Arun kumar R1* , Maignana Kumar R1
, Maignana Kumar R1 , Duraivel M1
, Duraivel M1 , Ahamed Basha A2
, Ahamed Basha A2 , Amalan Stanley V3
, Amalan Stanley V3 and Ruckmani A1
and Ruckmani A1
1Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
2Department of Physiology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
3Scientific and Academic advisor, International Institute of Biotechnology and Toxicology, Padappai, Kanchipuram-601301, Tamil Nadu, India.
Corresponding Author E-Mail: drarunvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2182
Abstract
The objective was to evaluate the efficacy of ViproTM, a polyherbal formulation in the management of uncomplicated respiratory infection towards reducing the severity of clinical symptoms, reducing the worsening of clinical symptoms&complications and to determine its safety by detectingadverse events. The study was started after obtaining approval from Institutional Human Ethics Committee. It was a prospective, randomized controlled study done in 60 patients. Patients having uncomplicated respiratory infections, at least for less than 5 days, with clinical symptoms and signs of fever, myalgia, rhinitis, sore throat, throat pain, cough, expectoration and head ache were enrolled and randomly allocated to one of the three groups, A, B and C. Group A received standard treatment, group B ViproTM and group C ViproTM along with the standard treatment. All the patients were followed up for a period of 7 days from starting treatment. Telephonic follow up was done daily for 7 days and physical follow up on day 0, day 4 and day 7. During physical follow up, vitals & body temperature were recorded, general and systemic examination done, adverse events were noted and improvement in constitutional symptoms was assessed. Complete blood count (CBC) and nasal / throat swab for culture were done at the baseline and at the end of study. Vipro TM has demonstrated efficacy in alleviating the clinical symptoms similar to standard treatment. With regard to safety, ViproTM is associated with a few adverse events and all of them are minor in nature and subsided within 24 hours.
Keywords
Poly Herbal Formulation; Respiratory Infection; Siddha; ViproTM
Download this article as:| Copy the following to cite this article: Kumar R. A, Kumar R. M, Duraivel M, Basha A. A, Stanley V. A, Ruckmani A. A Single Centre, Prospective, Randomized, Open Labelled Clinical Study to Evaluate the Effectiveness of Siddha Poly Herbal Formulation, Viprotm, Towards the Management of Uncomplicated Respiratory Infection. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Kumar R. A, Kumar R. M, Duraivel M, Basha A. A, Stanley V. A, Ruckmani A. A Single Centre, Prospective, Randomized, Open Labelled Clinical Study to Evaluate the Effectiveness of Siddha Poly Herbal Formulation, Viprotm, Towards the Management of Uncomplicated Respiratory Infection. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3u4hhKk |
Introduction
With the advent of worldwide SARS-CoV-2 pandemic and the desperation of humankind, to find a cure or a relief from the COVID-19 morbidity and to avoid mortality, especially the pharmaceutical and healthcare centers globally have been venturing various treatment options such as vaccines, drug combinations, and then alternative medicines such as AYUSH products in India.
With that purpose, ‘ViproTM’ has been developed and formulated based on the traditional system of medicine, the Siddha herbal alternatives. The product contains ten plant products as crude extracts based on the Materia medica of the Siddha literature and it is aimed to alleviate the respiratory implications of the patients, supposedly a COVID-19 infected or asymptomatic to it or any other common respiratory infections, and also as a prophylactic approach for the people who might get related respiratory infections.
