Manuscript accepted on :30-Jun-2021
Published online on: 06-07-2021
Plagiarism Check: Yes
Reviewed by: Sharad Kamble
Second Review by: Shaimaa Mutlak
Final Approval by: Dr. Ian James Martin
Prince Ekisha Gideon , Alphienes Stanley Xavier*
, Alphienes Stanley Xavier* , Punnagai Kumaravelu
, Punnagai Kumaravelu and Darling Chellathai David
and Darling Chellathai David
Department of Pharmacology, Sri Ramachandra Medical College and Research, Institute, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, Tamil Nadu, India.
Corresponding Author E-mail : alphclinpharm@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2211
Abstract
Topical preparations of allylamines and imidazoles are the commonly used medications for the treatment of tinea corporis, having extended efficacy and good safety profile. In our study, we have compared topical terbinafine, an allylamine and topical miconazole, which is an imidazole to assess their efficacy and safety in tinea corporis infection. Hundred patients with tinea corporis infection were equally randomized to receive either terbinafine (1%) or miconazole (2%) for 4 weeks. The reduction in clinical symptoms (pruritus, erythema, vesicle, and desquamation), attaining mycological cure, reduction in composite score, as well as physician global assessment were evaluated from baseline to the end of intervention, and at follow up visit, and compared between the groups. Incidence of adverse drug reactions was also noted for safety analysis. Baseline demographic characteristics of terbinafine and miconazole groups were similar. The study demonstrated statistical significance reduction in clinical symptom scores, attaining Mycological cure, composite score and Physician Global Assessment from baseline to 4th week and follow up week between both groups with a p value <0.05. Compared to terbinafine group, patients received miconazole as intervention had significant successful outcome at the end of treatment (4 weeks) and during the follow-up visit at 5 weeks. Topical miconazole is found to be better and an ideal drug in patients with tinea corporis for the improvement in clinical cure and successful treatment outcome compared to topical terbinafine. Both the drugs are well tolerated drug with the incidence of side effects like itching, irritation, dryness which are comparatively lesser in miconazole group.
Keywords
Antifungal; Clinical Trial; Ring Worm; Tinea Infection
Download this article as:| Copy the following to cite this article: Gideon P. E, Xavier A. S, Kumaravelu P, David D. C. A Randomized Open Labeled Parallel Group Study to Compare the Efficacy and Safety of Topical Terbinafine and Miconazole in Patients with Tinea Corporis. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Gideon P. E, Xavier A. S, Kumaravelu P, David D. C. A Randomized Open Labeled Parallel Group Study to Compare the Efficacy and Safety of Topical Terbinafine and Miconazole in Patients with Tinea Corporis. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/36hA4Zs |
Introduction
Tinea corporis, a ringworm infection also known as tinea carcinata, is a superficial dermatophytosis of arms and legs. It is characterized by either inflammatory or non-inflammatory lesions on the glabrous skin i.e., skin regions except the groin, palms, scalp and soles. Ringworm infection is caused by a type of fungi that require keratin for their growth called dermatophytes. Hence dermatophytes grow in body areas rich in keratin such as skin, scalp, hair and nails. These infections are communicable through contact with infected people.1
Tinea corporis is the common form of dermatophytosis observed. It begins usually on smooth, hairless areas with a pruritic patch or plaque as a circular, red spot gradually increasing in size varying from 1 to 10 cm in diameter, with a central clearing and raised red colored scaly border associated with itching, burning sensation or sometimes asymptomatic. It is commonly located on face, neck, trunk or limbs. Often with a macule and spreads radially.2,3 The risk factors are poor personal hygiene, lower socioeconomic group, age less than 15 years, hyperhidrosis, debilitated, and immune-compromised individuals.4
