Department of Pharmacology, Mandya Institute of Medical Sciences, Mandya, India.571401.
Corresponding Author E-mail: drkiranmaiya8@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2173
Abstract
Background: Tuberculosis is one of the major public health concerns in India. Treatment of tuberculosis need multidrug combinations, which is associated with increased incidence of adverse drug reactions (ADRs). Hence there is a need of active monitoring for adverse effects in patients who are on antitubercular treatment (ATT). Objectives: To study the pattern of ADRs caused by antitubercular drugs and to assess causality, severity and predisposing factors. Methodology: A prospective observational study was conducted for 6 months in tertiary care hospital of Mandya. A total of 74 patients of tuberculosis who experienced ADRs were included in the study after obtaining informed consent. Their demographic, treatment and ADR data were collected and analysed. Causality was assessed using WHO scale and Naranjo’s algorithm, whereas severity was assessed by Modified Hartwig and Siegel scale. Results: Among 74 patients, 55(74.32%) were males and 19 (25.67%) were females. A total of 86 ADRs were recorded amongst 74 patients, as 11 patients experienced two ADRs. During intensive and continuation phase of treatment, 65 (87.63%) and 9 (12.16%)patients experienced ADRs respectively. Gastrointestinal side effects and hepatotoxicity were the most frequently observed ADRs with 23 (26.7%) each, followed by pruritus and rashes in 18 (20.93%) patients.63.51% of ADRs had an association with fixed dose combination (FDC) of isoniazid, rifampicin, pyrazinamide and ethambutol. As per WHO scale and Naranjo’s algorithm majority of ADRs were probable with 44 (59.45%) and 58 (78.37%) respectively. Most of the ADRs belonged to mild (67.56%) category as per Modified Hartwig and Siegel scale. Conclusion: ADRs induced by ATT are common. Hence counselling of patients regarding their life style along with early detection and management will minimize the occurrence of ADRs and improvethe adherence to treatment.
Keywords
Antitubercular Drug Therapy; Naranjo’s Algorithm; Pharmacovigilance; Tuberculosis; WHO Causality Scale
Download this article as:| Copy the following to cite this article: Kiran M, Nagabushan H. Adverse Drug Reactions Monitoring in Patients on Antitubercular Treatment in Tertiary Care Hospital, Mandya. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Kiran M, Nagabushan H. Adverse Drug Reactions Monitoring in Patients on Antitubercular Treatment in Tertiary Care Hospital, Mandya. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3x9FOAA |
Introduction
Tuberculosis (TB) is a communicable disease which is one of the major causes of death globally and the leading cause of death from a single infectious agent.1 The World Health Organization (WHO) estimates that up to10 million people continue to fall ill with TB every year.2 Treatment of tuberculosis involves more than one drug which is consumed for a long duration.This can result in development ofADRs. The frequency and expression of ADRs may be influenced by factors like the demographic, genetic, nutritional, and co-morbidities in a population.3 The adverse effects occurring during treatment are classified as major or minor.4 Gastrointestinal side effects in the form of nausea, vomiting and abdominal pain are common especially during early phase ,whilst hepatotoxicity is the most common serious adverse reaction with first line drugs.5 ADRs can lead to interruption in treatment before completion, and can contribute to avoidable morbidity, drug-resistance, treatment failure, reduced quality of life, or death.The overall burden of ADRs directly attributable to anti-TB medicines is poorly quantified. Appropriate measures are required to ensure that harm is reduced and symptoms are relieved. Pharmaco vigilance will thus be an important part of global and national policy for addressing the safety of antitubercular treatment.3 Hence proactive monitoring of patientswith adequate training of healthcare workers will help in early identification, management and prevention of ADRs which helps in better adherence to the treatment.
Aims and Objectives
To study and describe the pattern of adverse drug reactions caused by anti-tubercular treatment.
To assess the causality, severity and predisposing factors for occurrence ofadverse drug reactions.
Materials and Methods
The present study was a prospective, observational study conducted in tertiary care hospital, Mandya during the period between December 2018 and May 2019. The study was started after getting approval from Institutional Ethics Committee (MIMS/IEC/2018/299) and written informed consent was taken from all the participants. Patients with ADRs due to ATT during the study period who qualify the inclusion criteria were considered in the sample. A total of 74 tuberculosis patients with ADRs of either sex, aged above 18 years were included in the study. Patients not willing to give consent to the study were excluded. In case record forms, demographic details and treatment profile of patients were recorded. Patient’s treatment record included disease classification, category of treatment, regimen of drug, treatment outcome and follow-up. Data was collected from the patients admitted in wards due to ADRs and by interviewing the patients when they came for follow-up, regarding any current or past ADRs and also voluntary reporting by the patient.
