossRef - Kumar S, Alagawadi KR, Raghavendra M. Effect of Argyreia speciosa root extract on cafeteria diet-induced obesity in rats. Indian J Pharmacol 2011; 43(2):163-167.
CrossRef - Shikova A, Pozharitskayaa O, Makarovaa M, Kovalevaa M, Laaksob I, Damien H, et al. Effect of Bergenia crassifolia L. extracts on weight gain and feeding behavior of rats with high-caloric diet-induced obesity. Phytomedicine 2012; 19(14): 1250–1255.
CrossRef - Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 1969; 22(2): 158- 161.
CrossRef - Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20(4): 470-475.
CrossRef - Jacobs NJ, Vandemark PJ. The purification and properties of the alpha-glycerophosphate-oxidizing enzyme of Streptococcus faecalis 10C1. Arch Biochem Biophys 1960; 88(2): 250-255.
CrossRef - Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res 1970; 11(6): 583-595.
CrossRef - Tietz NW. Clinical Guide to Laboratory Tests. 3rd Edition, W.B. Saunders, Co., Philadelphia. 1995; 22-23.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem 1979; 95(2): 359–364.
CrossRef - Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47(3): 469–474.
CrossRef - Caliborne A. Catalase activity. In: Greenwald RA, editor. Handbook of methods of oxygen radical research. London, UK: CRC Press. 1985; 283–285.
- Sedlak J, Lindsay RH. Estimation of total, protein bound and non protein sulfhydryl group in tissue with Ellman’s reagent. Anal Biochem 1968; 25:192–205.
CrossRef - Zhou JY, Zhou SW, Zhang KB , Tang JL, Guang XL, Ying Y, et al. Chronic Effects of Berberine on Blood, Liver Glucolipid Metabolism and Liver PPARs Expression in Diabetic Hyperlipidemic Rats. Biol Pharm Bull 2008; 31(6): 1169 -1176.
CrossRef - Chen Y, Wang Y, Zhang J, Sun C, Lopez A. Berberine Improves Glucose Homeostasis in Streptozotocin-Induced Diabetic Rats in Association with Multiple Factors of Insulin Resistance. Endocrinology 2011; 51: 1- 8.
CrossRef - Kaur J. A Comprehensive Review on Metabolic Syndrome. Cardiol Res Pract 2014; 18(1): 1-21.
CrossRef - He K, Ye X, Wu H. The safety and anti-hypercholesterolemic effect of coptisine in Syrian golden hamsters. Lipids 2015; 50(2): 185-194.
CrossRef - Kim SH, Shin EJ, Kim ED, Bayaraa T, Frost SC, Hyun CK. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol Pharm Bull 2007; 30(11): 2120 – 2125.
CrossRef - Hasty AH, Gruen ML, Terry ES, Surmi BK, Atkinson RD, Gao L, et al. Effects of vitamin E on oxidative stress and atherosclerosis in an obese hyperlipidemic mouse model. J Nutr Biochem 2007; 18(2):127–133.
CrossRef - Zhou J, Zhou S. Berberine regulates peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in diabetic adipocytes. Eur J Pharmacol 2010; 649(1-3): 390–397.
CrossRef - Zhou JY, Zhou SW. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 2011; 82(2):184–189.
CrossRef - Liu WH, Hei ZQ, Nie H, Tang FT, Huang HQ, Li XJ, et al. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Med J 2008; 121(8):706– 712.
CrossRef - Liu W, Liu P, Tao S, DengY, Li X, Lan T, et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch Biochem Biophys 2008; 475(2): 128–134.
CrossRef