According to World Health Organization (WHO), the worldwide incidence of superficial fungal infection is 20%.5 The severity of the inflammation mainly depends on the immunogenicity of the infective species. In typical cases, Trichophyton rubrum, Epidermophyton floccosum and Trichophyton mentagrophytes are the most frequent isolates, among which Trichophyton rubrum is the most common infectious agent in the world and is the cause for 47% of tinea corporis cases. The intensity of the reaction is influenced by the size of the infective inoculum, site of the body affected and immune status of the host.6
Most of the patients are diagnosed clinically for Tinea corporis. Confirmation of dermatophyte infections requires a mycological examination from skin scrapings in 10% to 15% potassium hydroxide (KOH) preparation.7 The differential diagnosis of tinea corporis includes drug eruptions, pityriasis rosea, nummular dermatitis, tinea versicolor, erythema multiforme, erythrasma, psoriasis and secondary syphilis.8
There are pharmacological and non-pharmacological management of tinea infection. Non pharmacological management includes personal hygiene, and keeping the skin dry. Site of infection, efficacy, safety, and cost of treatment are the important factors considered by the physician for choosing a drug. Topical antifungals are generally effective for uncomplicated tinea infection but in case of severe infection, large infected area with secondary infection, immunocompromised individuals and for the patients not responding to topical therapy, systemic medications are preferred.9 An appropriate topical agent for superficial dermatophytosis should have broad-spectrum activity, greater cure rate, easy dosing, minor side effects, and more economical. Though tinea infections are most commonly treated with topical preparations, therapeutic success is limited because of poor patient compliance, greater relapse rates at specific body sites, and long duration of treatment. The two principal pharmacologic groups of antifungal drugs are the azoles and the allylamines. Others include polyenes (amphotericin B and nystatin), tolnaftate, haloprogin and ciclopirox. Commonly used topical antifungals are allylamines such as terbinafine, butenafine and azoles like clotrimazole, fluconazole, miconazole, ketoconazole,etc.10,11
Terbinafine is a synthetic agent, a fungicidal with a broad spectrum of activity. Topical cream is indicated in localized tinea corporis, pedis, cruris, and pityriasis versicolor and is 80% efficacious.12 Topical miconazole, a synthetic imidazole, is a fungistatic, which is highly efficacious (>90%) in tinea infection with better side effect profile.13 It acts by inhibiting lanosterol-14 demethylase, an enzyme in ergosterol synthesis, whereas the fungicidal action of terbinafine is due to competitive inhibition of squalene epoxidase and subsequent accumulation of squalene. Common systemic antifungal agents used are oral griseofulvin, terbinafine, fluconazole and itraconazole which are indicated in severe and deep mycotic infections. Considering the increase in the incidence of tinea infection and its morbidity, as well as conflicting results observed in the clinical trials comparing azoles and allylamines from scientific literature the current study was undertaken to compare the efficacy, safety of terbinafine, and miconazole, the two widely used drugs as topical formulation for tinea corporis infection.
Materials and Methods
Study Setting
The study is a randomized, open label, parallel group study, conducted at out-patient department of Dermatology in Sri Ramachandra Medical College Hospital, Porur, Chennai. The research work was conducted after obtaining approval from institutional ethics committee (Register no: ECR/203/Inst/TN/2013/RR-16). Clinically diagnosed patients with tinea corporis were screened for eligibility using predefined criteria. Patients of age between 18 and 70 years, of either gender, not on treatment with topical antifungal drugs with involvement of less than 20% of body surface area, and willing to participate in the study were recruited after obtaining written informed consent. Patients with uncontrolled diabetes mellitus, hypertension, allergic to either of the study drugs, pregnant, lactating women, and hepatic or renal dysfunction were excluded from the study.