All the ADRs were evaluated for their causality using Naranjo’s algorithm6 and the WHO Causality scale7. Severity assessment was done using the Modified Hartwig and Siegel scale.8
Statistical Analysis
Data analysis was performed using Microsoft Excel and Statistical Package for Social Sciences (SPSS) version 20. Results were expressed as numbers and percentages. Descriptive statistics were used to analyse data regarding, causality and severity assessment of ADRs.
Results
A total of 74 tuberculosis patients who had experienced ADRs over a period of 6 months were included in the study. All the patients were recruited from tertiary care hospital Mandya, either directly from DOTS centre or from patients who were admitted in hospital wards due to adverse effect from ATT.
A total of 86 ADRs were reported from 74 patients. Amongst which 63 (85.13%) individuals developed only one ADR and 11 (14.86%) developed two ADRs. In this study, 55(74.32%) patients were males and 19 (25.67%) were females. Majority of ADRs belonged to age group 31-40 years (25.67%). Mean age of patients was 44.8 years.Overall mean weight was 46.43 kgs and mean height was 1.63 meters. Mean body mass index of patients (BMI) was 17.37 kg/m2.
Majority of patients were literate and from rural area. When occupation was considered majority were elementary workers (35.13%), followed by agriculturists(32.43%). Most of the patients were smokers (62.16%) and alcoholic (58.1%). (Table 1)
Table 1: Demographic details of study population
|
Parameters |
Number (n=74) |
Percentage (%) |
|
|
Gender |
Male | 55 | 74.32 |
|
Female |
19 |
25.67 |
|
|
Age Distribution |
Children andAdolescents (10-20 years) | 4 | 5.4 |
| Adults (20-60 years) | 58 | 78.37 | |
| Elderly (> 60 years) | 12 | 16.21 | |
|
Education |
Illiterate | 33 | 44.59 |
|
Literate |
41 |
55.4 |
|
|
Locality |
Rural | 55 | 74.3 |
|
Urban |
19 |
25.6 |
|
|
Socioeconomic Status |
Below Poverty Line | 70 | 94.59 |
| Above Poverty Line | 4 | 5.4 | |
|
Food habits |
Non-Vegetarian | 66 | 89.18 |
| Vegetarian | 8 | 10.81 | |
|
Smoking status |
Smoker |
46 |
62.16 |
| Non-Smoker | 28 | 37.83 | |
|
Alcoholism |
Alcoholic | 43 | 58.1 |
| Non-Alcoholic | 31 | 41.8 | |
Diagnosis of Tuberculosis was confirmed microbiologically in 63 (85.13%) patients and clinically in 11 (14.86%) patients. Patients with pulmonary tuberculosis(79.72%) were predominant followed by extrapulmonary tuberculosis(20.27%). When extrapulmonary tuberculosis was considered, out of 15 patients, majority had pleural effusion (8), followed by tubercular meningitis (5) and lymphadenitis (2).