The traditional medicinal ingredients of the product processed with essential oil from Cocus nucifera L, as base have been scientifically proven to ward off respiratory infectious diseases[1]. It includes,Ocimum basilicum L, Curcuma longa L, Citrus lemon L, Allium sativum L, Plectranthus amboinicus Lour, Momordica charantia L, Cinnamomum verum J.Presl, Zingiber officinale Rosc and Piper nigrum L. The crude extracts of these plant parts have been proven to have anti-bacterial, anti-viral and immunomodulatory as well as anti-inflammatory properties.
| No. | Botanical name | Common name | Part / % | Action |
| 1 | Ocimum basilicum L | Tulsi | Leaf, 15 | Antimicrobial in nature and helps to maintain throat health[2] |
| 2 | Curcuma longa L | Turmeric | Tuber, 5 | Rich in anti-oxidant, antimicrobial and helps to fight viral load[3] |
| 3 | Citrus lemon L | Lemon | Fruit, 10 | Rich in vitamin C and helps to fight cough, flu and cold[4] |
| 4 | Cocus nucifera L | Coconut | Base oil | Rich in antioxidant[5]
|
| 5 | Allium sativum L | Garlic | Seed, 10 | Antioxidant and helps to builds immunity[6] |
| 6 | Plectranthus amboinicus Lour | Citrus | Fruit, 10 | Expectorant , helps to recover from cold and cough[7] |
| 7 | Momordica charantia L | Bitter melon | Pod, 30 | Helps build immunity[8] |
| 8 | Piper nigrum L | Pepper | Seed, 5 | Helps build immunity against infections[9] |
| 9 | Cinnamomum verum J.Presl. | Cinnamon | Bark, 5 | Rich in antioxidant and antipyretic[10] |
| 10 | Zingiber officinale Rosc. | Ginger | Tuber, 10 | Antimicrobial properties and helps in build immunity[11] |
A preclinical acute oral toxicity study in Wistar rats, Rattus norvegicus, was conducted as per the OECD guideline 423with ViproTMand it was observed that the formulation was safe in animals upto the dose of 2000mg/kg as a limit dose with a cut off value of 5000 mg/kg body weight. Therefore, it is classified as Category 5 as per the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). When it was converted to human equivalent dose by applying rodent to human conversion factor, 322.5 mg/kg body weight, which provided wide safety index for the clinical trial dose.Further the poly herbal product was formulated at VIMAC, a World Health Organization (WHO)-certified GMP facility, Chennai and the product had the Batch No.001/2020; manufactured in April 2020. This study was done to evaluate the effectiveness of ViproTM in uncomplicated respiratory infection.
Objectives
To determine the efficacy of the poly herbal formulation ‘ViproTM’ towards
Reducing the severity of clinical symptoms such as fever, myalgia, rhinitis, sore throat, throat pain, cough and head ache
Reducing the worsening of clinical symptoms
Reducing the complications
To determine the safety by way of detection and reporting of adverse events
Materials and Methods
The study was initiated after obtaining approval from the Institutional Human Ethics Committee and after registering in Clinical Trials Registry- India (CTRI) on 02/06/2020 (Registration number- CTRI/2020/06/025559).It was a prospective, randomized, open labeled clinical study to evaluate the effectiveness of ViproTM in the management of uncomplicated respiratory infection including common cold with / without fever, flu like respiratory infection and bacterial respiratory infection.The patients, investigators and statisticians were not blinded. Simple random sampling was applied to all eligible subjects followed by random assignment of numbers between treated and control in each arm to ensure equal distribution of subjects.
The study was conducted in a tertiary care hospital in South India. 60 patients who fulfilled the selection criteria were enrolled in the study, after obtaining informed consent and they were grouped as per the treatment protocol defined into three as follows.
Group 1: Standard treatment (symptomatic management with antipyretics and / or antihistamines and / or nasal decongestants and / or Levofloxacin and / or cough syrups for duration of 3 to 5 days as decided by the treating physician)
Group 2: Liquid, oral, poly herbal formulation ‘ViproTM’, one teaspoon (20 drops) of formulation, mixed in a glass of water, mixed thoroughly and swallowed after food for 7 days, for four times a day, morning, afternoon, evening and night.