Randomization and Study Duration
Patients who met the eligibility criteria were randomized to two parallel intervention groups in 1:1 ratio by simple randomization method using computer generated random numbers, where group 1 received topical terbinafine 1% and group 2 received topical miconazole 2% twice daily, for a period of 3 weeks. The total study duration was 5 weeks, and the intervention period was 3 weeks. The participants were instructed to apply the medicine over the lesions as well as up to 2 cm around the lesion, two times a day. There were 5 scheduled visits during the study: visit 1 (0 weeks-beginning of the study), visit 2 (after 1 week), visit 3 (after 2 weeks), visit 4 (after 3 weeks), and visit 5 (after 4 weeks – follow up visit).
Study Procedure
During the screening visit at baseline, eligibility for the study, fasting blood glucose, HbA1C level, clinical as well as mycological assessment were done after obtaining written informed consent. Clinical examination included grading of pruritus, erythema severity assessment, and composite scoring consisted of assessment of vesicles and desquamation along with pruritus, and erythema. Skin scrapings from lesions were mounted with KOH and microscopically examined for the presence of thin filament forms of hyphae.7 Both clinical and mycological examinations were done at all study visits. During each visit after drug administration, study participants were observed for any occurrence of adverse reactions and reported. Physician Global Assessment (PGA) was performed at visit 4 and visit 5.
Pruritus Grading System
The grading for pruritus was in based on the distribution, frequency, severity of itch, and quality of sleep. The sum of the individual scores was calculated and the pruritus was graded as mild, moderate, and severe, according to the total score of 0-5, 6-11, and 12-19 respectively.14
Table 1: Pruritus Grading System.
| Pruritus Grading System | ||
| Distribution | Solitary site | 1 |
| Multiple sites | 2 | |
| Generalised | 3 | |
| Frequency | Episodic | 1 |
| Frequent | 3 | |
| Continuous | 5 | |
| Severity | Rubbing | 1 |
| Scratching | 1 | |
| Localised excoriation | 3 | |
| Generalised excorciation | 5 | |
| Sleep disturbance | Rare | 0 |
| Occassional | 2 | |
| Frequent | 4 | |
| Totally restless | 6 | |
Erythema Grading Scale
The erythema was graded as none, mild, moderate, or severe, based on clinical feature of no erythema, slight pinkness, defined redness, and intense redness respectively.
Composite Score
Apart from pruritus, erythema, other symptoms like vesicle, desquamation were also graded and total sum of all four clinical grades were totaled and noted as composite score.
Physician Global Assessment
In the global assessment, successful treatment outcome was defined as attaining clinical and mycological cure, and clinical success was defined as symptomatic relief and clinical cure.
Statistical Analysis
All statistical analysis was done with Statistical Package for Social Science (SPSS, version 17). Both parametric/Non Parametric tests were used due to mixed distribution of data. Descriptive statistics were presented as numbers and percentages. The data was expressed in Mean ± SD. To compare continuous variables between two groups, independent sample student t test/Mann Whitney test were used. A chi-squared test was used for comparison between two attributes. p value < 0.05 was considered statistically significant.
Results
150 patients attending the dermatology OPD were assessed for eligibility, based on the pre-defined criteria 100 patients were included for the trial (Figure 1). Baseline characteristics of both the intervention groups are tabulated (Table 2). No significant difference was observed between the groups. Severity of pruritus, erythema was graded during each study visit (Table 3, 4). Composite score of all clinical symptoms were compared between intervention groups at baseline and the end of treatment (Table 6). Significant reduction in clinical symptoms was noted in miconazole group compared to terbinafine.
At baseline, KOH test for mycological assessment was positive for all patients in both the groups. In second visit, the mycological resolution was higher proportion in miconazole group (90%) when compared with terbinafine group (57%). At 3rd visit, the mycological resolution in miconazole group was 94% and in terbinafine group, it was 89%. At 4th visit and Follow up visit, all patients showed negative mycological assessment. The statistical test comparison using Chi – square test shows statistical significance between the groups with a p value of 0.001. Higher prevalence of successful treatment outcome was also noted in miconazole group at the end of treatment (28% Vs 4%), and during follow-up visit at 5 weeks (90% Vs 70%). Patient reported treatment emergent side effects were noted, itching and irritation at the site of application were the commonly reported adverse effects. No serious effects were observed during the study period (Table 7).