Newly diagnosed cases were 59 (79.22%) patients, recurrent cases were around 11 (14.86%) and 4 (5.4%) were multidrug resistant cases. Most commonly prescribed fixed dose combination (FDC) was 3 FDC. Majority of ADRs occurred during intensive phase of treatment (87.63%) and it was observed that most of the ADRs occurred within first four weeks of treatment(74.32%). (Table 2)
Table 2: Clinical parameters of study population
| Sl No | Parameter | Number
(n=74) |
Percentage
(%) |
|
| 1 | Diagnosis | Microbiological | 63 | 85.13 |
| Clinical | 11 | 14.86 | ||
| 2 | Type | Pulmonary | 59 | 79.72 |
| Extra pulmonary | 15 | 20.27 | ||
|
3 |
Category |
Newly diagnosed cases | 59 |
79.72 |
|
Previously treated cases |
11 |
14.86 |
||
| MDR TB | 4 | 5.4 | ||
| 4 | Phase of treatment | Intensive | 65 | 87.83 |
| Continuation | 9 | 12.16 | ||
|
5 |
No. of FDC |
2 FDC | 17 | 22.97 |
| 3 FDC | 40 | 54.05 | ||
| 4 FDC | 13 | 17.56 | ||
| MDR | 4 | 5.4 | ||
|
6 |
Time of onset of ADR |
1-4 weeks | 55 | 74.32 |
| 5-8 weeks | 5 | 6.75 | ||
| 9-12 weeks | 6 | 8.1 | ||
| > 12 weeks | 8 | 10.8 | ||
Gastrointestinal side effects and hepatotoxicity were the most frequently observed ADRs with 23 (26.7%) each. When System Organ Class was considered majority of ADRs belonged to gastro-intestinal disorders and hepatobiliary disorders contributing 23 (26.74%) each followed by skin and subcutaneous disorders 17 (19.76%), blood and lymphatic system disorders and nervous system disorders contributed 6 (6.97%) each, whereas other system disorders were 11 (12.79%). (Table 3)
Table 3: Types of adverse drug reactions experienced by the patients
| Sl.No | Adverse Drug Reactions | No. of cases
(n=86) |
Percentage
(%) |
|
| 1 | Gastrointestinal system (Epigastric pain, nausea and vomiting) | 23 | 26.7 | |
|
2 |
Hepatobiliary system (Hepatitis and raised enzymes) | 23 | 26.7 | |
| 3 | Dermatological system (Pruritis and rashes) | 18 | 20.9 | |
| 4 | Hematological system (Anemiaand thrombocytopenia) | 6 | 6.97 | |
| 5 | Nervous system (Peripheral neuritis, stroke and dizziness) | 6 | 6.97 | |
| 6 | Ear and Labyrinthine System (Deafness andVestibulotoxicity) | 3 | 3.48 | |
|
7 |
Miscellaneous |
Pedal edema | 2 | 2.32 |
| Flu like syndrome | 2 | 2.32 | ||
| Nephrotoxicity | 1 | 1.16 | ||
| Hypothyroidism | 1 | 1.16 | ||
| Discoloration of tears and saliva | 1 | 1.16 | ||
As treatment of tuberculosis consists of fixed dose combination, 63.51% of ADRs was associated with FDC of isoniazid+ rifampicin+ pyrazinamide + ethambutol (HRZE), followed by isoniazid+rifampicin+pyrazinamide+ ethambutol with tenofovir +lamivudine +efavirenz (HRZE + TLE) with 6 (8.1%) patients. (Table 4)
Table 4: Suspected drugs implicated in Adverse Drug Reactions
| Sl No | Suspected drugs | Number | Percentage (%) |
| 1 | HRZE | 47 | 63.51 |
| 2 | HRZE + TLE | 6 | 8.1 |
| 3 | MDR Regimen | 5 | 6.7 |
| 4 | HRZES | 4 | 5.4 |
| 5 | HRE | 2 | 2.7 |
| 6 | HRZE + ZLE | 1 | 1.3 |
| 7 | HRZE + ZLN | 1 | 1.3 |
H- Isoniazid, R- Rifampicin, Z- Pyrazinamide, E- Ethambutol, S- Streptomycin, T- Tenofovir, L- Lamivudine E- Efavirenz, Z- Zidovudine, N- Nevirapine, MDR regimen- Multidrug resistant regimen
In the study population apart from tuberculosis, patients had various other comorbid conditions. Anaemia was the most common condition contributing to 24 (32.43%) patients followed by HIV infection and diabetes mellitus with 17 (22.97%) patients each.Cotrimoxazole was the most common concomitant medication associated with ADRs (18.91%).
Causality assessment was done using WHO scale and Naranjo’s algorithm. Majority of ADRs were classified as probable with WHO-causality scale and Naranjo’s algorithm contributing to 59.45% and 78.37% respectively. (Figure 1 & 2)
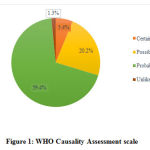 |
Figure 1: WHO Causality Assessment scale |
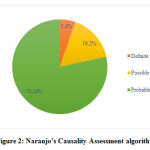 |
Figure 2: Naranjo’s Causality Assessment algorithm |
Modified Hartwig and Siegel Scale was used for assessment of severity of adverse drug reaction. Out of 74 patients, 50 (67.56%) belonged to mild category which required no change in medication followed by 23 (31.08%) patients who belonged to moderate class. Only 1 (1.3%) patient required intensive medical care due to ADR who belonged to severe class. (Table 5)
Table 5: Modified Hartwig and Siegel scale
| Sl No | Severity | Number | Percentage (%) |
| 1 | Mild | 50 | 67.56 |
| 2 | Moderate | 23 | 31.08 |
| 3 | Severe | 1 | 1.3 |
Discussion
The present study was done to find out the pattern of ADRs in patients under DOTS therapy.