Group 3: “Standard Treatment + ViproTM” combination was advised as in group 1 and 2
Selection criteria
Inclusion Criteria
Age >18 years <60 years, both male and female;
Having uncomplicated respiratory infections, at least for less than 5 days, diagnosed with clinical symptoms and signs of fever, myalgia, rhinitis, sore throat, throat pain, cough, expectoration and head ache
Consenting to participate in the study and sign the informed consent
Exclusion criteria
Patients with uncontrolled co-morbid conditions like Diabetes mellitus, Hypertension and Bronchial asthma
Patients with Lower respiratory tract infections, Chronic Bronchitis, Chronic obstructive airway problems
Patients with significant Cardiovascular, Neurological, Psychiatric, Gastro intestinal and other system infections or disorders and malignancies
Hypersensitivity to the herbal ingredients in the formulations and other medications prescribed in the study
Pregnant and feeding mothers
When the patients were interviewed for selection, all those patients who came under the testing criteria for COVID-19 as per ICMR guidelines were tested for COVID-19 and if they were found to be positive, they were excluded from participating in the study.
Study assessments
Baseline assessment on day 0:
Demographic profile – age, gender, height, weight and BMI were recorded
Blood pressure, Pulse, SpO2, Respiratory rate, Body temperature and Constitutional symptoms
General examination, systemic clinical examination
Complete blood count (with 5 ml of blood drawn) and Throat / Nasal swab for bacterial culture and sensitivity
After the baseline assessment, the subjects were randomly provided with any one of the treatments as detailed above for groups 1, 2 and 3.
Follow up assessment (days 1 to 7):
Telephonic follow up (days 1 to 7) – Reminder for medication intake (daily once), adverse events (daily once), clinical symptoms for improvement / worsening (daily once), body temperature and constitutional symptoms (daily once)
Physical follow up (On days 4 and 7) – Checking medication compliance, blood pressure, pulse, SpO2, respiratory rate, body temperature, constitutional symptoms, adverse events, general and systemic examination, clinical signs and symptoms
During follow up – Complete blood count (with 5 ml of blood drawn) and Throat / Nasal swab for culture and sensitivity on day 7.
If the participants in group 2 did not show improvement in 48 hours, they were to be provided standard treatment from day 3. If the subjects were found to deteriorate with the treatments administered, they were to be withdrawn from the study and suitable alternate treatment was to be provided.
The subjects who experienced AE (Adverse Event) / SAE (Serious Adverse Event), were to receive treatment at free of cost till the event subsided.
Composite measure of Constitutional symptoms – The seven constitutional symptoms (fever, myalgia, rhinitis, sore throat, throat pain, cough and head ache) were given a score of each 1 in case of having these symptoms and each 0 in case of not having these symptoms. The scores of every subject was added at baseline, day 4 and day 7 to get the composite measure of constitutional symptoms. The reduction in the score from baseline to day 4 and 7 was considered for primary outcome.
Statistical Analysis
The analysis was carried out in ITT (Intention to treat) population and the interpretations and conclusions were made using ITT population.
Among the demographic parameters, gender was analysed using Chi square test and the remaining parameters were analysed using one-way ANOVA. The vital parameters were analysed using repeated measures ANOVA within groups and one-way ANOVA between groups.
The blood parameters were analysed using paired t test within groups and one-way ANOVA between groups. The composite measure of constitutional symptoms was analysed using Friedman test within groups and Kruskal Wallis test between groups. The proportion of patients with adverse events was analysed using Chi square test between groups.
Results
Out of 60 subjects who were randomized to one of the three treatment groups, 58 completed the study and there were 2 drop outs due to ‘lost from follow up’. The participant flow diagram has been depicted according to the CONSORT (Consolidated Standards for Reporting Trials) guidelines in figure 1.
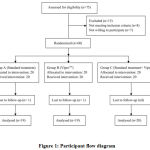 |
Figure 1: Participant flow diagram. |
Among the subjects enrolled, 36 were males and 24 females. None of the subjects had significant co-morbid conditions, though one subject had hypertension in group 3 and one subject diabetes mellitus in group 2, their clinical condition was stable and were included in the study.
The subjects were followed up effectively by telephonic communication and the medication reminders were precisely given and hence the drug compliance was more than 90% in all the subjects across three groups. All the 58 subjects qualified for analysis of data as ITT population (group 1- 19, group 2- 19 and group 3- 20 subjects).