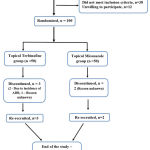 |
Figure 1: Participant Flow Diagram. |
Table 2: Baseline Demographic Characteristics of Study Participants.
| S.no | Characteristic | Terbinafine group (n=50) | Miconazole group (n=50) |
| 1 | Age (years) | 37.30 ± 15.19 | 37.50 ± 16.43 |
| 2 | Gender, male n (%) | 27 (54%) | 25 (50%) |
| 3 | HbA1c level | 4.78 ± 0.44 | 4.94 ± 0.36 |
| 4 | Weight (Kg) | 58.70 ± 13.36 | 57.07 ± 11.99 |
| 5 | Height (cm) | 161.81 ± 7.35 | 162.55 ± 8.30 |
| 6 | Smokers, n (%) | 2 (4%) | 7 (14%) |
| 7 | Alcohol use, n (%) | 1 (2%) | 7 (14%) |
| 8 | Diabetes mellitus, n (%) | 7 (14%) | 8 (16%) |
| 9 | Hypertension, n (%) | 5 (10%) | 4 (8%) |
| 10 | Percentage body surface area involved, median(range) | 10 (5, 15) | 10 (5, 15) |
Table 3: Grading of Pruritus Severity During Each Study Visit.
| Group | Terbinafine (N=50) | Miconazole (N=50) | ||||||
| Grade | Absent (0) | Mild (1) | Moderate (2) | Severe (3) | Absent (0) | Mild (1) | Moderate (2) | Severe (3) |
| Visit 1 | 0 | 3 | 43 | 4 | 0 | 0 | 25 | 25 |
| Visit 2 | 0 | 17 | 33 | 0 | 0 | 5 | 44 | 1 |
| Visit 3 | 0 | 38 | 12 | 0 | 0 | 38 | 12 | 0 |
| Visit 4 | 0 | 47 | 3 | 0 | 23 | 27 | 0 | 0 |
| Visit 5 | 0 | 50 | 0 | 0 | 43 | 7 | 0 | 0 |
Table 4: Grading of Erythema Severity During Each Study Visit.
| Group | Terbinafine (N=50) | Miconazole (N=50) | ||||||
| Grade | Absent (0) | Mild (1) | Moderate (2) | Severe (3) | Absent (0) | Mild (1) | Moderate (2) | Severe (3) |
| Visit 1 | 0 | 3 | 45 | 2 | 0 | 6 | 43 | 1 |
| Visit 2 | 0 | 0 | 50 | 0 | 0 | 1 | 49 | 0 |
| Visit 3 | 0 | 24 | 26 | 0 | 2 | 38 | 10 | 0 |
| Visit 4 | 1 | 45 | 4 | 0 | 29 | 21 | 0 | 0 |
| Visit 5 | 40 | 10 | 0 | 0 | 47 | 3 | 0 | 0 |
Table 5: Clinical Symptoms of Vesicles and Desquamation During Each Study Visit.
| Group | Terbinafine (N=50) | Miconazole (N=50) | ||
| Grade | Vesicle | Desquamation | Vesicle | Desquamation |
| Visit 1 | 21 | 8 | 45 | 38 |
| Visit 2 | 9 | 3 | 12 | 10 |
| Visit 3 | 1 | 0 | 0 | 0 |
| Visit 4 | 0 | 0 | 0 | 0 |
| Visit 5 | 0 | 0 | 0 | 0 |
P > 0.05, Chi-square test.
Table 6: Composite Score of All Clinical Symptoms.
| Terbinafine | Miconazole | p value | |
| Baseline | 5.60 ± 1.010 | 7.16 ± 1.017 | 0.834 |
| End of treatment | 2.12 ± 0.385 | 0.96 ± 0.699 | 0.002* |
| % Reduction | 78.57 | 97.2 |
*Paired t test, Comparison of two matched groups.