A total of 74 patients were included in the study over a period of 6 months. Males constituted most of the population in this study, which was 55 (74.32%) patients when compared to females who were 19 (25.67%) patients.These findings were similar to a study by Sinha K et al which also showed majority patients were males (76.47%).9
Majority of ADRs occurred in age group 31-40 years (25.67%) which was comparable to study by Edoh and Adjei who also found higher incidence of ADRs (29.7%) in same age group.10
In our study,non-vegetarians (89.18%)encountered higher incidence of ADRs than vegetarians (10.81%). Similar results were obtained by Nemagouda S where non vegetarians outnumbered vegetarians.11
It was observed that newly diagnosed (Category 1) cases had higher incidence of ADRs with 79.22% when compared to other regimens. A study by Sinha Ket al also showed that majority of cases belonged to Category 1 (64.71%).9
Occurrence of more than one ADR was observed in this study. But majority i.e. 85.1% patients experienced only one ADR while 14.86% experienced two ADRs. This was similar to a study by VenkateswaruluK et al, with 76.19% patients with only one ADR.12
Most common ADRs were from gastrointestinal (26.7%) and hepatobiliary (26.7%) system. ADRs under gastrointestinal system included nausea, epigastric pain and vomiting. A study by Dalal NP et al 13 and Sinha K et al 9also observed that majority of ADRs belonged to gastrointestinal system with 12.67% and 53.52% respectively. This increased incidence of ADRs may be due to the association of all the first line ATT drugs with gastrointestinal intolerance.
In our study, ADRs from hepatobiliary system constituted 26.7%, equivalent to gastrointestinal system. Hepatitis accounted for majority of reaction followed by raised serum transaminases.FaraziA et al also showed maximum incidence (35.7%) of ADRs related to hepatobiliary system.14Hepatotoxicity can occur with Isoniazid, Rifampicin and Pyrazinamide. Deranged liver functions did not progress to fulminant liver failure in any of the cases, unlike observation by Anand AC et al with 10% of cases progressing to acute liver failure.15This can be due to shorter study duration and lost to follow up in our study.
Second most common ADRs were from dermatological system which accounted for 20.93%. Pruritus, rashes, mucosal lesions and hair fall were the commonly observed reactions. Ramnath et al observed majority of ADRs from dermatological system (27.34%), unlike our observation.16 In this study, 2 female patients experienced hair fall. A study conducted by Garg et al also observed diffuse hair loss which was attributed to Isoniazid.17In patients receiving ATT, Isoniazid was thelikely cause for alopecia.18
ADRs from hematological system (anemia and thrombocytopenia) were 6.97%. In a study by Sadiq S et al ADRs from hematological system were least common with 2.7% of all cases.19Amongst the first line antitubercular drugs, Rifampicin is most commonly associated with thrombocytopenia.This is probably attributed to immunological basis and common with intermittent regimen.20Anemia due to first line antitubercular drugs is common with Isoniazid and Rifampicin. Isoniazid produces anemia in individuals with pyridoxine deficiency, which can be corrected with high doses of pyridoxine. Rifampicin also produces anemia by immunologically mediated hemolysis.18,21
In our study, pedal edema was seen in 2.32% patients which was similar to Chhetri AK et alwith 1.03% patients.22 In a signal detection study as a part of pharmacovigilance in Morocco it was observed that edema of lower limbs during ATT is a potential new signal.23 Occurrence of pedal edema can also be due to concomitant cardiac illness, renal diseases or hypoproteinaemia. This requires more investigations to establish cause and effect relationship with antitubercular drugs.
In our study, vertigo as an ADR accounted to 2.32% of cases which was similar to the incidence rate of 2.7% in a study by Sadiq S et al.19In a study by Qayyum et al vertigo was observed in 31.7% of cases.24 This difference in incidence of vertigo can be corelated with withdrawal of Streptomycin from the category II regimen.