Demographic profile
There was no statistically significant difference among the three groups for age, height, weight and BMI. The proportion of males was higher in group 1 and 2 compared to group 3 (p – 0.03, Chi square test), but it does not have any clinical significance.
Vital parameters
Blood pressure, Pulse, SpO2 and Respiratory rate were within the acceptable limits during baseline, on day 4 and day 7 and there was no clinically significant differences or variations observed, though few parameters showed statistical significance during analysis.
Body Temperature
The body temperature at baseline and on day 4 and 7 during follow up was analysed in terms of absolute body temperature and the proportion of patients who were febrile.
The mean body temperature was 99.4° F at baseline, 97.2° F on day 4 and 96.2° F on day 7 in group 1. It was 96.9° F at baseline, 96.8° F on day 4 and 96.3° F on day 7 in group 2 and 97.7° F at baseline, 96.7° F on day 4 and 96.1° F on day 7 in group 3.
The reduction in body temperature was observed in all groups, from baseline to day 4 and day 7. The reduction was significant in group 1 and 3 (repeated measures ANOVA, p value < 0.05) whereas in group 2, the reduction was not statistically significant (repeated measures ANOVA, p >0.05), though the body temperature remained normal. Comparative analysis of the body temperature among three groups showed that the reduction is highly significant in group 1 (One-way ANOVA, p <0.05).
The proportion of patients who were febrile at various time points showed that the number of patients who were febrile at the time of enrollment was 15 in group 1 (78.94%), 1 in group 2 (5.26%) and 7 in group 3 (35%). All the patients became afebrile on day 4 and remained afebrile on day 7. The proportion of patients who had fever during baseline was significantly high in group 1 compared group 2 and 3 (p – 0.00001, Chi square test).
Constitutional symptoms
These include myalgia, rhinitis, sore throat, throat pain, cough and head ache other than fever. These symptoms gradually improved in all groups and on day 7, 50 subjects had none of these symptoms. In group 1, one subject had cough on day 7. Five subjects in group 2 had constitutional symptoms such as head ache, sore throat and throat pain. Two subjects had symptoms like head ache and cough in group 3. Nevertheless, these symptoms improved from baseline and appropriate medical advice was given on day 7 to all these subjects. None of the subjects in this study exhibited worsening of symptoms or had any complications. The detailed data of symptomatic improvement in terms of proportion of subjects having constitutional symptoms is available in table 2.
Composite measure of constitutional symptoms
The mean composite score for constitutional symptoms is 4.21, 4.00 and 4.00 in group 1, 2 and 3 respectively. It was reduced to 0.53, 1.26 and 0.55 in the respective groups 1, 2 and 3 on day 4 and further reduced to 0.05, 0.26 and 0.10 on day 7. The reduction in the composite score was statistically significant in all the groups (Friedman test, p<0.00001). Between group analysis showed that the reduction is highly significant in group 1 compared to group 2 and 3 (Kruskal Wallis, p<0.00001).
The analysis of composite measure of constitutional symptoms indicated that all the three treatments were effective and standard treatment was superior to other two treatments.
Complete blood count (CBC)
The analysis of CBC parameters did not show clinically significant changes in various parameters, though few statistically significant changes in WBC, platelets and Eosinophils were observed. There was a significant increase in WBC count in group 1 on day 7 compared to baseline (paired t test, p < 0.05). Platelet count increased in group 3 significantly (paired t test, p < 0.05) and this was significant compared to other two groups also (one-way ANOVA, p <0.05). There was an increase in Eosinophil count in group 2 (paired t test, P<0.05).
Throat swab – Bacterial culture
Overall, 15 subjects had positive culture for pathological organisms during baseline. The organisms included Streptococcus pyogenes (4 subjects), Pseudomonas aeruginosa (1 subject), Klebsiella (7 subjects), Acinetobacter (1 subject), Citrobacterkoseri (1 subject) and Staphylococcus aureus (1 subject).