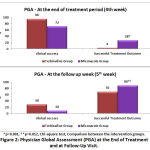 |
Figure 2: Physician Global Assessment (PGA) at the End of Treatment and at Follow-Up Visit. |
Table 7: Treatment Emergent Adverse Effects Observed During Study Period.
| Adverse Drug Reaction (ADR) | Terbinafine Group (N=50) |
Miconazole Group (N=50) |
| Itching | 7 | 4 |
| Irritation | 5 | 6 |
| Dry skin | 3 | 0 |
| Thinning of skin or atrophy | 0 | 0 |
| Skin pigmentation | 0 | 0 |
| Others | 0 | 0 |
Discussion
Tinea corporis is a superficial fungal infection, and our tertiary care hospital caters to the particular socioeconomic strata where this infection is significantly prevalent. The epidemiological features of dermatophyte infections vary according to the geographical area. Factors, such as lifestyle, socio-economic conditions, migratory streams and incidence of uncharacteristic co-morbidities have led to change in the epidemiological features of dermatophyte infections. Inadequate efficacy in treatment and re-infections in inter-triginous areas have led to recurrence of tinea infections.15
The clinical picture of dermatophyte infections results from a combination of the destruction of keratinized structures by the fungus and from the host inflammatory response. Other influences include the site of infection, the size of the inoculums and physical factors, such as humidity or friction. The concomitant inflammatory symptoms including pruritus affect the quality of life of patients suffering from dermatophyte infections. Mycoses have significant negative social, psychological, occupational and health effects.3,15 As persistent infections can compromise the quality of life to a larger extent, this study was taken up to compare the efficacy of topical terbinafine 1% and topical miconazole 2% in reducing the clinical symptoms of tinea corporis and thereby provide a better lifestyle for the patients.
In this study, the mean age was 37.30 ± 15.16 in terbinafine group. In miconazole group with a sample size of 50, the mean age was 37.50 ± 16.33. In a study conducted by Banerjee et al., in comparing the efficacy of a topical imidazole drug with amorolfine in tinea corporis, the mean age was 29.88 ± 10.86 and 30.42 ± 11.25 respectively, which is similar to our study. 64.9% patients in the study were in 20–50 years age group, 28.3% in 1–20 years age group and 6.8% patients were in above 50 years age group.16 In an epidemiological research on dermatophytosis in human patients conducted by Bhatia VK et al., also revealed a similar pattern of age distribution in their study.17
In our study, incidence of tinea corporis is almost equal in both sexes. In terbinafine group, out of 50 subjects, 27 patients were male and 23 participants were female. In Miconazole group, out of 50 subjects, 25 participants were male and 25 participants were female. But in some studies, incidence is more among men and in some it is more among women. In a clinical and mycological study of dermatophytic infections conducted by Surendran KA et al., a total of 100 patients were enrolled in the study, comprising of 62 males and 38 females, where males were affected to a more extent.18 But in a study conducted by Teklebirhan G et al., to evaluate the prevalence of dermatophytic infection and the spectrum of dermatophytes in patients attending a tertiary hospital, a total of 305 clinical samples were collected from suspected cases of dermatophytosis of which 97 (31.8%) were from males and 208 (68.2%) from females.19
In this present study there is a considerable improvement in the clinical symptoms in both groups from baseline to end of 4th week and at end of follow up week. At baseline in terbinafine group 6% were under mild category of pruritus, 85% were under moderate grade and 8% were severely affected with pruritus. In Miconazole group 50% were under moderate category, whereas 50% were under severe category. At week 4, in terbinafine amd miconazole group 94% and 46% were under mild grade respectively, but in Miconazole group 46% had no symptoms of pruritus. During follow up visit, 100% patients in terbinafine and 14% patients in miconazole group were under mild grade, but in Miconazole group 86% had no symptoms of pruritus.