Most common FDC associated with occurrence of ADRs was attributed to HRZE accounting to 63.51% which was similar to the results obtained by Marra F et al25 and Anusha N et al26
Most common FDC associated with occurrence of ADRs was attributed to HRZE accounting to 63.51% which was similar to the results obtained by Marra F et al25 and Anusha N et al26
The majority of the ADRs reported in this study were categorized as ‘probable’ as per Naranjo algorithm and WHO causality scale with 78.37% and 59.45% respectively. Gholami K et al and Reena V et al also observed that most of the ADRs were classified as ‘probable’ with 48.2% and 58.2% respectively.28,29ADRs classified as ‘definite’ constituted only 5.4% which can be explained as placebo effect was not studied and laboratory investigations were not done to determine the concentration of drug in body fluids.
There was disagreement in causality assessment between two scales with respect to “probable” and “possible” criteria. A study by Behlekar MN et al comparing the two-causality assessment showed that a poor agreement between the two scales.30 This can occur due to differences in dechallenge pattern, timing of event and alternative etiological factors.31
Naranjo’s algorithm is simple, of high clarity and brief, in addition to less inter-rater disagreementwhen compared to the other scales. But validity of this scale is not consistent with pediatric population. Even though WHO-causality scale is convenient to use, it is non-probabilistic and generates unpredictability during evaluation. But both the methods are valuable in assessment of ADRs and to understand its scientific basis.6,32
ADRs can result in discontinuation of drug or hospitalization or sometimes even death. To assess the severity of occurred reaction Modified Hartwig and Siegel scale was used. Majority of ADRs were mild (67.56%) and only one patient required critical care. These findings were similar to Maqusood M et al that majority of ADRs were categorized as mild (75.94%).33
The limitations of this study were, baseline biochemical andhematological parameters were not available to attribute whether ATT was the cause. Causality assessment which was claimed as certain or definite was based on the reintroduction of treatment and not on rechallenge and dechallenge test, as it could not be performed due to ethical issues.Also, the main flaw in the algorithm-based causality assessment is its dependability on “yes/no” response, which can be influenced by observer bias.
Conclusion
Gastrointestinal side effects and hepatotoxicity were the most frequently observed ADRs, followed by pruritus and rashes. As per WHO-causality scale and Naranjo’s causality algorithm majority of ADRs were probable. Most of the ADRs belonged to mild category according to the Modified Hartwig and Siegel scale for severity assessment.
ADRs induced by ATT are common, which can result in discontinuation of treatment and development of resistant bacilli. Hence counselling of patients regarding their life style with early detection and management will minimize the occurrence of ADRs and improve the adherence to treatment.
Acknowledgment
Authors would like to thank DOTS Centre and Department of Chest Medicine, MIMS, Mandya.
Conflicts of Interest
None declared
Funding source
There was no financial support from any source for the study.
References
- Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1(Accessed on 2020 Feb 12)
- Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1(Accessed on 2020 Apr 17)
- World Health Organisation. Pharmacovigilance in Tuberculosis care.2013.Availablefrom https://www.who.int/medicines/publications/PharmacoTB web_v3.pdf (Accessed on 2020 Apr 17)
- Central tuberculosis Division. Guidelines – technical and operational guidelines for TB control in India. 2016. Available on https://tbcindia.gov.in/showfile.php?lid=3220(Accessed on 2020 Feb 12)
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis.2016;63(7):147-195.
CrossRef - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin PharmacolTher. 1981;30:239–245.
CrossRef - The use of the WHO-UMC system for standardized case causality assessment. World Health Organization (WHO) — Uppsala Monitoring Centre. Available from: http://www.who-umc.org/Graphics/24734.pdf. (Accessed on 2020 Apr 13)
- Hartwig S, SiegelJ, Schneider P. Preventability and Severity Assessment in Reporting Adverse Drug Reactions. Am JHosp Pharm.1992;49:2229-2232.
CrossRef - Sinha K, Marak IR, Singh W A. Adverse drug reactions in tuberculosis patients due to directly observed treatment strategy therapy: Experience at an outpatient clinic of a teaching hospital in the city of Imphal, Manipur, India. J Assoc Chest Physicians. 2013;1:50-53.
CrossRef - Edoh D, Adjei R. Rapid assessment of a National Tuberculosis (TB) Control Programme in Eastern Ghana. Afr J Health Sci. 2002;9:59‑64.
- Nemagouda S.The antitubercular drug induced adverse effects in registered cases under RNTCP-dots, programme in Bijapur. J Evol Med Dent Sc.2014;3(19):5255-5262.
CrossRef - Venkateswarlu K,Tiwari K, Mamatha E, Sai Vivek, Shravan P. Study of adverse drug reactions in tuberculosis patients. J Pharm Res. 2017;6(2):61-65.