6 subjects (31.58%) had pathological organisms in group 1 and the same was 6 (31.58%) in group 2 and 3 (15%) in group 3. Upon repeating the culture on day 7, it was nil (0%) in group 1, 4 (21.05%) in group 2 and 3 (15%) in group 3.
Out of 6 subjects who had positive throat swab during baseline in group 2, 4 subjects returned to normal flora on day 7. The other two subjects remained culture positive, with one subject having the organism Klebsiella and the other subject having Acinetobacter at baseline and got into Streptococcus pyogenes on day 7. Two subjects, in group 2, who were culture negative during baseline became culture positive with Staphylococcus aureus and Pseudomonas aeruginosa.
Three subjects who had culture positive during baseline returned to normal flora on day 7, in group 3. Three subjects who were negative during baseline became culture positive with one subject each having Klebsiella, Staphylococcus aureus and Acinetobacter on day 7.
Adverse events
Six subjects (10.34%) had adverse events. Three subjects had nausea, two subjects vomiting and one subject loose stools. These events were managed symptomatically and they all became normal in 24 hours. The study treatments were not interrupted due to adverse events. Among these AEs, one AE occurred in group 1 (5.26%) and group 2 (5.26%) each and 4 events in group 3 (20%). There were no serious adverse events.
Chi square analysis of proportion of patients having AEs indicated that there was no statistically significant difference among three groups (p-0.21)
Palatable nature of ViproTM
8 (42.10%) patients in group 2 and 7 (35%) patients in group 3 expressed that ViproTM liquid formulation was bitter in taste even after diluting it in drinking water and it was difficult to consume it. This could be one of the reasons for less compliance observed in 6 patients in group 2 and 4 patients in group 3.
The summary of group wise demographic profile and baseline & follow up clinical data on day 4 and 7 including Blood pressure, Pulse, SpO2, respiratory rate, body temperature and constitutional symptoms are tabulated in table 1 and table 2 respectively.
Table 1: Demographic data
| Groups | Group 1 [Mean (SD)] | Group 2 [Mean (SD)] | Group 3 [Mean (SD)] | P-value (Inter group) | |
| Age (years) | 34.74 (10.82) | 40.05 (14.49) | 38.1 (13.31) | 0.44 | |
| Gender | Males | 16 (80%) | 12 (60%) | 8 (40%) | 0.03* |
| Females | 4 (20%) | 8 (40%) | 12 (60%) | ||
| Height (meters) | 1.61 (0.06) | 1.60 (0.12) | 1.57 (0.07) | 0.43 | |
| Weight (kg) | 64.77(12.85) | 63.97 (12.42) | 63.02 (11.88) | 0.09 | |
| BMI (kg/m2) | 25.14 (4.32) | 25.28 (5.30) | 25.58 (4.87) | 0.95 | |
 |
Table 2: Vital parameters and Constitutional symptoms |
The summary data of Blood parameters and bacterial culture of throat swab across groups in baseline and on day 7 is tabulated in Table 3.
 |
Table 3: Blood parameters and throat swab culture. |
Discussion
This study which evaluated the effectiveness of ViproTM in uncomplicated respiratory infection showed that ViproTM had similar efficacy in reducing the constitutional symptoms as that of standard treatment. With regard to body temperature, patients in all the three groups became afebrile on day 4 and remained afebrile on day 7. The proportion of patients with fever during baseline was significantly high in group 1 compared to group 2 and 3. Hence the statistical comparison of groups for reduction in the proportion of patients who are febrile will not be appropriate for assessment of efficacy. Nevertheless, it is pertinent to note that all the patients were afebrile on day 4 and day 7. The mean body temperature is in febrile range only in group 1 and in other two groups it is in normal range during baseline. Hence the reduction in body temperature and its clinical significance in assessing the efficacy of interventions may not be appropriate.
One subject in group 1, five subjects in group 2 and two subjects in group 3 had constitutional symptoms at the end of treatment but these symptoms improved from baseline and they did not show any worsening of symptoms or any complications.