At baseline, the erythema grading in terbinafine group are 6% (mild), 90% (moderate) and 4% (severe). In Miconazole group 12% (mild), 86% (moderate) and 12% (severe). At week 4, in terbinafine amd miconazole group 90% and 42% were under mild grade respectively, 2% of terbinafine group and 58% of miconazole group had no symptoms of erythema. In terbinafine group 8% were under moderate category. During follow up visit, 80% patients in terbinafine and 90% patients in miconazole group were free from erythema. 20% and 6% were under mild grade respectively in both groups.
In a study conducted by Choudhary S et al., to evaluate the efficacy and safety of terbinafine hydrochloride 1% cream vs eberconazole nitrate 1% cream in localized tinea corporis and tinea cruris, it is found that in both groups, a statistically significant complete cure (p <0.05) was observed between baseline to 2nd week, as well as baseline to 3rd week, which is similar to our study.20
But in some studies, both allylamine and azole group of drugs shows complete clinical cure and there would be no statistical significance. In a study by Choudhary S et al, they compared the efficacy and safety of terbinafine hydrochloride 1% cream with sertaconazole nitrate 2% cream against tinea corporis and tinea cruris. 100% cure was seen in both groups at the end of third week. And they also reported that there was no significant difference during the course of treatment between the two groups (first week, P = 0.461 and second week, P = 0.679). But, 80% cure rate was seen in terbinafine group compared to 73.35% cure rate in sertaconazole with no statistical significance, at the end of 2nd week.21
Azole drugs also have significant effect in reduction of clinical symptoms when compared to allylamines or with clotrimazole. In an observational study conducted by Shivamurthy RP et al., which compares sertaconazole with clotrimazole against tinea corporis infections, reported erythema grades of the patients after treatment period showing grade 0 (30% patients), 1(63.3% patients) for clotrimazole group and grade 0 (96.7% patients), grade 1 (0% patients) in sertaconazole group. They reported a significantly better reduction in erythema by sertaconazole (p<0.02).22 This might be attributed to anti-inflammatory property and better clinical efficacy of sertaconazole. Similarly in the sertaconazole showed better improvement in erythema, scaling and itching.
In our study, at baseline, all patients were positive for KOH test. The mycological grading was found to be in higher proportion in miconazole group (90%) when compared with terbinafine group (57%) at the end of second week. All patients were having negative mycological assessment after 4th week and follow up. The result shows statistical significance between the groups (p value = 0.001), which is highly significant.
Jerajani H et al., conducted a prospective, randomized, multicenter, open-label, parallel-group study with 62 patients of Tinea corporis and Tinea cruris infection. They compared sertaconazole 2% cream BD for 4 weeks, terbinafine 1% cream OD for 2 weeks and luliconazole 1% cream OD for 2 weeks. Their study revealed mycologic resolution using KOH microscopy as 100% for sertaconazole group when compared to terbinafine group which is 86.4%.23 Therefore azole group of drugs gives a better mycological cure when compared to terbinafine.
In a randomized, double-blind, vehicle-controlled study conducted by Jarratt M et al, the efficacy of 1% luliconazole cream was compared between 2 and 4 weeks treatment periods. They found a cure rate of 91.4% in 4-week treatment group when compared to 2 weeks treatment which is found to be 88.6%, which shows a cure rate in dose dependent manner.24
The composite score in this study were 5.60 (baseline), 3.90(visit 2), 2.78(visit 3), 2.12(visit 4), 1.20(follow up visit) in terbinafine group and the composite score were 7.16(baseline), 4.34(visit 2), 2.40(visit 3), 0.96(visit 4), 0.20(follow up visit) in miconazole group. At week 4, there is a statistical significance of p value – 0.002 and therefore it is found that there is a better percentage reduction of composite score mean was higher in miconazole group (97.2%) than terbinafine group (78.57%).