- Dalal NP, Karandikar YS, Pandit VA. Safety evaluation of directly observed treatment short course (DOTS) regimen in a tertiary care hospital Pune. Int J Basic Clin Pharmacol. 2014;3:369-376.
CrossRef - Farazi A,Sofian M, Jabbariasl M, Keshavarz S. Adverse Reactions to Antituberculosis Drugs in Iranian Tuberculosis Patients. Tuberc Res Treat.2014;2014:412893.
CrossRef - Anand AC, Seth AK, Paul M, Puri P. Risk Factors of Hepatotoxicity During Anti-tuberculosis Treatment. Med J Armed Forces India. 2006;62(1):45–49.
CrossRef - Ramanath KV, Ramesh S. A Study on Assessment of Adverse Drug Reaction in Tuberculosis Patients. Am J Pharm Tech Res. 2012;2(2):14–18.
- Garg T, Yadav P, Agarwal S, Mendiratta V. Drug-induced diffuse hair loss in females: An observational study. Astrocyte. 2014;1:80-83.
CrossRef - Alison B. Martindale: The complete drug reference. 38th London: Pharmaceutical Press;2014.311-314.
CrossRef - Sadiq S,Khajuria V, Vishal R, Annil M, Singh J. Adverse Drug Reaction Profile in Patients on Anti-tubercular Treatment Alone and in Combination with Highly Active Antiretroviral Therapy. J Clin Diagn Res. 2015; 9(10):01–04.
CrossRef - Aronson JK. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. 15th United Kingdom: Elsevier Science;2006.3040-3047.
- Laurence LB, Randa HL, Bjorn CK. Goodman and Gilman’s: The pharmacological basis of therapeutics. 13th New York: Tata McGraw Hill; 2018.1072-1074.
- Chhetri AK, Saha A, Verma SC, Palaian S, Mishra P, Shankar PR. Study of adverse drug reactions caused by first line anti-tubercular drugs used in directly observed treatment, short course (DOTS) therapy in Western Nepal,Pokhara. J Pak Med Assoc.2008;58(10):531-536.
- Tanani SD, Serragui S, Cherrah Y, Ait Moussa L, El Bouazzi O, SoulaymaniBencheikh R.Signal Management of Disproportionate Reporting in Moroccan Pharmacovigilance: The Lower Limb Edema Induced by Anti-Tuberculosis Drugs. J Pharmacovigilance. 2015;3:161.
CrossRef - Qayyum S, Ahmed I, Baig S, Rizvi N. Adverse events in the treatment of multi-drug resistant tuberculosis. Eur Respiratory Soc. 2011;38(55):4402.
CrossRef - Marra F, Marra CA,Bruchet N,Richardson K,Moadebi S,Elwood RKet al. Adverse drug reactions associated with first-line anti-tuberculosis drug regimens. Int J Tuberc Lung Dis. 2007;11(8):868–875.
CrossRef - Anusha N, Isabella T, Anil J P. Adverse drug reactions monitoring among TB patients on anti-tubercular drugs under RNTCP in Pondicherry. Int J Adv Res. 2015;2(12):165-173.
CrossRef - Lee SW,Kang YA,Yoon YS, Um SW, Lee SM, Yoo CG, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1032
CrossRef - Gholami K, Kamali E, Hajiabdolbagh Mi, Shalviri G. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Prac. 2006;4(3):134-138.
- Verma R, Gr M, Shrivastava AK, Pathak P. Adverse drug reactions associated with first line anti tubercular drugs in a tertiary care hospital of Central India: A study of clinical presentations, causality and severity. Asian J Pharm Clin Res. 2014;7(5):140-143.
- Belhekar MN, Taur SR, Munshi RP. A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014;46(1):117-120.
CrossRef - Pere JC, Begaud B, Haramburu F, Albin H. Computerized comparison of six adverse drug reaction assessment procedures. Clin PharmacolTher .1986;40(4):451-461.
CrossRef - Kahn LM, Al-Harthi SE, Osman AM, Sattar MA, Ali AS. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2016;24:485-492.
CrossRef - Maqusood M, Khan F A, Swaroop A. A Study on Incidence of Adverse Drug Reaction of AntiTubercular Drugs in New Cases of Pulmonary Tuberculosis in a Tertiary Care Teaching Hospital. Int J Med Res Prof. 2016;2(3):53-56.
CrossRef