The composite score of constitutional symptoms was used to assess the efficacy of interventions. It was noted that all the three treatments were effective and standard treatment was found to be superior over the other two treatments. The addition of ViproTM to the standard treatment resulted in more reduction of composite score compared to ViproTM alone. The reduction observed in the combination treatment was numerically close to the standard treatment. But the advantage of adding ViproTM to the standard treatment in routine clinical care for any additional benefit has to be explored further.
Complete blood count assessments did not show any clinically significant differences in all the three groups.
Throat swab bacterial culture showed that 4 subjects in group 2 and 3 subjects in group 3 had positive culture at the end of treatment. However, all these subjects who remained culture positive at the end of the study (Day 7) were not having any significant constitutional and clinical symptoms and they were managed according to the clinical judgement by the treating physician.
With regard to the safety, one subject in group 2 experienced mild gastrointestinal related adverse event and he became normal in next 24 hours. The treatment was not interrupted due to the adverse event and ViproTM was found to be safe in all the subjects.
6 patients in group 2 and 4 patients in group 3 had less compliance with ViproTM due to the bitter nature of the formulation.
Conclusion
This study evaluated the efficacy and safety the Siddha polyherbal formulation, ViproTM in 58 patients of uncomplicated upper respiratory infection. ViproTM has demonstrated efficacy in alleviating the clinical symptoms similar to standard treatment. With regard to safety, ViproTM is associated with a few adverse events and all of them are minor in nature and subsided within 24 hours.
Acknowledment
The authors thankfully acknowledge the clinical research associates Ms. Jenisha Mary, Ms. Mirra and Mr. Ajith for their valuable help in patient recruitment and data collection for this study.The authors are grateful to Chettinad Hospital and Research Institute (CHRI), Chettinad Academy of Research and Education (CARE) for supporting the study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
This study was externally funded by The Chairman, Buy Happy Marketing LLP, Chennai 600058 and monitored by QScience Therapeutics, Chennai.
References
- Effiong EB, Gwana DM, Okaro C. Antimicrobial Susceptibility Pattern of Coconut Oil Extract on Selected Bacterial and Fungi. Inter Ped Dent Open Acc J. 2018;1(3).
CrossRef - Eftekhar, N., Moghimi, A., Roshan, N.M., Saadat, S. and Boskabady, M.H., 2019. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimumbasilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complementary and Alternative Medicine, 19(1), p.349.
CrossRef - Cikrikci S, Mozioglu E, Yilmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Records of Natural Products. 2008;2(1):19.
- Klimek-Szczykutowicz M, Szopa A, Ekiert H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants. 2020 Jan;9(1):119.
CrossRef - Singla RK, Jaiswal N, Bhat VG, Jagani H. Antioxidant and antimicrobial activities of Cocos nucifera Linn.(Arecaceae) endocarp extracts. Indo Global J Pharm Sci. 2011;1(4):354-61.
- Londhe VP. Role of garlic (Allium sativum) in various diseases: An overview. angiogenesis. 2011;12:13.
- Arumugam G, Swamy MK, Sinniah UR. Plectranthusamboinicus (Lour.) Spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules. 2016 Apr;21(4):369.
CrossRef - Anilakumar KR, Kumar GP, Ilaiyaraja N. Nutritional, pharmacological and medicinal properties of Momordicacharantia. International Journal of Nutrition and Food Sciences. 2015 Jan 1;4(1):75-83.
CrossRef - Damanhouri ZA, Ahmad A. A review on therapeutic potential of Piper nigrum L. Black Pepper): The King of Spices. Med. Aromat. Plants. 2014;3:161.
CrossRef - Mazimba O, Wale K, Kwape TE, Mihigo SO, Kokengo BM. Cinnamomumverum: Ethylacetate and methanol extracts antioxidant and antimicrobial activity. J Med Plants Studies. 2015;3(3):28-32.
- Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. American journal of respiratory cell and molecular biology. 2013 Feb;48(2):157-63.
CrossRef