Jerajani H et al, study showed baseline composite score of 6.80 for sertaconazole group, 6.73 for terbinafine group and 7.05 for luliconazole group, which is found to be similar to our study results.23 After study period, there was a reduction in the mean total composite score showing 97.1% for sertaconazole group, 91.2% for terbinafine group and 92.9% for luliconazole group.
Physician global assessement is based on 3 criteria, i.e Successful treatment outcome which included clinical cure and negative mycology; Clinical success termed for symptomatic relief and clinical care; and Clinical failure termed for no clinical and mycological improvement. After 4th week of treatment, the ‘Successful treatment outcome’ was 4% and 28% in terbinafine and miconazole group respectively. At the follow up week, it was found that, ‘Successful treatment outcome’ in terbinafine group was 70% compared to that of 90% in miconazole group. The result shows a statistical significance between the groups with a p value of 0.001 and 0.012 at 4th week and follow-up week respectively.
In a study conducted by Banerjee et al, in comparing topical clotrimazole and topical amorolfine in patients with tinea corporis, it is found that at the end of the study, the Physician Global Assessment did not show any significant difference between the groups.16 But in our study there was a statistical significance between the groups. The study drugs were well tolerated in both the groups. All the adverse events that have occurred during the study period were mild to moderate, none were serious. The frequency of adverse effects observed was similar to the reported adverse effects for the interventions.
In our study we have included both patient reported symptoms and physician assessment to report the outcome measures. We have included all the major symptom scores, and mycological assessment to compare the efficacy of two interventions. We have not done pharmacoeconomic analysis of both the interventions, which could have added relevant information about the cost-benefit. Longer follow-up period after resolution can help to assess and compare the recurrence rate.
Conclusion
In conclusion, both the groups have shown significant effects in reducing the clinical symptoms, attaining mycological cure, reduction in composite score and Physician Global Assessment after the treatment period of 4 weeks. But topical miconazole was found to be better in reducing composite clinical symptom score, achieving mycological cure, hence the successful treatment outcome compared to topical terbinafine. Though there are certain studies which states that topical terbinafine and topical miconazole have the same profile in terms of efficacy and safety, but based on our study results, topical miconazole is found to be better and an ideal drug in tinea patients for the improvement in clinical cure and successful treatment outcome which could help to prevent the recurrence and further complications.
Conflict of Interest
None of the authors have any conflicts of interest to declare.
Acknowledgment
The author(s) received no specific funding to acknowledge for this research work.
References
- Yee G, Al Aboud AM. Tinea Corporis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 Sep 24]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK544360/
- Grover S, Roy P. Clinico-mycological Profile of Superficial Mycosis in a Hospital in North-East India. Med J Armed Forces India. 2003 Apr;59(2):114–6.
CrossRef - Lakshmanan A, Ganeshkumar P, Mohan SR, Hemamalini M, Madhavan R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015 Feb;33 Suppl:134–6.
CrossRef - Qadim HH, Golforoushan F, Azimi H, Goldust M. Factors leading to dermatophytosis. Ann Parasitol. 2013;59(2):99–102.
- WHO, 2005. Epidemiology and management of common skin diseases in children in developing countries. World Health Organization, Geneva.
- Jacyk WK. Four common infectious skin conditions Tinea corporis, Pityriasis versicolor, Scabies, Larva migrans. South African Family Practice. 2004 Sep 1;46(8):13–6.
CrossRef - Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006 Jun;72(3):238–41.
CrossRef - Hsu S, Le EH, Khoshevis MR. Differential Diagnosis of Annular Lesions. AFP. 2001 Jul 15;64(2):289.
- Cohn MS. Superficial fungal infections. Topical and oral treatment of common types. Postgrad Med. 1992 Feb 1;91(2):239–44, 249–52.
CrossRef - El-Gohary M, van Zuuren EJ, Fedorowicz Z, Burgess H, Doney L, Stuart B, Moore M, Little P. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014 Aug 4;(8):CD009992.
CrossRef - Poojary SA. Topical antifungals: A review and their role in current management of dermatophytoses. Clin Dermatol Rev 2017;1, Suppl S1:24-9.
CrossRef - Jg N, Sm A-R. Update on terbinafine with a focus on dermatophytoses. Clin Cosmet Investig Dermatol. 2009 Apr 21;2:49–63.
CrossRef - Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS. Miconazole: A Review of its Antifungal Activity and Therapeutic Efficacy. Drugs. 1975 Jun 1;9(6):406–23.
CrossRef - Al-Qarqaz FA, Aboosi MA, Al-Shiyab D, Bataineh A. Using Pruritus Grading System for Measurement of Pruritus in Patients with Diseases Associated with Itch. Jordan Medical Journal [Internet]. 2012 Jul 1 [cited 2020 Sep 24];46(1). Available from: https://journals.ju.edu.jo/JMJ/article/view/3023
- Nussipov Y, Markabayeva A, Gianfaldoni S, Tchernev G, Wollina U, Lotti J, Roccia MG, Fioranelli M, Lotti T. Clinical and Epidemiological Features of Dermatophyte Infections in Almaty, Kazakhstan. Open Access Maced J Med Sci. 2017 Jun 19;5(4):409–13.
CrossRef - Banerjee M, Ghosh AK, Basak S, Das KD, Gangopadhyay DN. Comparative evaluation of effectivity and safety of topical amorolfine and clotrimazole in the treatment of tinea corporis. Indian Journal of Dermatology. 2011 Nov 1;56(6):657.
CrossRef - Bhatia VK, Sharma PC. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014 Mar 9;3:134.
CrossRef - Surendran K, Bhat RM, Boloor R, Nandakishore B, Sukumar D. A Clinical and Mycological Study of Dermatophytic Infections. Indian J Dermatol. 2014;59(3):262–7.
CrossRef - Teklebirhan G, Bitew A. Prevalence of Dermatophytic Infection and the Spectrum of Dermatophytes in Patients Attending a Tertiary Hospital in Addis Ababa, Ethiopia. Int J Microbiol. 2015;2015:653419.
CrossRef - Choudhary SV, Aghi T, Bisati S. Efficacy and safety of terbinafine hydrochloride 1% cream vs eberconazole nitrate 1% cream in localised tinea corporis and tinea cruris. Indian Dermatol Online J. 2014 Apr;5(2):128–31.
CrossRef - Choudhary S, Bisati S, Singh A, Koley S. Efficacy and Safety of Terbinafine Hydrochloride 1% Cream vs. Sertaconazole Nitrate 2% Cream in Tinea Corporis and Tinea Cruris: A Comparative Therapeutic Trial. Indian J Dermatol. 2013;58(6):457–60.
CrossRef - Shivamurthy RPM, Reddy SGH, Kallappa R, Somashekar SA, Patil D, Patil UN. Comparison of Topical Anti- Fungal Agents Sertaconazole and Clotrimazole in the Treatment of Tinea Corporis-An Observational Study. J Clin Diagn Res. 2014 Sep;8(9):HC09-HC12.
CrossRef - Jerajani H, Janaki C, Kumar S, Phiske M. Comparative assessment of the efficacy and safety of sertaconazole (2%) cream versus terbinafine cream (1%) versus luliconazole (1%) cream in patients with dermatophytoses: a pilot study. Indian J Dermatol. 2013 Jan;58(1):34–8.
CrossRef - Jarratt M, Jones T, Kempers S, Rich P, Morton K, Nakamura N, Tavakkol A. Luliconazole for the treatment of interdigital tinea pedis: A double-blind, vehicle-controlled study. Cutis. 2013 Apr;91(4):203–10.







